Abstract
Background
Despite evidence that bright light can improve mood, the neurobiology remains poorly understood. Some evidence implicates the catecholamines. In the present study, we measured the effects of transiently decreasing dopamine (DA) synthesis on mood and motivational states in healthy women with mild seasonal mood changes who were tested in either bright or dim light.
Methods
On 2 test days, participants slept overnight in a light-controlled room. On the morning of each session, half of the participants awoke to gradual increases of bright light, up to 3000 lux, and half to dim light (10 lux). For all participants, DA was reduced on 1 of the test days using the acute phenylalanine/tyrosine depletion (APTD) method; on the other day, they ingested a nutritionally balanced control mixture (BAL). Beginning 4 hours postingestion, participants completed subjective mood questionnaires, psychological tests and a progressive ratio breakpoint task during which they worked for successive units of $5.
Results
Thirty-two women participated in our study. The APTD lowered mood, agreeableness, energy and the willingness to work for monetary reward. The effects on energy and motivation were independent of light, while the effects on mood and agreeableness were seen in the dim condition only, being prevented by bright light.
Limitations
Acute phenylalanine/tyrosine depletion might affect systems other than DA. The sample size was small.
Conclusion
These results suggest that increased DA function may be responsible for some of the beneficial effects of light, while adding to the evidence that the neurobiology of mood and motivational states can be dissociated.
Introduction
Bright light can alleviate low mood in patients with seasonal affective disorder (SAD),1 nonseasonal major depressive disorder,2,3 antepartum depression,4 eating disorders5,6 and those with subsyndromal winter mood disturbances7,8 (for contrasting results, see Rosenthal and colleagues9). Although the mechanism of action behind the therapeutic effect of bright light remains poorly understood, some potential candidates contributing to this effect have been identified. For example, altered diurnal rhythms may be involved, and administration of melatonin at certain times of day can shift rhythms and improve symptoms in patients with SAD.10 Cortisol is also implicated, but the effects have been inconsistent, as extended bright light exposure has been reported to decrease,11 increase12,13 and to have no effect14 on plasma cortisol levels in humans. A small but more consistent literature implicates serotonin (5-HT). Decreasing 5-HT synthesis with acute tryptophan depletion (ATD) temporarily reverses the effects of phototherapy,15,16 while in the converse experiment, bright light exposure protects against ATD-induced mood lowering in healthy women with mild seasonal changes to mood and behaviour.17
The SAD symptom cluster of lethargy, psychomotor retardation, weight gain and low motivation18 suggest a hypoactive dopamine (DA) system.19–22 However, evidence that light influences DA transmission remains limited and largely indirect. For example, acute bright light exposure (7000 lux for 10 min) increased striatal blood flow in healthy volunteers,23 and in a case series of patients with Parkinson disease, light exposure (1000–1500 lux, 1 hour daily for 2 weeks) improved mood, social activity and motor function and, in some cases, reduced DA replacement therapy dosages by 13%–100%.24 Consistent with this, DA D2/D3 receptor availability was greater in participants whose measurements were preceded by 30 days with more sunshine than in those tested after less sunshine.25 Patients with symptomatic SAD also show evidence of altered DA system function, and, compared with healthy controls,26 striatal DA transporter (DAT) availability is reduced. Moreover, in patients with remitted SAD, catecholamine depletion with α-methyl-para-tyrosine (AMPT) reversed the therapeutic efficacy of bright light.27 Finally, in a preclinical model, rodents kept in constant darkness showed increased monoamine cell body apoptosis — changes that were associated with behavioural alterations indicative of a depressed state.28
The purpose of the present study was to investigate the roles of DA and light on mood and motivational states using the acute phenylalanine/tyrosine depletion (APTD) method.29,30 The APTD method has a number of advantages. Unlike AMPT, evidence suggests that it preferentially affects the release of DA;29 compared with DA receptor antagonists, APTD influences presynaptic release and, consequently, the activation of all DA receptor subtypes. While APTD does not typically induce mood-lowering when participants are at rest,31 effects have been seen in women following a psychological challenge.32 Features of the study design were similar to that of aan het Rot and colleagues,17 with the exception of the transmitter targeted. In brief, healthy women with mild seasonal changes to mood and behaviour were tested in bright or dim light conditions after ingestion of amino acid mixtures that either did or did not contain the DA precursors phenylalanine and tyrosine. We hypothesized that APTD would lower both mood and motivation and that these effects would be prevented by exposure to bright light.
Methods
Participant recruitment
This study was approved by the Institutional Review Board of McGill University’s Faculty of Medicine, and all participants provided written informed consent. The study was carried out on women, as some depletion studies found women were more susceptible to mood-lowering effects than men.16 Participants were recruited using advertisements asking for women who felt less energetic in winter than in summer. Those individuals who expressed interest were scheduled for an assessment that included the Structured Clinical Interview for DSM-IV, Non-patient Edition (SCID-NP33), the Seasonal Pattern Assessment Questionnaire (SPAQ34), the Short Michigan Alcoholism Screening Test,35 the Beck Depression Inventory (BDI36) and a self-rated version of the Hamilton Rating Scale for Depression, Seasonal Affective Disorders self-rating version.37 The SPAQ included the Global Seasonality Scale (GSS), which assesses degree of seasonal change on 6 dimensions: sleep, appetite, mood, energy, weight and social activity. The inclusion criteria were age 18–40 years, because seasonality is more common in this age range,38 and a GSS score of 6 or more out of 24, which indicates at least mild seasonal changes in mood and behaviour. In addition participants must have never met criteria for major depressive disorder, including seasonal type (i.e., SAD). Exclusion criteria were any past or present Axis I disorders, as defined by the DSM-IV-TR; a BDI score of 15 or higher; use of hormonal contraceptives in the previous 3 months; abnormal sleep patterns; excessive caffeine, tobacco or alcohol use; and any current medical illness. Medical health was assessed through an exam performed by a physician.
Testing took place over 3 consecutive winter seasons (2008–2010) starting and ending with the daylight savings time changes, which occurred on the first Sunday in November and the second Sunday in March.
Study design
Participants were randomly assigned, in blocks of 4, to 2 groups: the dim and bright light conditions. In both conditions, participants received the APTD mixture and the nutritionally balanced (BAL) control mixture on different days using a double-blind crossover procedure. The order of treatment was randomized. The APTD mixture and the procedure for its administration was the same as that used previously.32 As participants were blind to which amino acid (AA) mixture they received on each day, the fact that they were not blind to the bright or dim light condition should not have biased the results.
On each test day, participants ingested the APTD or BAL mixture. Changes in plasma tyrosine and prolactin levels have been observed as soon as 1–3 hours after ingestion of the AA mixture, but effects may be maximal at 4–7 hours.39,40
Experimental procedure
Testing environment
The testing environment was a windowless, temperature-controlled and soundproof isolation suite free from external time cues, ensuring uniformity among sessions. Light intensity settings were verified with a calibrated light metre (IL1400A; International Light). Light was administered by ceiling-mounted banks of cool-white fluorescent lamps (4100 K, F32T8/TL841; Philips Lighting, and F032/841; Sylvania) covered with filters emitting less than 1% of radiant energy up to 400 nm (Uvalite Plus; Plaskolite Inc.).
Pre-experimental session protocol
We asked participants to keep a regular sleep/wake cycle for at least 1 week before each test day. Test days took place during the follicular phase of the menstrual cycle and at least 3 days apart. The day before each test day, participants were asked to consume a low-protein diet.
A prepackaged, precooked meal was either collected by the participant at McGill University or delivered to her home before each test day. These meals were the same as those used previously in a study conducted in our laboratory,41 and the protein (22.6 g/24 h), and caloric content (2212 kcal/24 h) were similar to those in the low protein diet used by Delgado and colleagues.42 The same diet was provided before both experimental sessions to maintain the double-blind and standardize the participant’s food intake the day before a test day.
Participants arrived at the laboratory on the evening before each test day, at least 1 hour before their normal sleep time. Upon arrival, participants handed in all personal devices that indicated the time, were placed in their isolation suite, were asked to complete a set of questionnaires, and were screened for recent illicit drug use (Triage Panel for Drugs of Abuse, Biosite Diagnostics) and pregnancy. Lights were turned off at their habitual bedtime, and participants slept in total darkness for their individual standard duration of sleep (7–9 h).
Experimental session
Test sessions began at each participant’s normal wake time with an initial light setting of 10 lux; if the participant was assigned to the dim light group they remained in this light setting. For participants in the bright light group, light was gradually increased in 3 increments over 15 minutes (10, 180 and 3000 lux) and then remained at 3000 lux for the remainder of the test day. Participants remained in their assigned light condition for the entire test day (460 min). Participants were given 30 minutes to get ready for the day, after which they completed morning questionnaires, had their vital signs recorded and provided a blood sample. They were then given 1 of the AA mixtures. Subjective rating questionnaires were completed 4, 5, 6 and 6.5 hours postingestion. Between 4 and 6 hours postingestion 2 computer tasks were administered: a challenging facial emotion recognition task and a progressive ratio (PR) breakpoint task. Data from the first task are not presented here. After completion of the computer tasks, participants had a second blood sample taken. At 6.5 hours post-ingestion, participants underwent a negative mood induction procedure, as outlined below. About 6.75 hours after being awoken, participants completed a final set of questionnaires, had their vital signs reassessed, had a high protein meal to normalize their tyrosine levels, and went home (Appendix 1, Fig. S1, available at cma.ca/jpn).
Negative mood induction procedure
During the initial screening, participants were asked to provide and rank 5–10 sad autobiographical memories, which were used in an established negative mood induction procedure.43 On each test day, participants listened to music known to induce a sad mood. While the music was playing, the researcher narrated a standardized script instructing the participant to listen to the music and recall the personal memory they had ranked as most sad. After reading the script, the participant was left alone for 20 minutes with the music playing.
Test measures
Self-reported mood ratings
Self-reported mood states were measured with the 72-item bipolar Profile of Mood States (POMS), a questionnaire sensitive to transient fluctuations in subclinical mood states.44 Raw POMS scores were normalized to t scores.
Incentive motivation
A PR breakpoint task was administered on a computer to measure motivation. Participants were offered the opportunity to work for $5 amounts by repeatedly pressing the letters “D” and “R;” each successful D–R combination counted as 1 press. Participants were told they could stop at any time, or when the time limit, unknown to them, was reached. To receive the first $5, participants had to complete 100 presses. Each subsequent breakpoint increased by a factor of 2.3. Participants could complete up to a maximum of 10 reward units ($50 maximum). A session ended when the ratio was too large to maintain responding or when the time ran out (50 min). The dollar amount used as a monetary reward for the incentive motivation task was determined by a pilot study (Appendix 1, supplementary results). Those data indicated that, first, participants were willing to work to complete PR breakpoints to earn units of monetary reward and that, second, those participants working for units of $5 reached higher breakpoints than those working for $1 units. Based on these results, and to avoid floor effects, we used the $5 reward unit for the present study.
Side effects
Dizziness, headache and nausea have been reported following AA mixture ingestion or exposure to bright light.17 These 3 items were assessed using visual analogue scales (VAS) 100 mm in length.
Plasma amino acid analysis
We obtained blood samples 15 minutes before and about 6 hours postingestion of the AA mixture (5 of 64 samples were not obtained). Blood samples were centrifuged immediately (10 min, 1500g, 0 ºC) and stored at −80 ºC until further analysis. We measured plasma AA levels, except for tryptophan, using high-pressure liquid chromatography with fluorometric detection (HPLC-FD) on an Ultrasphere ODS reverse-phase column (Beckman Coulter) with ophtalaldehyde precolumn derivatization and aminoadipic acid as an internal standard. Total tryptophan levels were measured by HPLC-FD on a Bondpak reverse-phase column (Phenomenex).
Statistical analysis
All analyses were performed using IBM SPSS Statistics Version 19 for Macintosh. We analyzed between-groups demographic variables using independent sample t tests. Changes to plasma AA levels, behavioural measures and subjective mood states were analyzed using repeated-measures analysis of variance (ANOVA). To rule out the potential influence of baseline differences on subjective mood ratings on the POMS or side-effect ratings on the VAS, we calculated Δ scores from evening baseline by subtracting data from each time point from the evening baseline to get a difference score; Δ scores were used for all analysis of participant mood states and side effects. The ANOVA included 2 within-subjects variables: AA mixture (APTD v. BAL) and time. The model also included 1 between-subjects variable: light (bright v. dim). Models considered AA mixture, light, time and their interactions. We considered main effects and interactions to be significant at p < 0.05. Significant interactions and planned comparisons were analyzed post hoc using a Bonferroni correction for multiple comparisons. We used Pearson correlations to evaluate the association between AA mixture–induced side effects and subjective mood and behavioural measures when significant results were found. We considered these results to be significant at p ≤ 0.05. All tests were 2-tailed.
All variables were screened for normality using the Shapiro–Wilks test, and equality of variance was assessed using the Levene test. These analyses indicated that the PR task data required a Log10 transformation to satisfy the assumption of normality required for parametric analyses.
Results
Participants
Eighteen women were initially assigned to the dim light condition. Of these, 2 withdrew after the first test day (1 stated side effects, 1 stated illicit drug use post–test day). Twenty women were initially assigned to the bright light condition. Of these, 4 withdrew after the first test day (3 stated side effects, 1 stated time constraints). Four of these 6 women had received the APTD mixture. Thirty-two women completed the study.
Participant GSS scores ranged from 7 to 16 out of 24 (mean 10.6, standard deviation [SD] 2.5). As none of the participants reported major depressive episodes, as determined by the SCID, but all described clinically relevant symptoms during the winter months, participants were classified as having subsyndromal SAD.7 Average participant age was 23.0 (SD 4.8) years, and BDI scores averaged 3.13 (SD 3.44). Participants in the 2 light conditions were similar on demographic variables with the exception of alcohol consumption (Table 1); however, all drinking histories were below clinically relevant levels, and individual differences in alcohol use did not correlate with any of the behavioural variables analyzed.
Table 1.
Demographic and clinical characteristics of participants
| Group, mean (SD)* | ||
|---|---|---|
|
|
||
| Characteristic | Bright light n = 16 |
Dim light n = 16 |
| Age, yr | 21.9 (2.6) | 24.2 (6.1) |
| Body mass index | 23.1 (2.3) | 22.3 (2.7) |
| Current alcohol use, drinks/wk† | 5.1 (2.7) | 2.0 (2.0) |
| BDI score | 3.3 (3.7) | 3.0 (3.3) |
| SPAQ: Global Seasonality Score | 10.4 (2.2) | 10.8 (2.8) |
| Occupation, % student | 50.0 | 68.8 |
| Bed time, hh:mm | 23:32 (0:54) | 00:00 (0:42) |
| Wake time, hh:mm | 8:10 (1:21) | 8:32 (0:35) |
Of the 16 women randomly assigned to the dim light group, 9 received BAL on their first test day and 7 received APTD. Of the 16 women assigned to the bright light group, 8 received BAL on their first day and 8 received APTD.
Plasma amino acids
Plasma concentrations of tyrosine and phenylalanine increased and decreased 4 hours postingestion of the BAL and APTD mixtures, respectively. There was no significant effect of light on plasma amino acid levels (F1,26 = 0.001, p = 0.51; Table 2).
Table 2 *.
Plasma levels of tyrosine and phenylalanine, and ratios of tyrosine and phenylalanine to large neutral amino acids
| BAL, mean (SD) | APTD, mean (SD) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Amino acid, μmol/L | Morning session | Afternoon session | Difference, % | Morning session | Afternoon session | Difference, % |
| Tyrosine | 49.8 (1.6) | 123.0 (10.0)‡ | 147.1 | 52.5 (1.5) | 13.6 (0.6)‡ | −74.1 |
| Phenylalanine | 44.8 (1.0) | 57.0 (4.0)‡ | 27.0 | 45.4 (1.1) | 14.8 (1.2)‡ | −67.5 |
| Tyrosine:LNAA† | 0.131:1 (0.004) | 0.151:1 (0.011) | 15.2 | 0.135:1 (0.003) | 0.015:1 (0.001)‡ | −88.8 |
| Phenylalanine:LNAA | 0.116:1 (0.002) | 0.063:1 (0.003)‡ | −45.9 | 0.115:1 (0.002) | 0.017:1 (0.002)‡ | −85.3 |
APTD = acute phenylalanine tyrosine depletion; BAL = balanced; LNAA = large neutral amino acid; SD = standard deviation.
On the APTD test session, plasma concentrations of tyrosine and phenylalanine decreased significantly (tyrosine F1,26 = 151.25, p < 0.001; phenylalanine F1,26 = 79.56, p < 0.001). The APTD mixture decreased tyrosine and phenylalanine levels by 74.09% and 67.46% from baseline. In comparison, the BAL mixture increased plasma tyrosine and phenylalanine by 147.11% and 27.01%. Ingestion of the APTD mixture also significantly decreased the ratio of tyrosine to other LNAAs by 88.77% (p < 0.001), whereas the BAL mixture caused a nonsignificant change in ratio (change of 15.18%, p = 0.06). Ingestion of both mixtures significantly decreased the ratio of plasma phenylalanine to other LNAAs (F1,26 = 155.30, p < 0.001), but the reductions were more pronounced following APTD than BAL with a decrease of 85.34% versus 45.90%, respectively (p < 0.001).
LNAAs include phenylalanine, tryptophan, leucine, isoleucine, valine and methionine.
p < 0.0001.
Effects of APTD: not prevented by light
Motivation
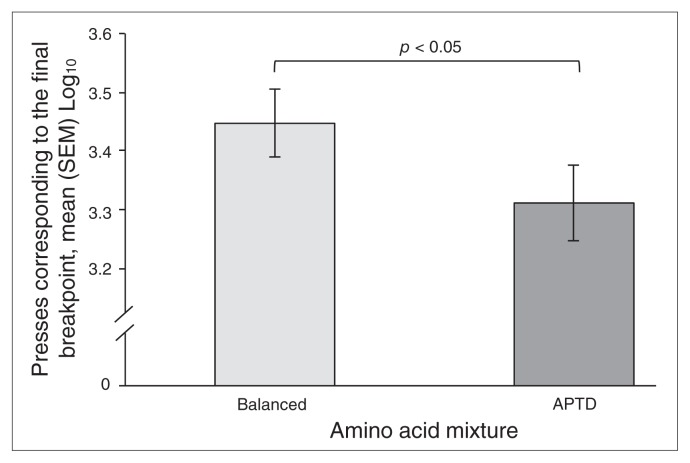
We found a significant main effect of AA mixture for PR breakpoint reached (F1,30 = 6.624, p = 0.015). On the APTD test session, the breakpoint achieved was lower than that achieved with the BAL session, and this effect was independent of light (light × AA mixture: F1,30 = 1.657, p = 0.21; Fig. 1).
Fig. 1.
Change on progressive ratio breakpoint task performance induced by ingestion of a control balanced (BAL) or acute phenylalanine/tyrosine depletion (APTD) amino acid mixtures. Plotted scores are mean number of presses corresponding to the final breakpoint and standard error of the mean (SEM). Participants, regardless of light condition, worked for fewer $5 units on the APTD test day than on the BAL test day.
Energy
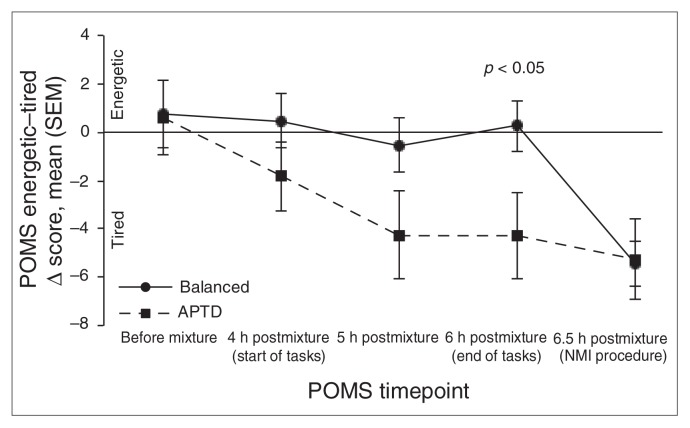
There was a significant AA mixture × time interaction for the POMS energetic–tired subscale (F4,120 = 4.711, p = 0.001; Table 3). Compared with the BAL test session, participants’ rating scores on the APTD session began to decrease during the psychological tasks (p = 0.10), an effect that was statistically significant immediately after their completion (p = 0.036). Again, this effect was independent of light (light × AA mixture × time: F4,120 = 1.007, p = 0.41; Fig. 2).
Table 3.
Summary of raw POMS subscale scores
| Time point, mean (SEM) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| POMS Item | Laboratory entry (evening baseline) | Before AA mixture | 4 h postingestion (pretasks) | 5 h postingestion (midtasks) | 6 h postingestion (posttasks) | 6.5 h postingestion (post-NMI) |
| Composed–anxious | ||||||
| Dim light condition | ||||||
| BAL | 56.8 (2.0) | 57.9 (2.2) | 60.0 (2.5) | 56.9 (2.5) | 57.5 (2.4) | 54.9 (2.3) |
| APTD | 60.1 (2.3) | 57.9 (2.3) | 59.2 (2.6) | 55.5 (2.3) | 55.4 (2.5) | 53.6 (2.2) |
| Bright light condition | ||||||
| BAL | 61.4 (2.3) | 61.6 (2.1) | 61.1 (2.0) | 61.3 (2.3) | 59.6 (2.1) | 57.4 (2.2) |
| APTD | 60.3 (2.7) | 60.1 (2.1) | 61.6 (2.1) | 58.8 (2.3) | 60.1 (2.5) | 56.0 (2.3) |
| Confident–unsure | ||||||
| Dim light condition | ||||||
| BAL | 53.9 (1.7) | 54.4 (1.7) | 51.3 (1.7) | 50.8 (1.9) | 52.6 (1.9) | 46.0 (2.2) |
| APTD | 54.8 (2.3) | 53.6 (2.3) | 51.1 (1.9) | 49.3 (1.9) | 48.8 ( 1.9) | 45.6 (2.3) |
| Bright light condition | ||||||
| BAL | 56.4 (1.3) | 52.9 (1.2) | 55.0 (1.5) | 51.8 (1.6) | 54.0 (1.5) | 49.7 (1.7) |
| APTD | 54.4 (1.6) | 53.6 (1.6) | 53.7 (1.6) | 49.8 (1.5) | 51.4 (1.4) | 48.2 (1.5) |
| Clearheaded–confused | ||||||
| Dim light condition | ||||||
| BAL | 51.6 (1.9) | 54.8 (2.0) | 52.1 (2.2) | 51.8 (2.2) | 53.4 (2.0) | 47.8 (2.4) |
| APTD | 53.8 (2.4) | 54.3 (2.0) | 51.0 (2.0) | 48.4 (1.7) | 48.0 (2.0) | 47.8 (2.7) |
| Bright light condition | ||||||
| BAL | 55.8 (1.7) | 54.9 (1.5) | 56.1 (1.8) | 54.5 (2.3) | 54.6 (1.9) | 51.1 (2.4) |
| APTD | 55.2 (2.0) | 54.5 (1.7) | 55.6 (1.7) | 53.4 (1.7) | 55.0 (1.6) | 50.3 (1.9) |
| Elated–depressed | ||||||
| Dim light condition | ||||||
| BAL | 52.9 (2.2) | 51.1 (2.2) | 48.8 (1.8) | 49.4 (2.1) | 52.4 (2.1) | 37.8 (1.9) |
| APTD | 52.6 (2.8) | 51.9 (2.5) | 48.4 (2.0) | 48.3 (2.3) | 47.4 (1.9) | 37.7 (2.2) |
| Bright light condition | ||||||
| BAL | 56.1 (1.6) | 54.1 (2.0) | 54.8 (2.0) | 53.8 (1.7) | 53.8 (1.5) | 42.9 (2.0) |
| APTD | 53.1 (2.2) | 52.6 (1.8) | 53.5 (1.8) | 52.1 (1.6) | 51.9 (1.6) | 41.4 (2.0) |
| Agreeable–hostile | ||||||
| Dim light condition | ||||||
| BAL | 50.6 (2.4) | 53.4 (2.6) | 53.3 (2.7) | 53.2 (2.9) | 54.4 (2.6) | 49.3 (3.1) |
| APTD | 53.7 (3.2) | 53.4 (2.6) | 53.0 (2.8) | 49.7 (2.4) | 48.8 (2.7) | 49.4 (3.1) |
| Bright light condition | ||||||
| BAL | 57.4 (2.4) | 57.1 (2.5) | 56.6 (2.5) | 55.1 (2.5) | 54.3 (2.7) | 50.3 (2.5) |
| APTD | 55.4 (2.5) | 55.0 (2.4) | 56.2 (2.3) | 54.0 (2.3) | 53.8 (2.5) | 51.4 (2.9) |
| Energetic–tired | ||||||
| Dim light condition | ||||||
| BAL | 49.4 (1.5) | 51.3 (1.8) | 49.4 (1.6) | 49.8 (1.7) | 50.8 (1.7) | 44.7 (1.6) |
| APTD | 50.2 (2.9) | 50.6 (2.0) | 46.6 (2.1) | 44.7 (2.0) | 44.0 (1.9) | 45.3 (1.9) |
| Bright light condition | ||||||
| BAL | 50.7 (2.1) | 50.3 (2.1) | 51.6 (1.9) | 49.3 (2.3) | 49.8 (1.8) | 44.4 (1.9) |
| APTD | 49.4 (2.2) | 50.2 (1.8) | 49.4 (1.7) | 46.4 (1.9) | 47.1 (2.2) | 43.9 (1.6) |
AA = amino acid; APTD = acute phenylalanine tyrosine depletion; BAL = control/balanced amino acid mixture; NMI = negative mood induction procedure; POMS = profile of mood states; SEM = standard error of the mean.
Fig. 2.
Changes in Profile of Mood States (POMS) energetic–tired mood scores induced by ingestion of control balanced (BAL) or acute phenylalanine/tyrosine depletion (APTD) amino acid mixtures. The plotted scores are the mean Δ score from evening baseline and standard error of the mean (SEM). A negative change in scores represents a lowering in mood. There was a significant main effect of amino acid mixture collapsed across light conditions, with participants reporting significantly lower energy levels 6 hours postingestion on the APTD day than on the BAL day. NMI = negative mood induction.
Effects of APTD: prevented by light
Agreeable–hostile
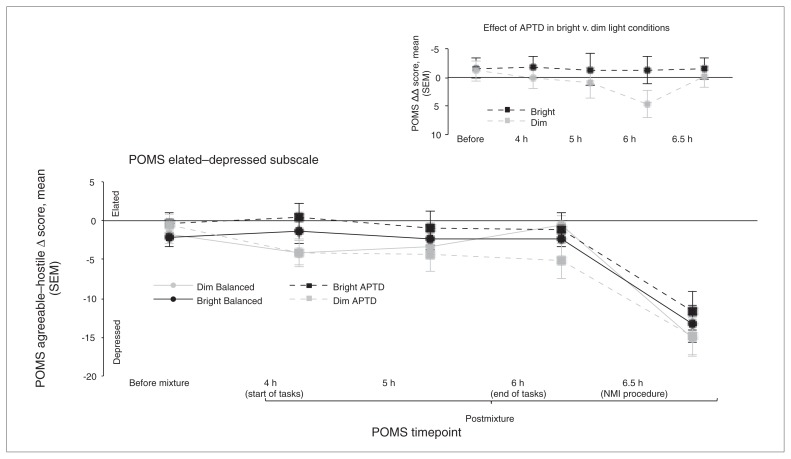
There was a significant 3-way light × AA mixture × time interaction for the POMS agreeable–hostile subscale (F4,120 = 2.580, p = 0.040). Participants in the dim light group reported significantly lower scores (i.e., greater hostility) with the APTD mixture than with the BAL mixture (Fig. 3). The decrease in agreeable–hostile scores was significant at 5 (p = 0.012) and 6 hours (p = 0.002) postingestion, corresponding to mid- and postpsychological task periods. This effect of APTD was not seen in the bright light group (p = 0.21). Visual inspection of the data also indicated a small decrease in agreeable–hostile scores for participants in the bright light group that was not predicated a priori. However, there was no significant main effect of light, and the more prominent difference in scores was between AA mixtures within the dim light condition.
Fig. 3.
Changes in Profile of Mood States (POMS) agreeable–hostile mood scores dependent on light condition (bright v. dim) and ingestion of a control balanced (BAL) or acute phenylalanine/tyrosine depletion (APTD) amino acid mixture. Values are shown as mean Δ score from evening baseline and standard error of the mean (SEM). A negative change in scores represents a lowering in mood. Significant results are between BAL and APTD within the dim light condition. No significant differences between amino acid mixtures were found within the bright light condition. *p < 0.05, †p < 0.01. The dim light group had significantly lower ratings of agreeableness on the APTD test session than on the BAL session at 5 hours and 6 hours postingestion. The inset graph depicts the same data represented as a double Δ, with difference scores calculated from morning baseline and net difference scores calculated between APTD and BAL at each time point within each light condition. The significant time points for the agreeable–hostile subscale remained consistent across both analyses. NMI = negative mood induction.
Elated–depressed
There was not a significant light × AA mixture × time interaction for the POMS elated–depressed subscale (F4,120 = 1.424, p = 0.23); however, as significant differences were anticipated on this scale a priori, we proceeded with planned comparisons. After completion of the psychosocial tasks, there was a trend for participants in the dim light group to report lower elated–depressed scores on the APTD day than on the BAL day (6 h postingestion, p = 0.05), an effect not seen in participants tested in the bright light group (p = 0.60; Fig. 4).
Fig. 4.
Profile of Mood States (POMS) elated–depressed mood scores by light condition (bright v. dim) comparing control balanced (BAL) and acute phenylalanine/tyrosine depletion (APTD) amino acid mixtures. Values are shown as mean Δ scores from evening baseline and standard errors of the mean (SEM). A negative change in scores represents a lowering in mood. Within the dim light group there was a trend toward increased ratings of depressed mood at 6 hours postingestion on the APTD test day compared with the BAL test day. There were no significant differences found between amino acid mixtures for the bright light condition. The inset graph depicts the same data represented as a double Δ, with difference scores calculated from morning baseline as well as net difference scores calculated between APTD and BAL at each time point within each light condition. The tendency toward a significant difference at 6 hours postingestion remained at the trend level when analyzed with the double Δ scores (p = 0.08). NMI = negative mood induction.
Nonsignificant POMS subscales
Other than a main effect of time (F5,150 = 9.148, p = 0.001), there were no significant main effects or interactions for the 3 remaining POMS subscales (composed–anxious, confident–unsure, clearheaded–confused).
Negative mood induction procedure
The negative mood induction (NMI) procedure was intended to act as a final stressor; however, the effects were potent, overwhelming any potential modest effects of APTD on mood. For all subscales of the POMS there was a significant effect of time (F4,120 = 8.947, p = 0.001), reflecting lower mood ratings following the NMI (6.5 h postingestion), as compared with all other time points (p = 0.027).
Discussion
In the present study, 2 main findings emerged. First, to our knowledge, this study provides the first evidence in humans that lowered DA transmission can reduce the motivation to work for a nonpharmacological reward, an effect produced irrespective of light condition. Second, APTD increased susceptibility to low mood following the mild stress of performing psychological tasks, an effect that was prevented by bright light exposure. Since bright light exposure prevented only some of the effects of APTD, the results support the suggestion that the influence of DA on mood and motivational states are dissociable. Moreover, the results add to the evidence that the effects of DA on motivational states do not require changes in mood.22,45 These results do not suggest, though, that mood and motivational states are unable to influence each other;46,47 negative mood states can decrease motivation, and susceptibility to low mood could be increased by a combination of low DA-related changes in motivation and energy. The present study though, suggests that neurobiological influences on mood and motivation are dissociable.
We have reported previously that APTD can induce a mild mood lowering effect when participants are psychologically stressed.32 The present study supports this observation following the administration of milder psychological challenges, and raises the possibility that studies where this mood-lowering effect was absent could reflect differential lighting conditions. Bolstering this interpretation, the present study benefitted from testing participants under carefully controlled conditions. Participants arrived the night before, slept in the laboratory and remained there throughout testing procedures without external influences or contact with individuals other than the researcher and nurse.
In previous studies, APTD reduced reward sensitivity48 as well as the willingness to work for pharmacological rewards.49,50 The present study indicates that lowered DA can also reduce the motivation to work for a nonpharmacological reward. The measure of motivation was the PR breakpoint task. Reductions in PR breakpoints could occur for many reasons, including increased fatigue, lowered mood or a decrease in the reward’s ability to sustain focused interest independent of mood.45,51 Notably, no significant correlations between changes in POMS energetic–tired scores and PR results were found for either light condition (r < 0.218, p > 0.15). While it cannot be ruled out that the effect of APTD on PR breakpoints may have reflected fatigue or psychomotor retardation, previous APTD studies reported no effect of APTD on response time or latency in either recovered depressed patients48,52 or healthy controls,53 whereas a significant increase in apathy has been observed.53 Our observations support this finding and suggest that motivation to obtain reward and subjective experience of energy are both DA-related but may be independent effects. This finding is also supported by animal and human research demonstrating that locomotor activation or fatigue can be behaviourally and anatomically dissociated from motivation to work for or seek out reward.54,55
Limitations
Our results should be interpreted in light of the following considerations. First, as with most studies using different light levels it is difficult to “blind” the condition of light. However, the influence of light condition was only seen in combination with AA mixture, which was administered in a double-blind fashion, and no independent effects of light were observed. Second, bright light did not prevent the effects of APTD on energy or motivation despite the fact that SAD summer remission is associated with marked increases in energy and goal-directed behaviour, sometimes to the point of hypomanic-like states.1 This noted, our study investigated the effects of an acute (1-day) change in light intensity only. More prolonged changes in light exposure lasting days or weeks might influence DA and DA-related behaviours more potently. Third, the mood-lowering effects of APTD did not appear until 5 hours postingestion — a delay that could reflect the influence of the psychological tests administered just before the assessment or the normal time-course of the effects of APTD. However, the latter interpretation seems unlikely because the subjective effects of APTD have been seen as early as 4 hours postingestion29,40,56 and because DA AA precursor use is known to be greater under challenge conditions.57,58 Fourth, the APTD mixture increased reporting of nausea and headache, which could have affected participants’ mood ratings. However, when analyzed, reported side-effects and mood ratings at the 2 significant time points (5 and 6 hours postingestion) were not associated. Fifth, the ability of bright light to prevent APTD-induced mood-lowering effects but not the decreases in energy levels and motivation to seek monetary reward could reflect either a positive mood–promoting mechanism that is nondopaminergic or effects in 2 separate DA pathways — 1 light sensitive, the other not. However, just as selective increases in DA synthesis do not elevate mood,59 APTD alone, before the addition of a psychological stressor, did not lower mood. Moreover, accumulating evidence suggests that the mechanisms regulating mood and motivational states are separable.21,45,51 Sixth, APTD might affect catecholamines other than DA. However, microdialysis,29 neuroendocrine40 and fos expression studies60 all suggest that APTD affects DA transmission over other catecholamines. Moreover, APTD has been shown to decrease extracellular DA levels in humans as indicated by increased striatal [11C]raclopride binding in imaging studies30,61 and to increase circulating prolactin levels (indicative of reduced DA function62), but to have no effect on plasma melatonin, a neuroendocrine index of noradrenergic neurotransmission.63 Finally, the sample size of 32 participants was modest, and only women were tested. Future studies will need to involve men to determine whether the present effects can be generalized or whether they are sex-specific.
Conclusion
Results from the present study suggest that enhancement of DA function may be responsible for some of the beneficial effects of light. In addition, these and findings from relevant studies support the hypothesis that the neurobiology of mood and motivational states can be dissociated in humans.
Acknowledgements
We acknowledge the valuable support of Abdel Azzoug, Dr. Zia Choudhry, Itamar Danziger, Dr. Mimi Israel, Milla Kerusenko and Franceen Lenoff during various stages of the study. We also thank Dr. Paul Clarke for his feedback on an earlier version of the manuscript.
Footnotes
Competing interests: None declared for E.I. Cawley, S. Park, K. Sancton and S.N. Young. Over the past 3 years, M. aan het Rot has received compensation from the Innovational Research Incentives Scheme Veni from the Netherlands Organisation for Scientific Research (NWO). D.B. Boivin has received travel grants and speaker honoraria from Servier and is Founder/CEO of Alpha Logik Consultants, Inc. C. Benkelfat and M. Leyton hold research chairs at McGill University.
Contributors: M. aan het Rot, C. Benkelfat, S.N. Young, D.B. Boivin and M. Leyton designed the study. E. Cawley, S. Park, K. Sancton and M. Leyton acquired the data, which E. Cawley, S. Park, M. aan het Rot, S.N. Young and M. Leyton analyzed. E. Cawley, S. Park and M. Leyton wrote the article, which all authors reviewed and approved for publication.
References
- 1.Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 2.Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression. Cochrane Database Syst Rev. 2004:CD004050. doi: 10.1002/14651858.CD004050.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieverse R, Van Someren EJW, Nielen MMA, et al. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68:61–70. doi: 10.1001/archgenpsychiatry.2010.183. [DOI] [PubMed] [Google Scholar]
- 4.Wirz-Justice A, Bader A, Frisch U, et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry. 2011;72:986–93. doi: 10.4088/JCP.10m06188blu. [DOI] [PubMed] [Google Scholar]
- 5.Janas-Kozik M, Krzystanek M, Stachowicz M, et al. Bright light treatment of depressive symptoms in patients with restrictive type of anorexia nervosa. J Affect Disord. 2011;130:462–5. doi: 10.1016/j.jad.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Braun DL, Sunday SR, Fornari VM. Bright light therapy decreases winter binge frequency in women with bulimia nervosa: a double-blind, placebo-controlled study. Compr Psychiatry. 1999;40:442–8. doi: 10.1016/s0010-440x(99)90088-3. [DOI] [PubMed] [Google Scholar]
- 7.Kasper S, Rogers SL, Yancey A, et al. Phototherapy in individuals with and without subsyndromal seasonal affective disorder. Arch Gen Psychiatry. 1989;46:837–44. doi: 10.1001/archpsyc.1989.01810090079011. [DOI] [PubMed] [Google Scholar]
- 8.Avery DH, Kizer D, Bolte MA, et al. Bright light therapy of subsyndromal seasonal affective disorder in the workplace: morning vs. afternoon exposure. Acta Psychiatr Scand. 2001;103:267–74. doi: 10.1034/j.1600-0447.2001.00078.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal NE, Rotter A, Jacobsen FM, et al. No mood-altering effects found after treatment of normal subjects with bright light in the morning. Psychiatry Res. 1987;22:1–9. doi: 10.1016/0165-1781(87)90044-8. [DOI] [PubMed] [Google Scholar]
- 10.Lewy AJ, Lefler BJ, Emens JS, et al. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–9. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung CM, Khalsa SBS, Scheer FAJL, et al. Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 2010;25:208–16. doi: 10.1177/0748730410368413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheer FA, Buijs RM. Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab. 1999;84:3395–8. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- 13.Leproult R, Colecchia EF, L’Hermite-Balériaux M, et al. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab. 2001;86:151–7. doi: 10.1210/jcem.86.1.7102. [DOI] [PubMed] [Google Scholar]
- 14.Leproult R, Van Reeth O, Byrne MM, et al. Sleepiness, performance, and neuroendocrine function during sleep deprivation: effects of exposure to bright light or exercise. J Biol Rhythms. 1997;12:245–58. doi: 10.1177/074873049701200306. [DOI] [PubMed] [Google Scholar]
- 15.White TL, Lejuez CW, de Wit H. Personality and gender differences in effects of d-amphetamine on risk taking. Exp Clin Psychopharmacol. 2007;15:599–609. doi: 10.1037/1064-1297.15.6.599. [DOI] [PubMed] [Google Scholar]
- 16.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002;71:857–65. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 17.aan het Rot M, Benkelfat C, Boivin DB, et al. Bright light exposure during acute tryptophan depletion prevents a lowering of mood in mildly seasonal women. Eur Neuropsychopharmacol. 2008;18:14–23. doi: 10.1016/j.euroneuro.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): The Association; 2000. text revised. [Google Scholar]
- 19.Willner P. Dopamine and depression. In: Di Chiara G, editor. Handbook of experimental pharmacology: dopamine in the CNS. Berlin: Springer; 2002. pp. 387–416. [Google Scholar]
- 20.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 21.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leyton M. The neurobiology of desire: dopamine and the regulation of mood and motivational states in humans. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. New York (NY): Oxford University Press; 2008. pp. 222–43. [Google Scholar]
- 23.Diehl DJ, Mintun MA, Kupfer DJ, et al. A likely in vivo probe of human circadian timing system function using PET. Biol Psychiatry. 1994;36:562–5. doi: 10.1016/0006-3223(94)90621-1. [DOI] [PubMed] [Google Scholar]
- 24.Willis GL, Turner EJD. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int. 2007;24:521–37. doi: 10.1080/07420520701420717. [DOI] [PubMed] [Google Scholar]
- 25.Tsai H-Y, Chen KC, Yang YK, et al. Sunshine-exposure variation of human striatal dopamine D2/D3 receptor availability in healthy volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:107–10. doi: 10.1016/j.pnpbp.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Neumeister A, Willeit M, Praschak-Rieder N, et al. Dopamine transporter availability in symptomatic depressed patients with seasonal affective disorder and healthy controls. Psychol Med. 2001;31:1467–73. doi: 10.1017/s003329170105434z. [DOI] [PubMed] [Google Scholar]
- 27.Neumeister A, Turner EH, Matthews JR, et al. Effects of tryptophan depletion vs catecholamine depletion in patients with seasonal affective disorder in remission with light therapy. Arch Gen Psychiatry. 1998;55:524–30. doi: 10.1001/archpsyc.55.6.524. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez MMC, Aston-Jones G. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci U S A. 2008;105:4898–903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McTavish SFB, Cowen PJ, Sharp T. Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology (Berl) 1999;141:182–8. doi: 10.1007/s002130050823. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery AJ, McTavish SFB, Cowen PJ, et al. Reduction of brain dopamine concentration with dietary tyrosine plus phenylalanine depletion: an [11C] raclopride PET study. Am J Psychiatry. 2003;160:1887–9. doi: 10.1176/appi.ajp.160.10.1887. [DOI] [PubMed] [Google Scholar]
- 31.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 32.Leyton M, Young SN, Pihl RO, et al. Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacology. 2000;22:52–63. doi: 10.1016/S0893-133X(99)00086-X. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. New York (NY): Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 34.Rosenthal NE, Bradt GH, Wehr TA. Seasonal Pattern Assessment Questionnaire. Bethesda (MD): National Institute of Mental Health; 1984. [Google Scholar]
- 35.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–26. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 37.Williams JBW. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–7. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 38.Kasper S, Wehr TA, Bartko JJ, et al. Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County, Maryland. Arch Gen Psychiatry. 1989;46:823–33. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- 39.McTavish SF, Callado L, Cowen PJ, et al. Comparison of the effects of amethyl-p-tyrosine and a tyrosine-free amino acid load on extracellular noradrenaline in the rat hippocampus in vivo. J Psychopharmacol. 1999;13:379–84. doi: 10.1177/026988119901300408. [DOI] [PubMed] [Google Scholar]
- 40.McTavish SFB, McPherson MH, Harmer CJ, et al. Antidopaminergic effects of dietary tyrosine depletion in healthy subjects and patients with manic illness. Br J Psychiatry. 2001;179:356–60. doi: 10.1192/bjp.179.4.356. [DOI] [PubMed] [Google Scholar]
- 41.Benkelfat C, Ellenbogen MA, Dean P, et al. Mood-lowering effect of tryptophan depletion: enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51:687–97. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- 42.Delgado PL, Charney DS, Price LH, et al. Serotonin function and the mechanism of antidepressant action: reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47:411–8. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez S, Vander Wal JS, Spring B. A negative mood induction procedure with efficacy across repeated administrations in women. J Psychopathol Behav Assess. 2003;25:49–55. [Google Scholar]
- 44.Lorr M, McNair DM, Fisher S. Evidence for bipolar mood states. J Pers Assess. 1982;46:432–6. doi: 10.1207/s15327752jpa4604_16. [DOI] [PubMed] [Google Scholar]
- 45.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 46.Lazarus RS. Emotion and adaptation. Oxford; Oxford University Press; 1994. [Google Scholar]
- 47.Rolls ET. Emotion explained. Oxford; Oxford University Press; 2005. [Google Scholar]
- 48.Roiser JP, McLean A, Ogilvie AD, et al. The subjective and cognitive effects of acute phenylalanine and tyrosine depletion in patients recovered from depression. Neuropsychopharmacology. 2005;30:775–85. doi: 10.1038/sj.npp.1300659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barrett SP, Pihl RO, Benkelfat C, et al. The role of dopamine in alcohol self-administration in humans: individual differences. Eur Neuropsychopharmacol. 2008;18:439–47. doi: 10.1016/j.euroneuro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Venugopalan VV, Casey KF, O’Hara C, et al. Acute phenylalanine/tyrosine depletion reduces motivation to smoke cigarettes across stages of addiction. Neuropsychopharmacology. 2011;36:2469–76. doi: 10.1038/npp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salamone JD, Correa M, Farrar AM, et al. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McTavish SFB, Mannie ZN, Harmer CJ, et al. Lack of effect of tyrosine depletion on mood in recovered depressed women. Neuropsychopharmacology. 2005;30:786–91. doi: 10.1038/sj.npp.1300665. [DOI] [PubMed] [Google Scholar]
- 53.McLean A, Rubinsztein JS, Robbins TW, et al. The effects of tyrosine depletion in normal healthy volunteers: implications for unipolar depression. Psychopharmacology (Berl) 2004;171:286–97. doi: 10.1007/s00213-003-1586-8. [DOI] [PubMed] [Google Scholar]
- 54.Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–8. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 55.Leyton M, aan het Rot M, Booij L, et al. Mood-elevating effects of d-amphetamine and incentive salience: the effect of acute dopamine precursor depletion. J Psychiatry Neurosci. 2007;32:129–36. [PMC free article] [PubMed] [Google Scholar]
- 56.Leyton M, Casey KF, Delaney JS, et al. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci. 2005;119:1619–27. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- 57.Carlsson A, Lindqvist M. Dependence of 5-HT and catecholamine synthesis on concentrations of precursor amino-acids in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1978;303:157–64. doi: 10.1007/BF00508062. [DOI] [PubMed] [Google Scholar]
- 58.Acworth IN, During MJ, Wurtman RJ. Tyrosine: effects on catecholamine release. Brain Res Bull. 1988;21:473–7. doi: 10.1016/0361-9230(88)90161-x. [DOI] [PubMed] [Google Scholar]
- 59.Liggins J, Pihl RO, Benkelfat C, et al. The dopamine augmenter L-DOPA does not affect positive mood in healthy human volunteers. PLoS ONE. 2012;7:e28370. doi: 10.1371/journal.pone.0028370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Masurier M, Cowen PJ, Sharp T. Fos immunocytochemical studies on the neuroanatomical sites of action of acute tyrosine depletion in the rat brain. Psychopharmacology (Berl) 2004;171:435–40. doi: 10.1007/s00213-003-1607-7. [DOI] [PubMed] [Google Scholar]
- 61.Leyton M, Dagher A, Boileau I, et al. Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2004;29:427–32. doi: 10.1038/sj.npp.1300328. [DOI] [PubMed] [Google Scholar]
- 62.Harmer CJ, McTavish SFB, Clark L, et al. Tyrosine depletion attenuates dopamine function in healthy volunteers. Psychopharmacology (Berl) 2001;154:105–11. doi: 10.1007/s002130000613. [DOI] [PubMed] [Google Scholar]
- 63.Sheehan BD, Tharyan P, McTavish SFB, et al. Use of a dietary manipulation to deplete plasma tyrosine and phenylalanine in healthy subjects. J Psychopharmacol. 1996;10:231–4. doi: 10.1177/026988119601000309. [DOI] [PubMed] [Google Scholar]