Abstract
Background
Children with bipolar disorder (BD) or severe mood dysregulation (SMD) show behavioural and neural deficits during facial emotion processing. In those with other psychiatric disorders, such deficits have been associated with reduced attention to eye regions while looking at faces.
Methods
We examined gaze fixation patterns during a facial emotion labelling task among children with pediatric BD and SMD and among healthy controls. Participants viewed facial expressions with varying emotions (anger, fear, sadness, happiness, neutral) and emotional levels (60%, 80%, 100%) and labelled emotional expressions.
Results
Our study included 22 children with BD, 28 with SMD and 22 controls. Across all facial emotions, children with BD and SMD made more labelling errors than controls. Compared with controls, children with BD spent less time looking at eyes and made fewer eye fixations across emotional expressions. Gaze patterns in children with SMD tended to fall between those of children with BD and controls, although they did not differ significantly from either of these groups on most measures. Decreased fixations to eyes correlated with lower labelling accuracy in children with BD, but not in those with SMD or in controls.
Limitations
Most children with BD were medicated, which precluded our ability to evaluate medication effects on gaze patterns.
Conclusion
Facial emotion labelling deficits in children with BD are associated with impaired attention to eyes. Future research should examine whether impaired attention to eyes is associated with neural dysfunction. Eye gaze deficits in children with BD during facial emotion labelling may also have treatment implications. Finally, children with SMD exhibited decreased attention to eyes to a lesser extent than those with BD, and these equivocal findings are worthy of further study.
Introduction
Severe mood disorders in children, specifically bipolar disorder (BD) and severe mood dysregulation (SMD), have recently received increased attention, as researchers and clinicians acknowledge the substantial impairment and treatment needs of these populations. The BD and SMD phenotypes differ in that BD is characterized by distinct episodes of mania (and, frequently, depression), whereas SMD is characterized by severe, chronic, impairing irritability.1 Importantly, however, the 2 phenotypes are similar in that both are severe pediatric mood disorders that share some neuropsychological deficits, including deficits in facial emotion labelling. Here, we used visual scan paths to compare children with BD and SMD during facial emotion labelling to explore the extent to which this shared behavioural deficit might be mediated by common abnormalities in eye gaze. We note that this approach of examining the pathophysiology of shared deficits across diagnostic categories, as well as in healthy populations, is consistent with the new Research Domain Criteria initiative of the National Institute of Mental Health.2
Children with BD and SMD show similar behavioural deficits in facial emotion labelling: they are are less accurate in labelling facial emotions, including happy, sad, fearful and angry expressions, than healthy control children.3–5 Behavioural deficits in facial emotion labelling can be mediated by perturbations within the neural circuit encompassing the ventral visual pathway, amygdala and ventrolateral prefrontal regions.6–8 In both adults and children with BD, amygdala dysfunction during facial emotion processing is among the most replicated findings.9–15 Given the associations between amygdala dysfunction and deficits in eye gaze,16 a study of visual scan paths in individuals with BD is a logical extension of the neuroimaging literature on this illness. With regard to SMD, the neural dysfunction data are considerably more limited, although data do suggest both amygdala and parietal dysfunction.17,18
The examination of visual scan patterns of emotional faces has been important to elucidate precise pathophysiological mechanisms of the aberrant face labelling behaviours across psychiatric disorders. In people with autism,19–22 psychopathy23 and schizophrenia,24–27 each in comparison with healthy controls only, eye tracking studies have revealed associations between emotion labelling deficits and decreased attention to eyes. However, few studies consider BD, and none include children. In 1 study, adults with mood disorders, including BD, compared with those with schizophrenia or no illness, exhibited fewer and shorter fixations on facial features (i.e., eyes, noses, mouths) across emotional facial expressions and degraded faces.28 Another study suggested that manic adults avoided looking at eyes during virtual social interactions.29 The only other relevant study found no scan path differences between healthy adults and those with BD.30 No prior eye movement study has attempted to differentiate healthy children from those with BD and SMD.
We tested the hypothesis that, across emotions, children with BD and SMD are less accurate than healthy children in facial emotion labelling and pay less attention to eye regions. Prior work found that children with BD and SMD manifested similar behavioural deficits resulting from different pathophysiology. Therefore, we expected disorder-common deficits in facial emotion labelling to reflect eye gaze patterns that are disorder-specific. However, because this is, to our knowledge, the first study of eye gaze in children with BD or SMD, no data exist to support specific hypotheses on the nature of these disorder-specific patterns. Thus, we used conservative statistical approaches, well protected against type 1 error, to test for between-group differences by comparing 3 groups in 3-way interaction omnibus statistical tests.
Methods
Participants
The children (ages 8–18 yr) enrolled in an Institutional Review Board–approved protocol at the National Institute of Mental Health. Parents and children provided written informed consent and assent, respectively. We recruited children with BD and SMD through advertisements posted on support groups’ websites and distributed to psychiatrists nationwide. Healthy control children were recruited by local advertisement.
All children were assessed with the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime version (K-SADS-PL).31 Clinicians administered the K-SADS-PL, including a module for SMD, separately to children and parents, with established inter-rater reliability, κ ≥ 0.9 for all diagnoses, including the differentiation of BD from SMD.32 All of the interviewing staff members were graduate-level (masters or doctoral) clinicians who had received extensive training. The final diagnosis was generated using a best-estimate procedure33 with a consensus conference chaired by a psychiatrist with extensive experience evaluating children with SMD and BD that included discussion with the interviewing clinicians and review of medical records where appropriate.
To be included in the study, children with BD had to meet the criteria for “narrow phenotype,”32 with at least 1 full-duration (hypo)manic episode characterized by abnormally elevated mood and at least 3 DSM-IV-TR “B” mania symptoms. Children with SMD had to have nonepisodic irritability, characterized by an angry mood at least half the day on most days and noticeable by others; over-reactivity to negative emotional stimuli 3 times a week or more; and hyperarousal (≥ 3 of insomnia, intrusiveness, pressured speech, flight of ideas/racing thoughts, distractibility, psychomotor agitation).32 Symptoms of SMD must have begun before age 12 years, must have occurred for 1 year or more without remission exceeding 2 months, and must have caused severe impairment in at least 1 setting (i.e., home, school, peers) and mild impairment in another. Those with euphoric mood or distinct (hypo)manic episodes lasting 1 day or more were excluded.
For patients with BD and SMD, we assessed mood state within 48 hours of the eye-tracking session. Depressive symptoms were assessed in both groups using the Children’s Depression Rating Scale (CDRS).34 Manic symptoms were assessed in children with BD using the Young Mania Rating Scale (YMRS).35 To evaluate symptom severity, clinicians administered the Clinical Global Impression for bipolar illness (CGI-BP)36 for the patients with BD. For those with SMD, the Clinical Global Impression for SMD (CGI-SMD) was adapted to assess associated symptoms.
Healthy control children were medication-free, had no lifetime psychiatric diagnoses and no first-degree relatives with a mood disorder. All participants had an IQ greater than 70 (determined by the Wechsler Abbreviated Scale of Intelligence37), with no history of neurologic disorder, pervasive developmental disorder, chronic medical illness or substance abuse/dependence.
Stimuli
Stimuli consisted of 130 black and white photographs of 4 facial emotions (happiness, sadness, anger, fear) and a neutral facial expression, taken from the Picture of Facial Affect set.38 There were 10 exemplars: 6 female and 4 male. Each emotional face was morphed with a neutral face from the same exemplar, so that each emotion had 3 emotional levels: 60% (40% neutral), 80% (20% neutral) and 100% (0% neutral; prototypic expressions).
Eye tracking
Eye movements were measured with the EyeLink II head-mounted, video-based eye tracker (SR Research). Eye movements were sampled at 500 Hz. Before each experiment, both eyes were calibrated and validated using 9-point calibration sequences. Calibration was repeated when maximum error at validation was more than 1.5°;39 however, average error was below 1° in all 3 groups, and there was no group difference in the mean calibration error (p > 0.10). During the experiment, we performed drift correction every 5 trials. Fixations were defined with the default settings of EyeLink II, including a velocity threshold of 30°/s, acceleration threshold of 8000°/s2 and distance threshold of greater than 0.1°.
Procedure
After calibration, each participant received task instructions and performed 10 practice trials. Faces presented in the practice trials were excluded from the main task. Participants repeated the 10 practice trials until they understood the task instructions.
During the task, each face appeared for 2000 ms, followed by a 3000 ms response period. During the response period, 5 words (1 = happy, 2 = angry, 3 = fearful, 4 = sad, 5 = neutral) were presented on the screen. To identify the face emotion, participants pressed a button on a 5-button box using the right hand. A blank screen appeared during the inter-stimulus interval (range 800–1800 ms). We used a blank screen instead of a fixation cross to avoid any experimental bias that might be introduced by a fixed starting position of the eyes before face presentation.40 Each of the 130 faces was presented once and the total duration of the task was approximately 15 minutes.
Statistical analysis
Behavioural data
We compared accuracy among the groups with 2 omnibus analyses, with age as a covariate, using IBM SPSS Statistics version 19.0. First, accuracy was compared with a 3-way repeated-measures analysis of covariance (ANCOVA), with group (BD, SMD, control) as a between-subjects factor and emotion (anger, fear, sadness, happiness) and emotional level (60%, 80%, 100%) as within-subjects factors. Second, since neutral expressions had only 1 emotional level (100%), we included neutral faces in a 2-way repeated-measures ANCOVA with group (BD, SMD, control) as a between-subjects factor and emotion (100% level expression of anger, fear, sadness, happiness, neutral) as a within-subjects factor. Hereafter, we refer to this analysis as the 2-way 100%-level omnibus analysis. We performed post hoc univariate ANCOVAs to decompose all significant interactions and main effects. Only main effects and interactions that involve group differences are discussed here; other findings are included in supplemental materials (Appendix, available at cma.ca/jpn; for all results, see Table S1). Age differed among the groups (F2,69 = 3.18, p < 0.05) and was included as a covariate in all analyses. Because responses were made during the response period after the face disappeared, reaction time did not necessarily reflect individual differences validly, and reaction times were therefore not compared among participants.
Eye movement data
The 3 primary eye movement variables (total duration of fixations, number of fixations, average fixation duration) in each of 3 interest areas (eyes, nose and mouth) were calculated using EyeLink Data Viewer software (SR Research). We performed subsequent analyses with IBM SPSS Statistics version 19.0.
As with the behavioural data, we conducted 2 omnibus ANCOVA analyses for each variable, with age as a covariate. First, a 4-way repeated-measures ANCOVA included group (BD, SMD, control) as a between-subjects factor and emotion (anger, fear, sadness, happiness), emotional level (60%, 80%, 100%) and interest areas (eyes, nose, mouth) as within-subjects factors. Second, because the neutral expression had only 1 level of emotion (100%), a separate omnibus analysis compared outcome variables for only 100%-level expressions, including neutral. This 3-way repeated-measures ANCOVA included group (BD, SMD, control) as a between-subjects factor, and emotion (100% emotional expression of anger, fear, sadness, happiness, neutral) and interest areas (eyes, nose, mouth) as within-subjects factors. Hereafter, we refer to this analysis as the 3-way 100%-level omnibus analysis.
All significant interactions and main effects were decomposed by post hoc ANCOVA analyses. However, because group differences are the primary focus of this paper, we present and discuss only interactions and main effects involving between-group differences; other findings are included in the Appendix (for all results, see Tables S2–S4). In each group, we examined associations between accuracy and eye movement data with bivariate Pearson correlations. The Fisher z transformation was used to assess the significance of the differences in correlation coefficients among the groups. In addition, we used bivariate Pearson correlations to explore associations between eye movement data and clinical measures. In children with BD, eye movement data correlated with YMRS, CDRS, CGI-BP Depression or CGI-BP Mania scores. In children with SMD, eye movement data were correlated with CDRS and overall CGI-SMD scores. Finally, we conducted post hoc exploratory univariate ANCOVAs to test potentially confounding effects of comorbid illnesses, mood state or medications on the eye movement findings (see the Appendix for details).
Results
Demographic and clinical characteristics
We enrolled 72 participants: 22 children with BD, 28 children with SMD and 22 healthy controls. Groups did not differ in sex, race or IQ (all p > 0.10). On average, children with BD were older than those with SMD (p = 0.018) and controls (p = 0.07; Table 1), thus age was included as a covariate in all analyses.
Table 1.
Demographic and clinical characteristics of children with bipolar disorder, those with severe mood dysregulation and healthy controls
| Characteristic | BD, n = 22 | SMD, n = 28 | Control, n = 22 |
|---|---|---|---|
| Mean (SD) | |||
| Age, yr* | 15.42 (1.97) | 13.70 (2.72) | 14.14 (2.26) |
| Wechsler Abbreviated Scale of Intelligence37 score | 107.18 (13.72) | 105.78 (15.41) | 114.00 (17.03) |
| 2-scale IQ† | |||
| Young Mania Rating Scale35 score | 8.05 (6.11) | — | — |
| Children’s Depression Rating Scale34 score‡ | 30.74 (13.91) | 27.04 (4.62) | — |
| No. medications§ | 2.55 (1.57) | 1.71 (1.46) | — |
| No. coexisting diagnoses | 2.59 (1.44) | 2.82 (1.56) | — |
| No. (%) | |||
| Male sex | 13 (59.1) | 19 (67.9) | 12 (55) |
| Race | |||
| White | 19 (86.4) | 26 (92.8) | 17 (77.3) |
| African-American | 1 (4.5) | 0 (0.0) | 3 (13.6) |
| Hispanic | 0 (0.0) | 0 (0.0) | 1 (4.5) |
| Asian | 0 (0.0) | 1 (3.6) | 0 (0.0) |
| Other | 2 (9.1) | 1 (3.6) | 1 (4.5) |
| BD I | 17 (77.3) | — | — |
| BD II | 5 (22.7) | — | — |
| Mood state | |||
| Euthymic¶ | 16 (72.7) | 28 (100.0) | — |
| Depressed | 1 (4.5) | 0 (0) | — |
| Manic/hypomanic | 3 (13.6) | — | — |
| Mixed | 2 (9.1) | — | — |
| Coexisting diagnoses | |||
| ADHD | 15 (68.2) | 22 (78.6) | — |
| Oppositional defiant disorder or | 6 (27.3) | 16 (57.1) | — |
| conduct disorder** | |||
| Anxiety disorder | 14 (63.6) | 17 (60.7) | — |
| Medication | |||
| Unmedicated | 3 (13.6) | 9 (32.1) | — |
| Atypical antipsychotic | 11 (50.0) | 10 (35.7) | — |
| Lithium†† | 9 (40.9) | 2 (7.1) | — |
| Antiepileptic | 8 (36.4) | 7 (25.0) | — |
| Antidepressant | 5 (22.7) | 9 (32.1) | — |
| Stimulant | 11 (50.0) | 14 (50.0) | — |
ADHD = attention-deficit/hyperactivity disorder; BD = bipolar disorder; CDRS = Children’s Depression Rating Scale; SIGH-SAD = Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders;41 SMD = severe mood dysregulation; YMRS = Young Mania Rating Scale.
BD v. SMD (t48 = 2.42, p < 0.05); BD v. HC (t42 = 2.01, p < 0.10).
Data missing for 1 patient with SMD. The SIGH-SAD was collected instead of the CDRS for 3 patients with BD (mean score 16.67 [SD 4.73]) and 1 patient with SMD (score 13) who were older than 18 years at the time of the study.
Data missing for 3 patients with BD and 1 patient with SMD.
BD v. SMD (t48 = 1.93, p < 0.10).
BD v. SMD (χ2 [1, n = 50] = 8.68, p < 0.01). For 3 patients with BD and 1 patient with SMD who were older than 18 years, SIGH-SAD was used instead of CDRS to determine mood state. Euthymia was defined as CDRS < 40 (or SIGH-SAD ≤ 20) and YMRS ≤ 12. Depression was defined as CDRS ≥ 40 (or SIGH-SAD > 20) and YMRS ≤ 12. Mania/hypomania was defined as CDRS < 40 (or SIGH-SAD ≤ 20) and YMRS > 12. Mixed state was defined as CDRS ≥ 40 (or SIGH-SAD > 20) and YMRS > 12.
BD v. SMD (χ2 [1, n = 50] = 4.46, p < 0.05).
BD v. SMD (χ2 [1, n = 50] = 8.19, p < 0.01).
Children with BD were more likely to be taking lithium than those with SMD (p = 0.004). Conversely, children with SMD were more likely to be euthymic (p = 0.003) and to have comorbid oppositional defiant disorder (ODD) or other conduct disorders than those with BD (p = 0.035; Table 1).
Emotion labelling accuracy
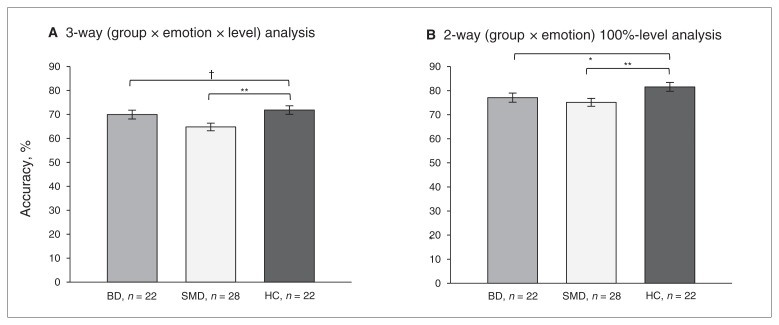
No 3-way or 2-way interactions were found in either of the omnibus ANCOVA analyses. We found a main effect of group in both the 3-way omnibus (F2,68 = 3.81, p = 0.027) and the 2-way 100%-level omnibus (F2,68 = 4.54, p = 0.014) analyses. Post hoc analyses indicated that children with SMD were less accurate than controls in both analyses (all F1,47 > 5.92, all p < 0.019). Children with BD were also less accurate than controls in the 2-way 100%-level analysis (F1,41 = 8.96, p = 0.005), with a similar trend in the 3-way analysis (F1,41 = 3.00, p = 0.09; Fig. 1).
Fig. 1.
Accuracy of emotion labelling in children with bipolar disorder (BD), those with severe mood dysregulation (SMD) and healthy controls (HC). A) Accuracy differed among the groups across expressions in 3-way (group × emotion × level) omnibus analysis (F2,68 = 3.81, p = 0.027). Post hoc results of the main effect of group are presented. Accuracy on the y axis is the mean accuracy across all emotional levels of expressions: anger, fear, sadness and happiness. B) Since the neutral expression has only the 100% emotional level, we conducted a separate analysis comparing groups on only the 100% emotional levels. Accuracy differed among the groups across expressions in this 2-way (group × emotion) 100%-level omnibus analysis (F2,68 = 4.54, p = 0.014). Post hoc results of the main effect of group are presented. Accuracy on the y axis is the mean accuracy across all 100%-level expressions: anger, fear, sadness, happiness and neutral. †p < 0.10, **p < 0.01, *p < 0.05.
Eye movement data
Total fixation durations
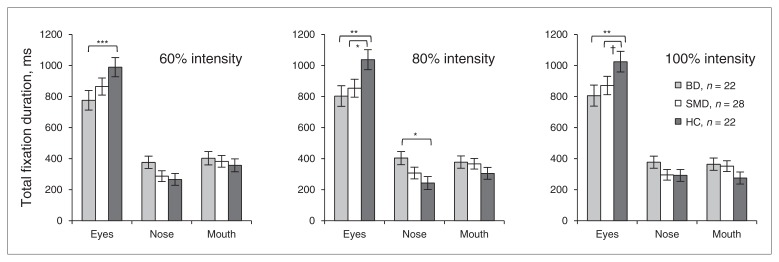
In the 4-way omnibus ANCOVA, a group × interest areas × emotional level interaction was significant (F8,272 = 2.15, p = 0.032). Post hoc analyses revealed that children with BD spent significantly less time than controls fixating on eyes for all emotional levels, regardless of specific facial emotion (all F1,41 > 8.30, all p < 0.006; Fig. 2A–C). Children with SMD also spent less time than controls fixating on eyes, but this was only significant at 80% morphed and 100% emotional expressions across all emotions (all F1,48 > 2.85, all p < 0.10; Fig. 2BC). In all 80% morphed emotional expressions, children with BD spent more time than controls fixating on the nose (F1,41 = 7.01, p = 0.011; Fig. 2B). No other group differences were found in fixations on the nose or mouth.
Fig. 2.
Total duration of fixations on interest areas (eyes, nose, mouth) in children with bipolar disorder (BD), those with severe mood dysregulation (SMD) and healthy controls (HC). The group × interest area × level interaction was significant in the 4-way omnibus analysis of covariance (F8,272 = 2.15, p = 0.032). Panels A–C show group × interest area interactions at each emotional level across emotions (all F4,136 > 2.55, all p < 0.042): (A) 60% morphed emotional level, (B) 80% morphed emotional level and (C) 100% emotional level. *** p < 0.005, ** p < 0.01, *p < 0.05, †p < 0.10.
In the 3-way 100%-level omnibus ANCOVA analysis, a group × interest areas interaction emerged (F4,136 = 3.13, p = 0.017). Post hoc analyses were consistent with the 4-way omnibus analysis. Specifically, across emotions, children with BD spent less time fixating on eyes than controls (F1,41 = 8.57, p = 0.006) or children with SMD (F1,48 = 2.87, p = 0.10).
Therefore, across all 5 emotional expressions, children with BD spent less time than controls fixating on eyes. For some expressions, children with SMD also spent less time than controls fixating on eyes (Fig. 2).
Number of fixations
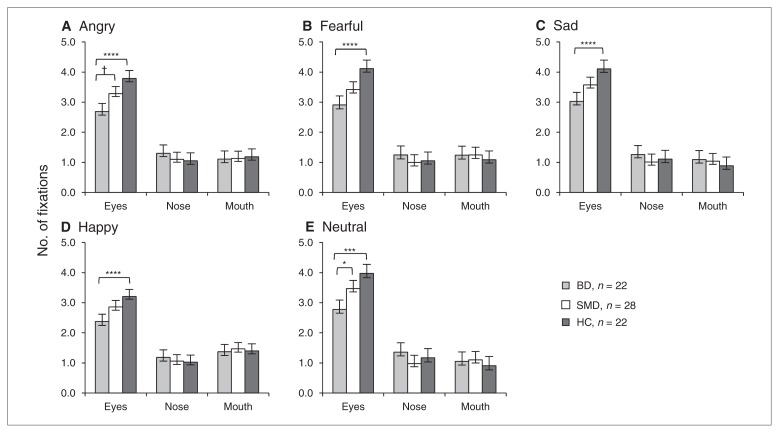
In the 4-way omnibus analysis, a group × interest areas × emotion interaction emerged (F12,408 = 2.11, p = 0.015). Post hoc analyses indicated that, for all emotional expressions, children with BD made fewer fixations than controls on eyes (all F1,41 > 13.32, all p < 0.001; Fig. 3A–D). For angry expressions only, children with BD tended to make fewer fixations than those with SMD on eyes (all F1,47 > 3.43, all p < 0.07; Fig. 3A).
Fig. 3.
Number of fixations on interest areas (eyes, nose, mouth) in children with bipolar disorder (BD), those with severe mood dysregulation (SMD) and healthy controls (HC). The group × interest areas × emotion interaction was significant in the 4-way omnibus analysis of covariance including 4 emotions (anger, fear, sadness, happiness; F12,408 = 2.11, p = 0.015). The group × interest areas interactions were also significant for neutral expressions (F4,136 = 3.93, p = 0.005). Figures A–E depict group differences in mean number of fixations on eyes in (A) angry, (B) fearful, (C) sad, (D) happy and (E) neutral expressions. ****p < 0.001, *** p < 0.005, ** p < 0.01, *p < 0.05, †p < 0.10.
Similarly, in the 3-way 100%-level omnibus ANCOVA, the group × interest areas interaction was significant (F4,136 = 3.66, p = 0.007), indicating that, regardless of specific facial emotion, children with BD made fewer fixations on eyes than controls (F1,41 = 15.50, p = 0.001) or children with SMD (F1,47 = 3.30, p = 0.08). When neutral expression was analyzed separately, findings were consistent with those for other emotions: children with BD made fewer fixations on eyes than controls (F1,41 = 13.75, p = 0.001) or children with SMD (F1,47 = 4.13, p = 0.048; Fig. 3E).
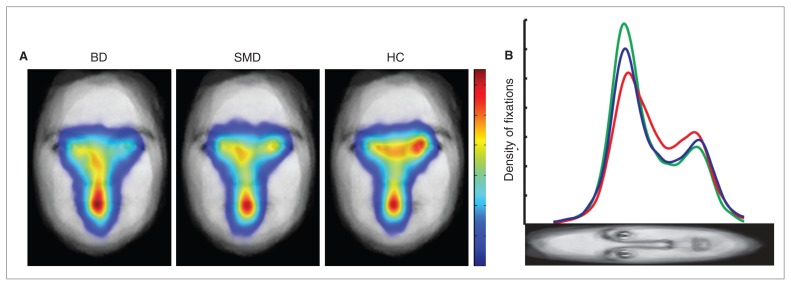
Therefore, across all emotions, children with BD made fewer fixations on eyes than controls, and the number of eye fixations made by children with SMD fell between those of children with BD and controls (Fig. 4).
Fig. 4.
Spatial density maps and profile plot of fixations across all expressions in children with bipolar disorder (BD), those with severe mood dysregulation (SMD) and healthy controls (HC). (A) Spatial density maps. The face plotted beneath the spatial density is the average, after alignment, of all faces used in the task. Fixations are plotted as Gaussian densities summed across trials. Maps were normalized for each participant, then averaged by the number of participants in each group. All spatial density maps are scaled the same from zero fixation density (blue) to maximal fixation density (red). (B) Profile plots of fixations. Each profile plot shows horizontal summations of the spatial densities for each group. The red, blue and green lines represent the mean in the BD, SMD and control groups, respectively.
Mean fixation duration
In both 4-way and 3-way 100%-level omnibus ANCOVA analysis, there were no interactions and no main effect of group.
Associations between emotion labelling accuracy and fixation patterns
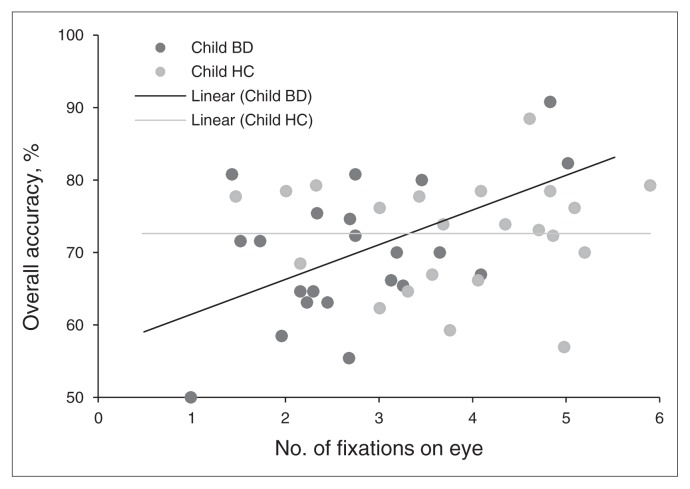
We examined whether fixations on the eyes were associated with emotion labelling accuracy in each group. First, we examined the correlation between accuracy and the number of eye fixations across expressions. We found a significant correlation in children with BD (r22 = 0.52, p = 0.014), but not in those with SMD (r28 = 0.08) or controls (r22 = 0.03; Fig. 5). The Fisher z test revealed that the correlation coefficients of children with BD and controls were significantly different (Fisher z = 1.81, p = 0.035, 1-tailed). The correlation coefficients of children with BD and those with SMD showed a trend difference (Fisher z = 1.46, p = 0.07, 1-tailed), but those of children with SMD did not differ from those of controls.
Fig. 5.
Correlation between accuracy and number of eye fixations across all expressions in children with bipolar disorder (BD) and healthy controls (HC). A significant correlation was found in children with BD (r22 = 0.52, p = 0.014), but not in controls (r22 = 0.03). The correlation coefficients of children with BD and controls differed (Fisher z = 1.81, p = 0.035; 1-tailed).
Second, we examined the association between total duration of fixations on the eyes and accuracy. The correlation between accuracy and total time fixated on eyes was a trend in children with BD (r22 = 0.37, p = 0.09), but not in those with SMD (r22 = 0.02) or in controls (r22 = −0.15). The Fisher z test revealed that the correlation coefficients of children with BD and controls differed (Fisher z = 1.66, p = 0.049, 1-tailed). Those of children with BD versus SMD or of SMD versus controls did not differ.
Thus, decreased attention to eyes was associated with lower accuracy in emotion labelling in children with BD (Fig. 5), but not in those with SMD or in controls.
Associations between fixation patterns and clinical measures
Among children with SMD, overall symptom severity, as measured by the CGI-SMD, was positively associated with both total duration of mouth fixations (r28 = 0.44, p = 0.021) and mean number of mouth fixations (r22 = 0.49, p = 0.008). Thus, more severe SMD symptoms were associated with greater attention to the mouth during emotion labelling. No association between fixation patterns and CDRS scores was found among children with SMD. No association between fixation patterns and clinical measures (YMRS, CDRS, CGI-BP depression or mania) was found among children with BD.
Effects of mood state, medication or comorbid illnesses
Post hoc exploratory analyses examined whether gaze pattern differences between children with BD and controls could be driven by comorbid illnesses, mood state or medications (see the Appendix for details). The differences in total fixation durations and mean number of fixations between children with BD and controls largely remained significant at a trend level or better, even after controlling for comorbid illnesses and mood states (all p < 0.06). Since the number of unmedicated children with BD was very small (n = 3), we were unable to test for confounding effects of medication. The difference in total fixation duration on eyes of 80% morphed emotional expressions between children with SMD and controls was significant when the analysis was limited to unmedicated children (p = 0.009), but not when effects of co-occurring illnesses were tested.
Discussion
We examined the gaze patterns of children with BD or SMD during a face emotion labelling task to examine whether dysfunctional gaze patterns contribute to the previously observed emotion labelling deficits in these populations. Consistent with the results of previous studies,3,14 across emotions, children with BD and those with SMD were less accurate than healthy children in identifying facial emotions. As hypothesized, children with BD exhibited reduced attention to eyes; they spent less time fixating on eyes and made fewer eye fixations than controls across emotions. Gaze patterns in children with SMD tended to fall between those of children with BD and controls. Increased eyes fixations were associated with increased accuracy across emotions only in children with BD. To our knowledge, this is the first study to demonstrate that reduced attention to eyes is associated with emotion labelling deficits in children with BD. Although children with SMD exhibited some abnormal gaze patterns, they were limited to specific conditions.
Our findings in children with BD were particularly clear: they showed decreased attention to eyes (compared with controls) in all emotional expressions. Furthermore, among children with BD, decreased attention to eyes was associated with lower accuracy in emotion labelling across emotions. This is consistent with the observation that when gathering emotional information the most effective visual scanning strategies involve attending to eyes.42 We did not observe a similar association among controls. Perhaps 1 or 2 fixations are sufficient for accurate labelling in controls,43 whereas children with BD require more fixations. Similar findings have been reported previously: greater attention to the eye region was associated with increased accuracy of fear labelling in children with severe psychopathic traits, but not in children with mild psychopathic traits.23
Our findings in children with SMD were less clear than those in children with BD. Like children with BD, those with SMD were less accurate than controls when labelling emotional expressions. Visual attention to eyes among children with SMD fell consistently between that among children with BD and controls, and on most measures, children with SMD did not differ from those with BD or controls. These equivocal findings in children with SMD should prompt further study. Comorbid attention-deficit/hyperactivity disorder (ADHD) may contribute to the gaze patterns in children with SMD. Given that the SMD criteria required hyperarousal symptoms that overlap with ADHD, the latter is a common comorbid disorder in children with SMD.44 Evidence is mixed about whether children with ADHD are less accurate than healthy individuals and exhibit neural abnormalities during facial emotion labelling.3,18,45–47 The only existing study of gaze patterns during facial emotion labelling in an ADHD population showed that adult patients made more fixations of shorter durations across facial features than healthy adults,48 which is distinct from the pattern we observed in the present study. However, more work in a larger sample is needed to dissociate the effects of chronic irritability from those of comorbid ADHD on gaze patterns during face processing.
Emotion labelling recruits several brain regions, including the amygdala, ventral visual pathway (including fusiform gyrus) and ventrolateral prefrontal cortex (VLPFC).6–8 The aberrant gaze patterns we observed in children with BD may reflect abnormalities in these regions. For example, the amygdala plays a role in directing attention to eye regions during facial emotion processing,16,39,49 and amygdala hyperactivity is associated with decreased attention to eye regions in children with a history of early deprivation50 and in adults with schizophrenia24 or social anxiety disorder.51 Amygdala hyperactivity has been reported consistently in individuals with BD during facial emotion processing.9,10,13,14 In children and adults with BD, VLPFC hypoactivity in response to emotional stimuli is also well documented.12,13 Therefore, the reduced attention to eye regions that we observed in children with BD may be associated with amygdala and VLPFC dysfunction.
Literature on neural abnormalities in people with SMD has been limited and inconsistent. One study reported decreased amygdala responses in individuals with SMD compared with both controls and individuals with BD during fear rating of neutral faces.18 Another study demonstrated that, while participants with BD and SMD exhibited similar deficits in amygdala modulation in response to facial expressions of increasing anger intensity, the 2 patient groups exhibited different patterns of abnormalities in ventral visual pathway and frontoparietal activity.17 Future work combining functional magnetic resonance imaging and eye tracking is needed to elucidate associations among neural dysfunction, impaired eye gaze and facial emotion labelling deficits in children with BD and SMD.
The present study may provide evidence to support abnormal gaze patterns as a biomarker of a potential endophenotype for emotion labelling deficits in patients with BD and in those with SMD. Moreover, the fact that gaze patterns in children with SMD fell between those of children with BD and controls may suggest that such an endophenotype is on a continuum and is state-dependent. In the present study, there was an association between gaze pattern and current symptom severity in children with SMD, but not in those with BD. Thus, abnormal eye gaze during facial emotion processing may be influenced by clinical state in individuals with SMD. Although current symptom severity was not associated with gaze patterns in children with BD, a potentially more restricted range of symptom severity in these children may preclude a similar association between symptom severity and gaze patterns.
Gaze pattern abnormalities in individuals with BD and SMD should also be considered in light of similar abnormalities observed in individuals with other psychiatric disorders. Social cognition deficits have also been suggested as a potential endophenotype for schizophrenia24,52 and anxiety disorders.53 Several lines of research, particularly molecular genetics, suggest pathophysiological links between BD and schizophrenia. With regard to SMD, childhood irritability predicts anxiety disorders and depression in adulthood.54,55 Therefore, abnormal gaze patterns associated with childhood irritability may be a biomarker of the increased risk for anxiety disorders developing in adulthood. Thus, our findings are consistent with the growing trend in psychiatric research to acknowledge shared pathophysiological links across diagnoses previously considered distinct. Paranoid delusions during mania may be associated with aberrant gaze patterns in individuals with BD. Studies suggest that paranoid delusions are associated with decreased attention to threatening cues in a scene.56,57 In the present study, no child with BD was manic at the time of the study. However, the fewer and shorter fixations on eyes that we observed in the children with BD may become more severe during manic states with paranoid delusions. Therefore, studies including a larger sample of children with BD are needed to examine associations between eye gaze and different mood states.
Limitations
Although the findings from the present study are promising, some limitations are worth noting. First, most children with BD were medicated, and the BD sample was heterogeneous in mood state and comorbid diagnoses. Post hoc analyses suggest that decreased attention to eyes among children with BD was largely unrelated to this clinical heterogeneity, although we were unable to assess the role of medication owing to insufficient numbers of unmedicated patients with BD. Second, although children with BD and SMD exhibited similar rates of comorbid ADHD and anxiety disorders, the 2 groups differed in the rate of ODD. Children with SMD had a higher rate of comorbid ODD because criteria for ODD include nonepisodic irritability symptoms, such as “often loses temper” and “is often touchy and easily annoyed by others.” However, our post hoc analysis comparing children with SMD but without ODD or other conduct disorders and healthy controls revealed that comorbid ODD status did not drive the gaze pattern differences that we identified between children with SMD and controls. Third, we do not have data to test whether decreased attention to eyes is related to social functioning in children with BD and SMD; this is worthy of investigation. Fourth, our findings differ from those of a previous study in which adults with BD did not exhibit abnormal gaze patterns during face scanning.30 While deficits in facial emotion labelling are present in both adults and children with BD, evidence suggests that these abnormalities may be more consistent and marked in children11,58 and may result in measurable eye gaze impairments. Future work comparing gaze patterns and facial emotion labelling deficits in pediatric and adult BD is important to better understand developmental differences. Fifth, the present study included children across a wide age span from childhood to adolescence. The limited sample size does not allow us to examine associations between age and eye gaze abnormalities. However, given the literature about prefrontal cortical development in adolescence,59 future studies should examine associations among age, prefrontal cortex function, emotion labelling deficits and eye gaze in these patient groups. Last, group differences in socioeconomic status or race may influence eye gaze patterns. There was no difference in race ratio among the groups, but we did not obtain information on participants’ socioeconomic status. Future studies should examine the effects of socioeconomic status on eye gaze in individuals with BD and SMD.
Conclusion
The present study demonstrated the associations between facial emotion labelling deficits and impaired attention to eyes in children with BD. Gaze patterns in children with SMD tended to fall between those of children with BD and healthy controls. The link between decreased attention to eyes and emotion labelling accuracy in children with BD may have clinical implications. It suggests that teaching patients to look at eyes while processing emotional expressions may help improve their ability to recognize facial expressions. Previous studies demonstrate that instructing individuals with emotion labelling deficits to focus on eyes improved their ability to identify emotions correctly,16,23 suggesting possible future interventions in children with BD.
Acknowledgements
Funding for this study was provided exclusively by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health. We thank the staff of the Emotion and Development Branch at NIMH and the children and families for their participation.
Footnotes
Conflict of interest: None declared.
Contributors: P. Kim, J. Blair, D. Pine, C. Baker, E. Leibenluft and J. Arizpe designed the study. P. Kim, B. Rosen, V. Razdan, C. Haring, S. Jenkins, C. Deveney, M.A. Brotman and D. Pine acquired the data, which P. Kim, V. Razdan, C. Haring, M.A. Brotman, D. Pine, C. Baker, E. Leibenluft and J. Arizpe analyzed. P. Kim, C. Haring, J. Blair and D. Pine wrote the article, which all authors reviewed and approved for publication.
References
- 1.Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168:129–42. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 3.Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48:863–71. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 4.McClure EB, Treland JE, Snow J, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–51. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 5.Rich BA, Grimley ME, Schmajuk M, et al. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20:529–46. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman MD, Eisenberger NI, Crockett MJ, et al. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 7.Critchley H, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 9.Pavuluri MN, O’Connor MM, Harral E, et al. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–67. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Kalmar JH, Wang F, Chepenik LG, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–42. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moscicki AM, Rosen BH, Zarate CA, Jr, et al. Differing amygdala responses to facial expressions in children and adults with bipolar disorder. Am J Psychiatry. 2012;169:642–9. doi: 10.1176/appi.ajp.2012.11081245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavuluri MN, Passarotti AM, Harral EM, et al. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:308–19. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CH, Suckling J, Lennox BR, et al. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 14.Kohler CG, Hoffman LJ, Eastman LB, et al. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res. 2011;188:303–9. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14:326–39. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 16.Adolphs R, Gosselin F, Buchanan TW, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 17.Thomas LA, Brotman MA, Muhrer EM, et al. Parametric modulation of neural activity by emotion in youth with bipolar disorder, severe mood dysregulation, and healthy subjects. Arch Gen Psychiatry. 2012;69:1257–66. doi: 10.1001/archgenpsychiatry.2012.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–9. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelphrey KA, Sasson N, Reznick JS, et al. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–61. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 20.Spezio ML, Adolphs R, Hurley RS, et al. Abnormal use of facial information in high-functioning autism. J Autism Dev Disord. 2007;37:929–39. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- 21.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spezio ML, Adolphs R, Hurley RSE, et al. Analysis of face gaze in autism using “Bubbles”. Neuropsychologia. 2007;45:144–51. doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Dadds MR, El Masry Y, Wimalaweera S, et al. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. J Am Acad Child Adolesc Psychiatry. 2008;47:455–63. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- 24.Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Curr Opin Psychiatry. 2009;22:140–6. doi: 10.1097/YCO.0b013e328324f895. [DOI] [PubMed] [Google Scholar]
- 25.Phillips ML, David AS. Visual scan paths are abnormal in deluded schizophrenics. Neuropsychologia. 1997;35:99–105. doi: 10.1016/s0028-3932(96)00061-9. [DOI] [PubMed] [Google Scholar]
- 26.Williams LM, Loughland CM, Gordon E, et al. Visual scanpaths in schizophrenia: is there a deficit in face recognition? Schizophr Res. 1999;40:189–99. doi: 10.1016/s0920-9964(99)00056-0. [DOI] [PubMed] [Google Scholar]
- 27.Loughland CM, Williams LM, Gordon E. Visual scanpaths to positive and negative facial emotions in an outpatient schizophrenia sample. Schizophr Res. 2002;55:159–70. doi: 10.1016/s0920-9964(01)00186-4. [DOI] [PubMed] [Google Scholar]
- 28.Loughland CM, Williams LM, Gordon E. Schizophrenia and affective disorder show different visual scanning behavior for faces: A trait versus state-based distinction? Biol Psychiatry. 2002;52:338–48. doi: 10.1016/s0006-3223(02)01356-2. [DOI] [PubMed] [Google Scholar]
- 29.Kim E, Ku J, Kim J-J, et al. Nonverbal social behaviors of patients with bipolar mania during interactions with virtual humans. J Nerv Ment Dis. 2009;197:412–8. doi: 10.1097/NMD.0b013e3181a61c3d. [DOI] [PubMed] [Google Scholar]
- 30.Bestelmeyer PE, Tatler BW, Phillips LH, et al. Global visual scanning abnormalities in schizophrenia and bipolar disorder. Schizophr Res. 2006;87:212–22. doi: 10.1016/j.schres.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Leibenluft E, Charney DS, Towbin KE, et al. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–7. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 33.Leckman JF, Sholomskas D, Thompson WD, et al. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–83. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 34.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–50. [PubMed] [Google Scholar]
- 35.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 36.Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159–71. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 37.Weschler D. Weschler Abbreviated Scale of Intelligence. Austin, (TX): The Psychological Corporation; 1999. [Google Scholar]
- 38.Ekman P, Friesen W. Pictures of Facial Affect. Palo Alto (Cali): Consulting Psychologists; 1976. [Google Scholar]
- 39.Kennedy DP, Adolphs R. Impaired fixation to eyes following amygdala damage arises from abnormal bottom-up attention. Neuropsychologia. 2010;48:3392–8. doi: 10.1016/j.neuropsychologia.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arizpe J, Kravitz DJ, Yovel G, et al. Start position strongly influences fixation patterns during face processing: difficulties with eye movements as a measure of information use. PLoS ONE. 2012;7:e31106. doi: 10.1371/journal.pone.0031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams JBW, Link MJ, Rosenthal NE, et al. Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD) New York (NY): New York Psychiatric Institute; 1988. [Google Scholar]
- 42.Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 43.Hsiao JH, Cottrell G. Two fixations suffice in face recognition. Psychol Sci. 2008;19:998–1006. doi: 10.1111/j.1467-9280.2008.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brotman MA, Schmajuk M, Rich BA, et al. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991–7. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 45.Uekermann J, Kraemer M, Abdel-Hamid M, et al. Social cognition in attention-deficit hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2010;34:734–43. doi: 10.1016/j.neubiorev.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Williams LM, Hermens DF, Palmer D, et al. Misinterpreting emotional expressions in attention-deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects. Biol Psychiatry. 2008;63:917–26. doi: 10.1016/j.biopsych.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Marsh AA, Finger EC, Mitchell DG, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 48.Marsh PJ, Lazzaro I, Manor BR, et al. Facial expressions of emotion and visual scanpaths in attention-deficit hyperactivity disorder (ADHD) and first-episode psychosis (FEP) [abstract] Schizophr Res. 2000;41:288. [Google Scholar]
- 49.Gamer M, Buchel C. Amygdala activation predicts gaze toward fearful eyes. J Neurosci. 2009;29:9123–6. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tottenham N, Hare TA, Millner A, et al. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneier FR, Kent JM, Star A, et al. Neural circuitry of submissive behavior in social anxiety disorder: a preliminary study of response to direct eye gaze. Psychiatry Res. 2009;173:248–50. doi: 10.1016/j.pscychresns.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loughland CM, Williams LM, Harris AW. Visual scanpath dysfunction in first-degree relatives of schizophrenia probands: evidence for a vulnerability marker? Schizophr Res. 2004;67:11–21. doi: 10.1016/s0920-9964(03)00094-x. [DOI] [PubMed] [Google Scholar]
- 53.Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J Abnorm Psychol. 2000;109:695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- 54.Stringaris A, Baroni A, Haimm C, et al. Pediatric bipolar disorder versus severe mood dysregulation: risk for manic episodes on follow-up. J Am Acad Child Adolesc Psychiatry. 2010;49:397–405. [PMC free article] [PubMed] [Google Scholar]
- 55.Stringaris A, Cohen P, Pine DS, et al. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166:1048–54. doi: 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips ML, Senior C, David AS. Perception of threat in schizophrenics with persecutory delusions: an investigation using visual scan paths. Psychol Med. 2000;30:157–67. doi: 10.1017/s0033291799001397. [DOI] [PubMed] [Google Scholar]
- 57.Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neurosci Biobehav Rev. 2004;28:333–42. doi: 10.1016/j.neubiorev.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Venn HR, Gray JM, Montagne B, et al. Perception of facial expressions of emotion in bipolar disorder. Bipolar Disord. 2004;6:286–93. doi: 10.1111/j.1399-5618.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 59.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]