Abstract
Background
Convergent evidence suggests dysfunction within the prefrontal cortex (PFC) and amygdala, important components of a neural system that subserves emotional processing, in individuals with major depressive disorder (MDD). Abnormalities in this system in the left hemisphere and during processing of negative emotional stimuli are especially implicated. In this study, we used functional magnetic resonance imaging (fMRI) to investigate amygdala–PFC functional connectivity during emotional face processing in medication-naive individuals with MDD.
Methods
Individuals with MDD and healthy controls underwent fMRI scanning while processing 3 types of emotional face stimuli. We compared the strength of functional connectivity from the amygdala between the MDD and control groups.
Results
Our study included 28 individuals with MDD and 30 controls. Decreased amygdala–left rostral PFC (rPFC) functional connectivity was observed in the MDD group compared with controls for the fear condition (p < 0.05, corrected). No significant differences were found in amygdala connectivity to any cerebral regions between the MDD and control groups for the happy or neutral conditions.
Limitations
All participants with MDD were experiencing acute episodes, therefore the findings could not be generalized to the entire MDD population.
Conclusion
Medication-naive individuals with MDD showed decreased amygdala–left rPFC functional connectivity in response to negative emotional stimuli, suggesting that abnormalities in amygdala–left rPFC neural circuitry responses to negative emotional stimuli might play an important role in the pathophysiology of MDD.
Introduction
Episodes of major depressive disorder (MDD) are characterized by negative affect and negative biases in processing emotional stimuli, implicating abnormalities in the neural system that processes negative emotional stimuli.1,2 The prefrontal cortex (PFC) shares extensive connections with the amygdala. These structures are central to a neural system that processes emotional stimuli, especially negatively valenced stimuli.3 Accumulating studies implicate this PFC–amygdala neural system in the disordered emotional processes of individuals with MDD.4
Morphological magnetic resonance imaging (MRI) and conventional functional MRI (fMRI) activation analyses provide evidence of morphological and functional abnormalities within the PFC and the amygdala in adults with MDD.5,6 We previously found morphological abnormalities in the amygdala in medication-naive individuals with MDD.5 Other groups have demonstrated excessive responses of the amygdala to negative emotion, especially fearful facial expressions, in medicated individuals with MDD.7,8 Studies of the role of the PFC suggest that functional imbalance between the left and right PFC in emotion processing may also be involved in the neuropathophysiology of MDD.9 Consistent with this idea, lesions in the left PFC and left PFC dysfunction, which lead to deficits in the capacity to experience positive affect, a key feature of depression, have been reported in association with depression,10,11 particularly during negative emotion processing,12 possibly indicating greater dysfunction in the left than the right PFC in individuals with MDD.
The conventional fMRI activation studies mentioned previously provide information about the functioning within specific brain regions. To study the ability of brain regions to work together, specialized measures can be used to examine the coordinated activity between brain regions or their “functional connectivity.” Connectivity fMRI (cfMRI) assesses activity in different brain regions that are coupled in time. Several cfMRI studies have shown connectivity abnormalities in individuals with MDD. For example, studies have shown decreased connectivity between the anterior cingulate cortex (ACC) and amygdala in response to negative stimuli in unmedicated individuals with MDD.13,14 Almeida and colleagues15 reported reduced amygdala–left orbitomedial prefrontal functional connectivity during processing of happy and sad faces in medicated patients with MDD. However, increased connectivity between frontal cortices, including the medial PFC and rostral ACC, and the amygdala when processing negative words has also been reported in depressed patients.16 The conflicting findings among studies may be related to differences in tasks performed during scanning and/or differences in patient samples, particularly in terms of illness chronicity or medication. Importantly, some studies suggest that antidepressant treatment influences the functioning of the amygdala and frontal cortices and their connectivity in individuals with MDD.17,18
In the present fMRI study, medication-naive individuals with MDD were scanned while processing emotional facial stimuli depicting fearful, happy and neutral expressions. We assessed the strength of the correlation in time between the blood oxygen level–dependent (BOLD) responses from the amygdala to the PFC. We hypothesized that participants with MDD would demonstrate deficits in functional connectivity between the amygdala and PFC, particularly the left PFC, and especially when processing negative emotional stimuli.
Methods
Participants
We recruited medication-naive patients with MDD aged 19–46 years from the outpatient clinic at the Department of Psychiatry, First Affiliated Hospital of China Medical University and the Mental Health Center of Shenyang. Some of these participants had been included in a previously published resting-state fMRI study.19 The diagnosis of MDD was confirmed by 2 trained psychiatrists (L.K. and F.W.) using the Structured Clinical Interview for DSM-IV disorders. To be included in our study, individuals with MDD had to fulfil the DSM-IV criteria for MDD; have a current depressive episode; have no comorbid Axis I or II diagnoses; have a score of at least 24 on the 17-item Hamilton Rating Scale for Depression (HAMD-17); and have no history of psychopharmacotherapy, electroconvulsive therapy or psychotherapy.
We recruited healthy controls from Shenyang, China, via advertisement. Some of these participants had also been included in the previously published resting-state fMRI study.19 The absence of DSM-IV Axis I disorders in controls was confirmed by 2 independent psychiatrists (L.K. and F.W.) using the Structured Clinical Interview for DSM-IV disorders. Individuals with first-degree family members who had a history of DSM-IV Axis I disorders were excluded.
Additional exclusion critera for both individuals with MDD and controls were the presence of any MRI contraindications, history of head injury or neurologic disorder, history of drug abuse or dependence, and any concomitant medical disorder. All participants were scanned within 24 hours of initial contact with the research team. The participants provided written informed consent after receiving a detailed description of the study. The Institutional Review Board of the China Medical University approved our study protocol.
MRI data acquisition
The fMRI data were acquired using a GE Signa HDX 3.0 T MRI scanner at the First Affiliated Hospital of China Medical University, Shenyang, China. Head motion was minimized with restraining foam pads. We used a standard head coil for radiofrequency transmission and reception of the nuclear magnetic resonance signal. The fMRI images were acquired using a spin–echo planar imaging sequence, parallel to the anterior commissure–posterior commissure plane with the following scan parameters: repetition time 2000 ms, echo time 40 ms, image matrix 64 × 64, field of view 24 × 24 cm2, 35 contiguous slices of 3 mm and without gap.
Emotional face paradigm
During the fMRI runs, each participant completed an event-related facial emotion task, which has been described previously.20,21 Participants viewed faces from the Ekman series depicting fearful, happy or neutral expressions, and they were instructed to press a button to make a male–female determination.22 In brief, 5 male and 5 female faces were each presented for 2 s with interstimulus intervals of 4, 8 or 12 s. Each of the 3 expressions was shown for each individual, for a total of 30 facial stimuli and a run time of 5 min, 6 s. The order of the facial stimuli varied to control for sequential dependencies.
Functional connectivity processing
Statistical Parametric Mapping 8 (SPM8) software (www.fil.ion.ucl.ac.uk/spm) was used for BOLD fMRI preprocessing. We discarded the initial 2 images. The remaining images were corrected for within-scan acquisition time differences between slices and realigned to the first volume to correct interscan movements. Linear motion (x, y, z planes) for all participants was below 2.5 mm and rotational motion (pitch, roll, yaw) below 2.5 degrees. The fMRI data were then spatially normalized to Montreal Neurological Institute (MNI) space, resampled to 3 × 3 × 3 mm3 and spatially smoothed (8 mm full-width at half-maximum).
The bilateral amygdala seed region of interest (ROI) was defined with the WFU PickAtlas Tool (www.fmri.wfubmc.edu/download.htm). For each participant, we calculated a mean time series for the amygdala seed ROI by averaging the time series for all voxels within the amygdala ROI separately for each facial emotion type (fearful, happy, neutral). We then performed correlational analyses between the amygdala time series and the time series for each brain voxel,22 resulting in 3 correlation maps for each participant, 1 for each facial emotion type. The correlation coefficients in each map were transformed to z values using Fisher r-to-z transformation for further statistical testing.
Statistical analyses
We used independent-sample t tests and χ2 tests to compare demographic data, HAMD-17 and Hamilton Anxiety Rating Scale (HARS) scores between the MDD and control groups using SPSS 13.0 software (SPSS Inc.). Group differences in functional connectivity were analyzed using 2-sample (MDD v. control) t tests in SPM, with the functional connectivity correlation coefficients (z scores) from the amygdala to all brain voxels as the dependent variables for each face condition (fearful, happy, neutral). The threshold for contrast maps was set at p < 0.005 and a cluster size of at least 729 mm3 (27 voxels) for the hypothesized PFC region; this was equal to a corrected threshold of p < 0.05, determined by the Monte Carlo simulation (AlphaSim command line in AFNI [analysis of functional neuroimaging; Cox, 1996]). The PFC was defined with the WFU PickAtlas Tool (www.fmri.wfubmc.edu/download.htm), including Brodmann Areas (BA) 9–12, 24, 25, 32 and 44–47. A linear mixed model was conducted to assess the effects of diagnostic group and the 3 emotional stimulus types on functional connectivity measurement (z values in the PFC regions showing significant differences between the control and MDD groups) with SPSS. In this model, diagnostic group (control v. MDD) represented a between-subjects factor, and emotional type (fear, happy, neutral) was included as a within-subjects factor. The interaction between diagnostic group and emotion was modelled. If the diagnostic group effect and interaction between diagnostic group and emotion demonstrated significance, we performed 2-sample t tests separately for the 3 emotional types in the post hoc analyses, and we considered results to be significant at p < 0.05, Bonferroni corrected. We conducted whole brain analyses to explore other possible brain regions not hypothesized a priori. Findings in these regions were considered to be significant at p < 0.05, family wise error–corrected for multiple comparisons. We performed post hoc exploratory Pearson correlation analyses in MDD participants to assess the correlation of HAMD-17 and HARS scores with z scores in the PFC regions that were significantly different between the MDD and control groups.
Results
The MDD group comprised 28 participants with a mean age of 30.6 ± standard deviation (SD) 8.7 years. Half of them were women, and 22 had been included in an earlier study.19 The mean years of education was 12.9 ± 3.2, the mean duration of illness was 13.0 ± 15.1 months, the mean HAMD-17 score was 28.5 ± 5.2 and the mean HARS score was 20.1 ± 8.3. The control group comprised 30 participants with a mean age of 29.5 ± 8.0 years. Half of them were women, and 23 had been included in a earlier study.19 All participants were right-handed. There were no significant differences in age (p = 0.62), sex (p > 0.99) and education (p = 0.19) between the MDD and control groups. The MDD group had significantly higher HAMD-17 and HARS scores than the control group (p < 0.001, Table 1).
Table 1.
Demographic and clinical characteristics of participants with major depressive disorder and healthy controls
| Group; mean ± SD* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | MDD n = 28 | Control n = 30 | t value | p value |
| Age, yr | 30.6 ± 8.7 | 29.5 ± 8.0 | t56 = 0.50 | 0.62 |
| Sex, male:female | 14:14 | 15:15 | χ21 = 0.000 | > 0.99 |
| Education, yr | 12.9 ± 3.2 | 13.9 ± 2.8 | t56 = 1.32 | 0.19 |
| HAMD-17 score | 28.5 ± 5.2 | 0.6 ± 1.0 | t = 29.09 | < 0.001 |
| HARS score | 20.1 ± 8.8 | 1.1 ± 1.6 | t = 12.27 | < 0.001 |
| Illness duration, mo | 13.0 ± 15.1 | — | — | — |
HAMD-17 = 17-item Hamilton Rating Scale for Depression; HARS = Hamilton Anxiety Rating Scale; MDD = major depressive disorder; SD = standard deviation.
Unless otherwise indicated.
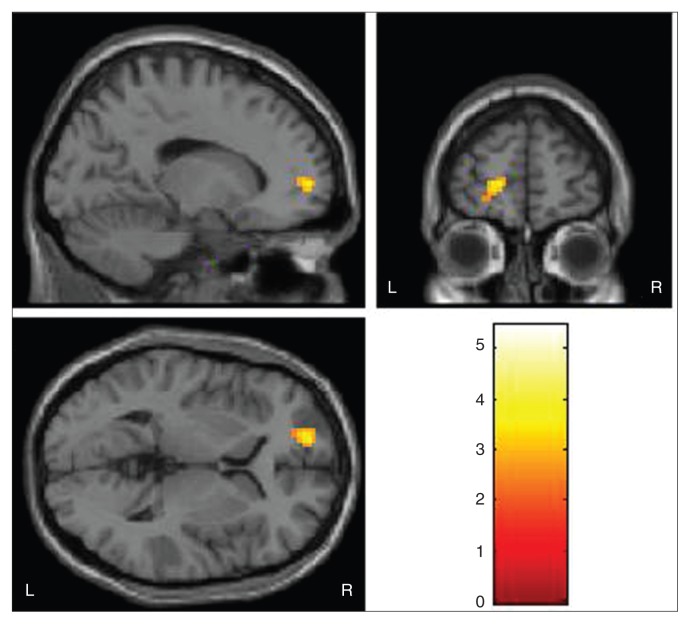
We observed decreased amygdala–left rostral PFC (rPFC; BA 10) functional connectivity in the MDD group compared with the control group for the fearful face condition (maximal MNI coordinates: x, y, z = –15, 57, 3; 37 voxels (999mm3); t = 3.65; p < 0.005, uncorrected; Fig. 1). These findings correspond to a corrected p < 0.05 by AlphaSim correction. Analysis of z values in the rPFC showed that the main effect of diagnosis was significant (F1,56 = 6.63, p = 0.010). In addition, there was a significant group × emotion interaction (F1,56 = 13.31, p = 0.001). The post hoc 2-sample t tests demonstrated that the contribution to group difference and interaction between group and emotion types was derived mainly from increased z values for the fearful condition (t1,56 = 3.64, p = 0.003, Bonferroni corrected) in the MDD compared with the control group. The z values for the other 2 emotion types were not significant (all p > 0.05, Bonferroni corrected; Table 2). No significant group decreases in functional connectivity were detected for the happy or neutral conditions. No significant group increases in functional connectivity were detected for any condition. Our whole brain analysis revealed no group differences in altered functional connectivity between the amygdala and other brain regions for any of the conditions. In post hoc correlation analyses, neither HAMD-17 scores nor HARS scores had significant associations with rPFC functional connectivity in participants with MDD.
Fig. 1.
The images display the region of the left rostral prefrontal cortex that showed decreased functional connectivity to the amygdala in 28 medication-naive participants with major depressive disorder compared with 30 healthy controls during fearful face processing (Montreal Neurological Institute coordinates for the point of maximal association x, y, z = –15, 57, 3; 37 voxels; t = 3.65, p < 0.005, uncorrected). The colour bar represents the range of t values. L = left; R = right.
Table 2.
Z scores of functional connectivity in rostral prefrontal cortex with 3 conditions
| Group; mean ± SD* | ||
|---|---|---|
|
|
||
| Condition | MDD, n = 28 | Control, n = 30 |
| Fear | 0.36 ± 0.33 | 0.66 ± 0.29 |
| Happiness | 0.47 ± 0.27 | 0.49 ± 0.25 |
| Neutral | 0.38 ± 0.46 | 0.61 ± 0.29 |
MDD = major depressive disorder; SD = standard deviation.
Unless otherwise indicated.
Discussion
In this study, we detected a deficit in amygdala–left rPFC functional connectivity in response to fearful face processing in individuals with MDD. To our knowledge, this is the first study to detect such deficits in medication-naive individuals with MDD.
Alhough rPFC is the single cytoarchitectonic subregion of the frontal lobes in the human brain, studies have shown the rPFC plays multiple roles in brain functions. It has been implicated in the integration of information from several emotional and cognitive domains.23–26 Recently, Okada and colleagues27 reported reduced rPFC activation during a verbal fluency task in individuals with remitted MDD. The similar executive dysfunction was also reported in an event-related potentials study in an MDD population.28 The amygdala has been proven to play an important role during emotion processing in animal and human research.29,30 Overactivity of the amygdala may correlate with depressive, ruminative thoughts31 and may be present in the early stages of MDD.32 Morphological and functional abnormalities within the amygdala in individuals with MDD have also been consistently demonstrated.13,33,34 As it is possible that the decreased functional connectivity of rPFC–amygdala circuitry detected in our MDD group may relate to both executive and emotional dysfunction, future neuro-imaging studies using tasks that investigate these functions would be of interest.
The decreased amygdala–rPFC functional connectivity in individuals with MDD detected in our study could reflect a reduction in the PFC’s inhibitory control over the amygdala and could delay the extinction of negative emotion.35 A study by Petrides and Pandya36 in the macaque monkey demonstrated that the rPFC has connections with the amygdala, ventral PFC and cingulate cortex, all areas that have been linked with emotion processing.37 Recently, an fMRI study reported that correct recall of negative faces was associated with increased activity in the amygdala and rPFC, indicating that both areas are intimately involved in the retrieval of emotional stimuli during recognition memory.38 In the present study, we used the task inducing implicit rather than explicit processing of emotional faces,39 suggesting that altered functional connectivity between the amygdala and rPFC might play a key role during implicit emotional processing in individuals with MDD and that this dysfunction might contribute to negative automatic thoughts in patients with MDD.40
We found abnormality in the functional connectivity to the left, but not to the right, rPFC. The decreased amygdyala–left rPFC functional connectivity in response to fearful emotion may be related to the hemispheric asymmetry, which has been demonstrated in individuals with MDD.41–44 The balance between the right and left hemispheres is very important to adaptive emotion regulation, and hemispheric asymmetry has been observed in normal affective processing of positive and negative emotions.45,46 Previous studies have indicated that the left PFC activates more during the regulation of negative affect47 and approach-related positive affect.48 Studies involving depressed individuals have shown decreased activation in the left PFC and increased activation in the right PFC in response to sad mood induction.49 Our results indicated that the left rPFC might contribute more than the right rPFC to the disturbed emotional processing in individuals with MDD and that abnormalities in hemispheric asymmetry might be related to the pathophysiology of MDD.
Consistent with the previous behavioural, electroencephalogram and functional activation findings of dysfunctional negative emotional processing in individuals with MDD,7,8,50,51 our finding of decreased amygdala–PFC functional connectivity during fearful face processing and not during happy or neutral face processing supports the involvement of abnormalities in amygdala–PFC functional connectivity in negative emotional processing in individuals with MDD, and it further implicates abnormal negative emotional processing in the neuropathophysiology of MDD. Taken together with the findings of our recent report of decreased amygdala–PFC functional connectivity during a resting-state fMRI in individuals with MDD,19 our current findings suggest that altered amygdala–PFC functional connectivity may be a key feature of the disorder. These studies also raise interesting questions about the association between amygdala–PFC functional connectivity during the resting state and tasks in individuals wtih MDD. Activation findings from both resting-state and task-related fMRI suggest that intrinsic resting-state activity may be involved in specific brain circuit engagement to perform a cognitive task and that resting activity can predict subsequent task-evoked brain responses in healthy individuals.52 To our knowledge, no studies have investigated the association of the functional connectivity within a specific neural circuitry during resting-state and task-related fMRI in individuals with MDD. Unfortunately, we were not able to examine this association within the amygdala–PFC circuitry in this study because of the differences in study design between the present task-related fMRI study and our previously published resting-state fMRI study, such as seed ROI selection (the bilateral amygdala was selected as a single ROI in the present study, whereas the left and right amygdala were selected as 2 separate ROIs in our previous resting-state study). We speculate that altered amygdala–PFC functional connectivity during the resting state might contribute to decreased amygdala–PFC functional connectivity during fearful face processing in individuals with MDD, and that altered functional connectivity during the resting state and negative emotional processing tasks reflect the dysfunctional negative emotional processing observed in individuals with MDD. Future studies with careful design to examine the association of functional connectivity within certain neural circuits during the resting state and emotional face processing tasks in individuals with MDD will be important in understanding the neuropathophysiology of MDD. Furthermore, the association between these functional connectivity disturbances and structural connectivity could be explored using diffusion tensor imaging. The connections between the amygdala and rPFC are bidirectional,53 but our study did not assess the direction of the functional connectivity. Since individuals were acutely ill at the time of study, we cannot determine whether the abnormality is state- or trait-related or whether successful treatment alters this disturbance.
Limitations
All participants with MDD were already experiencing acute episodes, therefore the findings could not be generalized to the entire MDD population. Future studies of participants in the euthymic state or individuals at risk for MDD are warranted to elucidate the amygdala–left rPFC functional connectivity abnormalities associated with MDD neuropatho-physiology or with predisposition to MDD. Furthermore, participants with MDD in the present study had high HARS scores. Although no association between HARS scores and amygdala–rPFC functional connectivity was detected, the abnormalities of functional connectivity within different anxiety levels in individuals with MDD need to be further investigated. In addition, only fear was used as a negative emotion in the study; future studies including more negative emotions, such as sadness, anger and disgust, which have been used in some fMRI studies,54–56 are needed to fully understand the neuropathophysiology of MDD.
Conclusion
Our study provides critical evidence supporting abnormalities of amygdala–left rPFC functional connectivity in response to negative emotion in individuals with MDD. The findings in medication-naive individuals with this disorder suggest that they may play an important role in the pathophysiology of MDD.
Acknowledgments
Funding for this study was provided by the National Natural Science Foundation of China (81101012, F. Wu; 81071099 and 81271499, Y. Tang), the Liaoning Doctor Scientific Foundation (20111099, F. Wu), the Liaoning Science and Technology Foundation (2008225010-14, Y. Tang), National Institute of Health (K01MH086621, F. Wang), the National Alliance for Research on Schizophrenia and Depression (F. Wang) and the Klingenstein Foundation (F. Wang).
Footnotes
Competing interests: Y. Tang declares grants from the National Natural Science Foundation of China (81071099) and the Liaoning Sciecne and Technology Foundation (2008225010-14). F. Wu declares grants from the National Natural Science Foundation of China (81101012) and the Liaoning Doctor Scientific Foundation (20111099). F. Wang declares grants from the National Institutes of Health (01MH086621), the National Alliance for Research on Schizophrenia and Depression and the Klingenstein Foundation. No other competing interests declared.
Contributors: Y. Tang, G. Fan, H.P. Blumberg, K. Xu and F. Wang designed the study. L. Kong, K. Chen, F. Wu, G. Fan, L. Ren, W. Jiang and Y. Cao acquired the data, which L. Kong, N. Driesen, F. Womer, W. Jiang, and F. Wang analyzed. L. Kong, K. Chen, Y. Tang, N. Driesen, F. Womer, H.P. Blumberg, K. Xu and F. Wang wrote the article. All authors reviewed the article and provided approval for publication.
References
- 1.Victor TA, Furey ML, Fromm SJ, et al. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–38. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dannlowski U, Ohrmann P, Bauer J, et al. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. J Psychiatry Neurosci. 2007;32:423–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y, Wang F, Xie G, et al. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: a voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 2007;156:83–6. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Wagner G, Koch K, Schachtzabel C, et al. Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J Psychiatry Neurosci. 2008;33:199–208. [PMC free article] [PubMed] [Google Scholar]
- 7.Veer IM, Beckmann CF, van Tol MJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:41. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 9.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 10.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J Abnorm Psychol. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 12.Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci. 2006;6:110–26. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- 13.Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–88. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Suckling J, Ooi C, et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33:1909–18. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- 15.Almeida JR, Versace A, Mechelli A, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–9. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimura S, Okamoto Y, Onoda K, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord. 2010;122:76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsycho-pharmacology. 2005;30:1334–44. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Ridler K, Suckling J, et al. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry. 2007;62:407–14. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Kong L, Wu F, et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychol Med. 2012;30:1–7. doi: 10.1017/S0033291712002759. [DOI] [PubMed] [Google Scholar]
- 20.Kerestes R, Bhagwagar Z, Nathan PJ, et al. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Res. 2012;202:30–7. doi: 10.1016/j.pscychresns.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalmar JH, Wang F, Chepenik LG, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–42. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–21. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop S, Duncan J, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 24.Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–86. [Google Scholar]
- 25.Gilbert SJ, Spengler S, Simons JS, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–48. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 26.Benoit RG, Gilbert SJ, Frith CD, et al. Rostral prefrontal cortex and the focus of attention in prospective memory. Cereb Cortex. 2012;22:1876–86. doi: 10.1093/cercor/bhr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada G, Okamoto Y, Yamashita H, et al. Attenuated prefrontal activation during a verbal fluency task in remitted major depression. Psychiatry Clin Neurosci. 2009;63:423–5. doi: 10.1111/j.1440-1819.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 28.Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65:179–88. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costafreda SG, Brammer MJ, David AS, et al. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 30.LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–7. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 31.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–9. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 32.Matthews SC, Strigo IA, Simmons AN, et al. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Dougherty DD, Rauch SL, Deckersbach T, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Arch Gen Psychiatry. 2004;61:795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- 34.Davey CG, Allen NB, Harrison BJ, et al. Increased amygdala response to positive social feedback in young people with major depressive disorder. Biol Psychiatry. 2011;69:734–41. doi: 10.1016/j.biopsych.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 36.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–86. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Keightley ML, Chiew KS, Anderson JA, et al. Neural correlates of recognition memory for emotional faces and scenes. Soc Cogn Affect Neurosci. 2011;6:24–37. doi: 10.1093/scan/nsq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whalen PJ, Rauch SL, Etcoff NL, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walther S, Hofle O, Federspiel A, et al. Neural correlates of disbalanced motor control in major depression. J Affect Disord. 2012;136:124–33. doi: 10.1016/j.jad.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Bajwa S, Bermpohl F, Rigonatti SP, et al. Impaired interhemispheric interactions in patients with major depression. J Nerv Ment Dis. 2008;196:671–7. doi: 10.1097/NMD.0b013e318183f86f. [DOI] [PubMed] [Google Scholar]
- 42.Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2006;9:641–54. doi: 10.1017/S1461145705006280. [DOI] [PubMed] [Google Scholar]
- 43.Stewart JL, Bismark AW, Towers DN, et al. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. 2010;119:502–12. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killgore WD, Gruber SA, Yurgelun-Todd DA. Depressed mood and lateralized prefrontal activity during a Stroop task in adolescent children. Neurosci Lett. 2007;416:43–8. doi: 10.1016/j.neulet.2007.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 46.Sackeim HA, Greenberg MS, Weiman AL, et al. Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Arch Neurol. 1982;39:210–8. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- 47.Jackson DC, Mueller CJ, Dolski I, et al. Now you feel it, now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci. 2003;14:612–7. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- 48.Tomarken AJ, Davidson RJ, Wheeler RE, et al. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. J Pers Soc Psychol. 1992;62:676–87. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- 49.Keedwell PA, Andrew C, Williams SC, et al. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 50.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80–5. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- 51.Segrave RA, Thomson RH, Cooper NR, et al. Emotive interference during cognitive processing in major depression: an investigation of lower alpha 1 activity. J Affect Disord. 2012;141:185–93. doi: 10.1016/j.jad.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Zou Q, Ross TJ, Gu H, et al. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp. 2012 Jun 19; doi: 10.1002/hbm.22136. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Victor TA, Furey ML, Fromm SJ, et al. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–38. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- 56.Keedwell PA, Drapier D, Surguladze S, et al. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J Affect Disord. 2010;120:120–5. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]