Abstract
The CS1 antigen provides a unique target for the development of an immunotherapeutic strategy to treat patients with multiple myeloma (MM). This study aimed to identify HLA-A2+ immunogenic peptides from the CS1 antigen, which induce peptide-specific cytotoxic T lymphocytes (CTL) against HLA-A2+ MM cells. We identified a novel immunogenic HLA-A2-specific CS1239–247 (SLFVLGLFL) peptide, which induced CS1-specific CTL (CS1-CTL) to MM cells. The CS1-CTL showed a distinct phenotype, with an increased percentage of effector memory and activated CTL and a decreased percentage of naive CTL. CS1239–247 peptide-specific CD8+ T cells were detected by DimerX analyses and demonstrated functional activities specific to the peptide. The CTL displayed HLA-A2-restricted and antigen-specific cytotoxicity, proliferation, degranulation and γ-interferon (IFN-γ)production against both primary MM cells and MM cell lines. In addition, the effector memory cells subset (CD45RO−CCR7−/CD3+CD8+) within CS1-CTL showed a higher level of CD107a degranulation and IFN-γproduction as compared to effector cells (CD45RO−CCR7−/CD3+CD8+) against HLA-A2+ primary MM cells or MM cell lines. In conclusion, this study introduced a novel immunogenic HLA-A2-specific CS1239–247 peptide capable of inducing antigen-specific CTL against MM cells that will provide a framework for its application as a novel MM immunotherapy.
Keywords: Multiple myeloma, CS1, Epitope, Peptide Vaccine
INTRODUCTION
Haematological malignancies are the most immune-responsive of human cancers. Among the novel immune treatment options in development, active-specific immunotherapy has the distinct advantage of inducing highly effective cytotoxic T lymphocytes (CTL) that target cancer cells, with an associated durable immune response (Westers, et al 2011; Speiser & Romero 2010; Silk & Finn 2007). An attractive active-specific immunotherapy approach is to trigger CTL with immunogenic peptides, which have specific activities against target antigens on tumour cells, ease of production, low toxicity, and broader applicability with a major histocompatibility complex (MHC)-restriction (Dudek, et al 2010).
Multiple myeloma (MM) is characterized by excess bone marrow plasma cells in association with monoclonal protein in the blood and/or urine in most cases. Despite recent advances in therapy including stem cell transplantation, two immunomodulatory drugs (thalidomide, lenalidomide), proteasome inhibitors (bortezomib), and new biological agents, MM remains an incurable disease (Anderson, 2005, Munshi, et al 2011); thus development of a targeted immunotherapy offers an attractive novel treatment approach.
A candidate target antigen in MM is the cell surface glycoprotein CS1 (CD2 subset 1, SLAMF7, CRACC, CD319). CS1, a member of the signalling lymphocyte activating-molecule-related receptor family, is highly expressed on MM cells and is absent in the vast majority of acute leukemia, B-cell lymphoma, and Hodgkin lymphomas (Hsi, et al 2008). In addition, CS1 antigen is not expressed by normal tissues or stem cells, but is expressed at low levels on natural killer (NK) cells and a subset of T lymphocytes compared with malignant plasma cells (Hsi, et al 2008). CS1 expression was observed on MM cells from all patients, including MM with high-risk and low-risk molecular profiles and those with and without cytogenetic abnormalities, suggesting that this antigen is not restricted to any particular MM subgroup (Zhan, et al 2006). Equally important for the development of immunotherapy, CS1 expression is maintained on patients’ MM cells even after disease relapse. The gene encoding CS1, (SLAMF7) is located on chromosome 1q, which is frequently amplified in aggressive MM and linked to early death (Shaughnessy, et al 2007). In a preclinical study, anti-CS1 monoclonal antibody (elotuzumab) delayed or abrogated the growth of human MM cell lines in mouse xenograft models. Histological analysis confirmed that all MM cells were targeted in the antibody-treated, but not in control, animals (van Rhee, et al 2009). An additional study demonstrated that anti-CS1 monoclonal antibody mediated allogeneic and autologous NK cell–mediated ADCC toward primary MM cells (Hsi, et al 2008), supporting CS1 as a potential therapeutic target in MM.
Here we report the identification of a novel immunogenic HLA-A2-specific epitope, CS1239–247 peptide (SLFVLGLFL), which is derived from the CS1 antigen andhas the ability to evoke MM-specific CTL. These CS1 peptide-specific CTL demonstrated HLA-A2-restricted anti-tumour cytotoxicity and degranulation against HLA-A2+ primary MM cells and MM cell lines. In addition, the CS1-CTL demonstrated cell proliferation and IFN-γ secretion in response to antigen restimulation, which is alsoHLA-A2-restricted and antigen-specific. We also observed distinct immunological activities specific to MM cells within the CD8 effector memory (CD45RO−CCR7−/CD3+CD8+) T cell subset. Therefore, the immunogenic CS1239–247 peptide described inthese studies offers a unique immunotherapeutic approach to effectively target MM cells and improve treatment outcome in patients with MM.
MATERIALS AND METHODS
Cell lines
The MM cell lines (McCAR, MM1S and U266) as well as the breast cancer cell line MCF-7 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The T2 cell line, a human B and T cell hybrid expressing HLA-A2 molecules, was provided by Dr. J. Molldrem (University of Texas M. D. Anderson Cancer Center, Houston, TX) and was used as antigen-presenting cells (APC). The K562 cell line transduced with HLA-A*0201 cDNA (K562-A*0201 cells) was provided by Dr. K. Anderson (Dana Farber Cancer Institute, Boston, MA). All cell lines were cultured in RPMI-1640 medium (Gibco-Life Technologies, Rockville, MD) supplemented with 10% fetal calf serum (FCS; BioWhittaker, Walkersville, MD), 100 iu/ml penicillin and 100 μg/ml streptomycin (Gibco-Life Technologies).
Reagents
Mouse anti-human CD3, CD4, CD8, CCR7, CD45RO, CD69, CD107a, IFN-γ and HLA-A2 monoclonal antibodies (mAbs) conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll (PerCP), PerCP-cyanin 5.5 PerCP-Cy5.5, allophycocyanin, Pacific Blue, allophycocyanin -H7 or PE-Cy7 were purchased from Becton Dickinson (BD)/Pharmingen or BD/Biosciences (San Diego, CA). Recombinant human interleukin (IL)-2, IL-4, αinterferon (IFN-α)and tumour necrosis factor a α(TNF-α)were purchased from R&D Systems (Minneapolis, MN), and granulocyte-macrophage colony-stimulating factor (GM-CSF) was obtained from Immunex (Seattle, WA).
Synthetic peptides
Four CS1 peptides were selected as potential HLA-A2-specific peptides: CS19–17 (TLIYILWQL), CS1232–240 (LLVPLLLSL), CS1236–245 (LLLSLFVLGL), and CS1239–247 (SLFVLGLFL). Influenza virus matrix protein58–66 (GILGFVFTL), MAGE3 (FLWGPRALV) and CMV pp65 (NLVPMVATV) were utilized as HLA-A2-specific positive control peptides. All peptides were synthesized by standard fmoc (9-fluorenylmethyl-oxycarbonyl) chemistry, purified to >90% using reverse-phase chromatography, and validated by mass-spectrometry for molecular weight (Biosynthesis, Lewisville, TX). Lyophilized peptides were dissolved in dimethyl sulphoxide (Sigma, St. Louis, MO), diluted in AIM-V medium (Gibco-Life Technologies), and stored at −140°C until needed.
Isolation of CD138+ plasma cells from bone marrow mononuclear cells
Bone marrow mononuclear cells (BMMC) were isolated by standard density gradient centrifugation over Ficoll-Paque™ Plus (Amersham Pharmacia Biotech AB, Uppsala Sweden) from bone marrow obtained from MM patients or normal donors. CD138+ plasma cells were isolated from BMMC using CD138 microbeads (Miltenyi Biotech, Auburn, CA) or RoboSep®CD138 (StemCell Technologies, Vancouver, Canada) positive immunomagnetic selection technology according to the manufacturer’s protocols. Primary CD138+ cells were used as target or stimulatory cells to evaluate the anti-tumour activity of CS1 peptide-specific CTL (CS1-CTL). Informed consent was obtained from all donors and the protocol was approved by the Institutional Review Board of Dana-Farber Cancer Institute (Boston, MA).
SLAMF7 gene expression
Plasma cells isolated from bone marrow obtained from either MM patients (n = 154) or normal donors (n = 9) were lysed using Trizol, and total RNA was collected using the Qiagen RNeasy kit (Qiagen, Valencia, CA). Gene expression was measured using human genome U133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA) scanned on a GeneArray scanner (Affymetrix). Affymetrix U133Plus2 arrays were hybridized with biotinylated in vitro transcription products (10 μg/chip) per manufacturer’s instructions and microarray expression profiling was analysed by the DNA-Chip Analyzer (Dchip) (Li et al., 2001) to determine SLAMF7 mRNA levels.
Surface protein expression of CS1 or HLA-A2
Primary CD138+ cells isolated from myeloma patients or MM cell lines (McCAR, U266 and MM1) were analysed for CS1 or HLA-A2 cell surface expression. The cells were stained with fluorochrome conjugated mouse anti-human CS1 or anti-human HLA-A2 mAbs for 15 min at 4°C, washed, and analysed using a FACSCanto™flow cytometer with CellQuest™ v2.1 software (Becton Dickinson, San Jose, CA). The primary CD138+ cells and MM cell lines were used as target or stimulatory cells to assess the immune function of CS1-CTL.
CS1 peptides binding assay
CS1 peptides were evaluated for HLA-A2-specific binding using the T2 cell line as described previously (Bae, et al 2004). In the assay, T2 cells were washed, resuspended in serum-free AIM-V medium to a final concentration of 1 × 106 cells/ml, and transferred into wells of a 24-well tissue culture plate. The cells were pulsed with 50 μg/ml of respective CS1 peptide [CS1236–245 (LLLSLFVLGL), CS1239–247 (SLFVLGLFL), CS1232–240 (LLVPLLLSL), CS19–17 (TLIYILWQL)] or 30 μg/ml of influenza virus matrix protein (IVMP)58–66 (GILGFVFTL) peptide plus 3 μg/ml human β2-microglobulin (Sigma) andincubated at 37°C, 5% CO2 in humidified air. Following overnight incubation, the cells were washed three times in PBS (Gibco-BRL) containing 5% FCS, stained with mouse anti-human HLA-A2-FITC mAb, washed, and analysed using a FACSCanto™flow cytometer. CS1 peptide binding affinity to HLA-A2 was determined by the up-regulation of HLA-A2 molecules on the T2 cells caused by specific peptide binding. Results are expressed as the Fluorescence Index (FI: [mean channel fluorescence of T2 cells pulsed with the peptide plus β2-microglobulin ÷ mean channel fluorescence of T2 cells pulsed with β2–microglobulin]) and demonstrated as the mean FI ± standard error (SE) for three separate experiments.
CS1 peptides stability assay
To assess HLA-A2/peptide complex stability, T2 cells were pulsed with CS1 peptide and human β2-microglobulin as described above. After overnight incubation, the T2 cells were washed to remove unbound peptide and incubated with 10 μg/ml Brefeldin A (Sigma) at 37°C for 1 h to block cell surface expression of newly synthesized HLA-A2 molecules. The HLA-A2/CS1 peptide complex stability was then evaluated at 0, 2, 4, 6 and 14 h post-Brefeldin A treatment. Following each incubation period, the cells were stained with HLA-A2-FITC mAb and analysed by flow cytometry. The results are shown as the HLA-A2 FITC mean fluorescence intensity (mean HLA-A2 MFI ± SE) for three separate experiments.
Generation of monocyte-derived dendritic cells
Peripheral blood mononuclear cells (PBMC) were isolated by standard density gradient centrifugation over Ficoll-Paque™ Plus (Amersham Pharmacia Biotech AB, Uppsala Sweden) from leucopaks obtained from HLA-A2+ normal donors. Dendritic cells (DC) were generated from monocytes obtained as the adherent cell fraction. To generate DC, the monocytes were cultured for 7 days in the presence of 1,000 u/ml GM-CSF and 1,000 u/ml IL-4 in RPMI-1640 medium (Gibco-Life Technologies) supplemented with 10% FCS. Fresh media plus GM-CSF and IL-4 was added to the cultures every other day. Mature DC (mDC) were obtained by adding 1,000 u/ml IFN-αplus 10 ng/ml TNF-αalong with fresh GM-CSF and IL-4 in 10% FCS-RPMI on day 7 and incubating an additional three days. The mDC were harvested and used as APC to generate CS1-CTL.
Isolation of CD3+ T cells
CD3+ T cells were obtained by negative selection from the non-adherent cell fraction post-monocyte adherence using the EasySep®magnet and Robosep®from StemCell Technologies. In brief, T cell enrichment was accomplished by depletion of non-CD3 T cells by labelling with mAbs directed against CD14, CD16, CD19, CD20, CD36, CD56, CD66b, CD123, and glycophorin A. After the removal of unwanted magnetically labelled cells, the enriched CD3+ T cells were washed and used to generate CS1-CTL. The purity of the enriched CD3+ T cells was found to be 97±2% (mean ± standard deviation [SD]) by flow cytometry.
Measurement of IFN-γ secretion by T cells stimulated with a respective CS1 peptide
The T cells, stimulated three times with matured DCs pulsed with a respective CS1 peptide, once a week for a total of three cycles, were evaluated for IFN-γ secretion by enzyme-linked immunosorbent assay (ELISA). For the assay, HLA-A2+ T lymphocytes were stimulated with T2 cells alone or T2 cells pulsed with respective CS1 peptide for 72 h at 37°C, 5% CO2 in humidified air. Following incubation, the supernatants were harvested and analysed for IFN-γ release using a human IFN-γ ELISA kit (R&D Systems). Briefly, purified IFN-γ standards or supernatants were transferred into wells of a 96-well plate pre-coated with a monoclonal anti-human IFN-γ capture antibody and incubated for 2 h at room temperature. After washing, the buffer containing detection antibody and avidin-horseradish peroxidase conjugate was added to each well and incubated for 1 h at room temperature. The wells were washed and developed by incubation with 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate solution for 30 min. Stop solution was added to each well and the absorbance was determined at 450 nm with a VICTOR2-1420 multi-label counter (PerkinElmer, Wellesley, MA). The amount of IFN-γ (pg/ml) present in the CTL culture supernatant was calculated based on the standard curve.
Induction of CS1 peptide-specific CTL
CS1-CTL were generated ex vivo by repeated stimulation of CD3+ T lymphocytes obtained from HLA-A2+ normal donors with CS1 peptide-pulsed APC (mDC or T2 cells). In brief, APC were washed and resuspended in serum-free medium AIM-V medium and pulsed overnight at 37°C and 5% CO2 in humidified air with 50 μg/ml of the CS1 peptide. Peptide-pulsed APC were washed, counted, irradiated at 10 Gy, and used to prime CD3+ T cells at a 1:20 APC/peptide (stimulator)-to-CD3+ T cell (responder) ratio in AIM-V medium supplemented with 10% human AB serum (BioWhittaker). The T cell cultures were restimulated once a week with irradiated APC/peptide for a total of 4 cycles. To maintain the T cells ex vivo, IL-2 (50 u/ml) was added to the cultures two days after the second stimulation. Control CD3+ T cell cultures were maintained under the same culture conditions, but without APC/peptide stimulation.
Phenotypic analysis and identification of CS1 peptide-specific CTL using DimerX HLA-A2: immunoglobulin (Ig) fusion protein technology
CS-CTL were stained with CD3, CD8, CD69, CD45RO and CCR7 mouse anti-human mAbs, washed, and analysed using a FACSCanto or Fortesa flow cytometer. The CD3+CD8+ cells were gated and evaluated for activation (CD69+) and memory/naive (CD45RO+CCR7−/CD45RO−CCR7+) cell subsets.
The presence of CS1 peptide-specific CD8+ T cells was assessed in the CS1-CTL using the DimerX assay, according to the manufacturer’s instructions. DimerX HLA-A2:Ig fusion protein (BD) was loaded with CS1 or control CMV pp65 peptide by overnight incubation of 2 μg dimer with 3 μg peptide and 3 μg β2-microglobulin (BD) at 37°C. As an additional control, we prepared DimerX HLA-A2:Ig fusion protein with β2-microglobulin alone. CS1-CTL (1×106 cells) were treated for 10 min with 2 μg of human IgG to block non-specific binding and then incubated with either empty or peptide-loaded DimerX HLA-A2:Ig fusion protein. Following incubation, the CS1-CTL were washed and stained with CD8-FITC anti-mouse IgG2a mAb and PE-conjugated anti-mouse IgG1 mAb (BD), washed, and analysed on a FACSCanto flow cytometer to evaluate the presence of CS1-peptide specific CD8+ T cells.
IFN-γ production and CD107a degranulation by CS1-CTL
Intracellular IFN-γ production and CD107a degranulation was analysed by flow cytometry to evaluate the functional activity of the CS1-CTL in stimulation with MM cell lines, primary MM cells or K562-A*0201 cells pulsed with respective peptide. In the assay, CS1-CTL were co-incubated at a 1:1 ratio with stimulator cells in 96-well round-bottom plates. CD107a PE-Cy5 mAb was added to the cultures, and the plates were placed into a 37°C, 5% CO2 incubator. After 1 h incubation, the protein transport inhibitors Brefeldin A (BD) and Monensin (BD) were added to the cells and incubated for an additional 5 h. As a baseline control, CS1-CTL were cultured in media alone with CD107a PE-Cy5 MAb, Brefeldin A, and Monensin, but without further stimulation. After incubation, the cells were harvested, washed, and surface stained with CD3-Pacific Blue, CD8-allophycocyanin-H7, CCR7-PeCy7, CD45RO-PE and CD69-PerCP anti-human mAbs. Cells were washed, fixed/permeabilized using Cytofix/Cytoperm (BD), and stained with anti-human IFN-γ FITC mAb, washed, and analysed on a Fortesa flow cytometer. The CS1-CTL cells were analysed for intracellular IFN-γ cytokine production and CD107a degranulation within the specific subsets of CD3+/CD8+ T cell population.
Cell proliferation by Carboxy Fluorescein Succinimidyl Ester (CFSE) tracking
Specific cell proliferation of CS1-CTL was measured by flow cytometry using the CFSE tracking dye. CS1-CTL were labelled with CFSE (Molecular Probes, Eugene, OR) at a final concentration of 5 μM and incubated for 10 min at 37°C in a 5% CO2 incubator protected from light. After quenching the reaction with 5 volumes of ice-cold PBS with 2% FCS-PBS and incubation for 5 min on ice, the cells were washed 3 times with 2% FCS-PBS and resuspended in fresh AIM-V media supplemented with 10% human AB serum. CFSE-labelled CS1-CTL (1 × 106 cells/ml) were co-incubated with stimulator cells (HLA-A2+ MM cell lines or primary CD138+ MM cells; 2 × 105 cells/ml) at 37°C and 5% CO2 in humidified air. CFSE-labelled CS1-CTL in media alone or the unstimulated control T cells were used as controls to establish baseline proliferation levels. After 5 or 6 days stimulation, the cells were harvested, washed, stained with human anti-CD3 and anti-CD8 mAbs, and evaluated by flow cytometry to determine the CTL proliferation to the respective stimulator cells. The specific cell proliferation was measured by gating on the CD3+CD8+ T cells and analysing their reduction in CFSE fluorescence intensity.
Calcein-release cytotoxicity assay
CS1-CTL were analysed for their cytotoxic activity using a calcein-release assay. Briefly, 3 × 105 target cells (McCAR, U266, MCF7, MM1S) were incubated with 10 mM calcein-AM (Molecular Probes) for 30 min at 37°C, washed 3 times, and resuspended in PBS containing 5% FCS. The calcein-AM labelled target cells (5 × 103 cells/well) were incubated with the CS1-CTL at various effector:target cell ratios in 96-well U-bottomed microtitre plates (triplicate wells/sample). Plates were incubated for 4 h at 37°C, 5% CO2. After incubation, the cells were pelleted by centrifugation and 100 μl of the supernatant was transferred from each well into 96-well flat-bottomed plates. The fluorescence of each supernatant was monitored at 490 nm excitation and 520 nm emission wavelengths using a VICTOR2-1420 multilabel counter (Perkin-Elmer). Maximum release was obtained from detergent-released target cells, and spontaneous release from target cells incubated in the absence of effector cells. The cytotoxicity of the CS1-CTL was calculated as follows: % Specific Lysis = [(experimental release − spontaneous release) ÷(maximum release − spontaneous release)].
Statistical analysis
Results are presented as mean ± SE. Groups were compared using unpaired Student’s t-test. Differences were considered significant when p<0.05.
RESULTS
SLAMF7 mRNA expression is universal on MM cells and the expression level is higher on primary myeloma cells than normal plasma cells
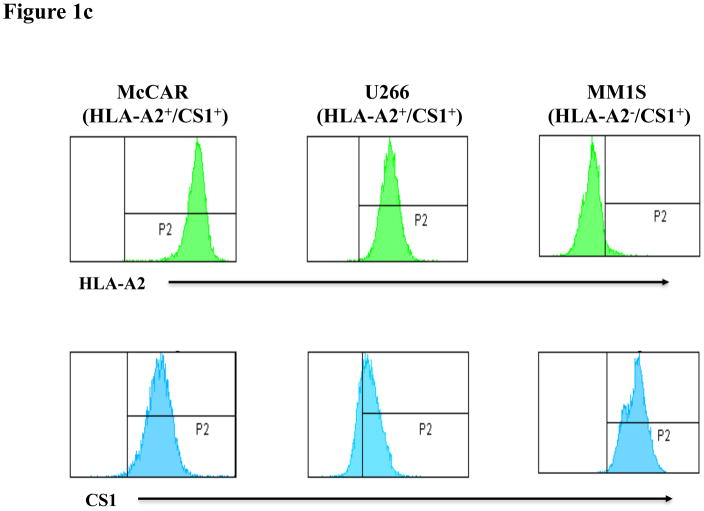
SLAMF7 mRNA expression was analysed in CD138-purified tumour cells isolated from MM patients (n = 154) or plasma cells from normal donors (n = 9). The microarray data (Figure 1a) demonstrates a significantly (p = 0.0045) higher level of SLAMF7 mRNA expression (A.U.) in MM patients’ plasma cells as compared to normal donors’ plasma cells detected by a probe 219159_s_at (SLAMF7). We confirmed surface expression of CS1 protein on primary CD138+ MM patient cells and MM cell lines by flow cytometry. CS1 protein expression was detected on primary CD138+ cells isolated from six MM patients although the level of CS1 expression was somewhat variable among patient samples (Figure 1b). CS1 protein and HLA-A2 expression were evaluated on three different MM cell lines [McCAR, U266, MM1S] by flow cytometry. Among the MM cells lines, McCAR and U266 showed HLA-A2+/CS1+ expression, while the MM1S was shown to be HLA-A2−/CS1+ (Figure 1c). Our results demonstrate that the MM cell lines and primary CD138+ cells isolated from MM patients express CS1 antigen and are suitable for use as target or stimulator cells to evaluate the functional activities of CS1 peptide-specific CTL.
Figure 1. CS1 expression on primary MM cells and MM cell lines.
Figure 1a. Difference in SLAMF7 mRNA expression between primary MM cells and normal plasma cells.
RNA isolated from CD138-purified plasma cells from MM patients (n = 154) or normal donors (n = 9) was evaluated for SLAMF7mRNA expression (probe 219159_s_at (SLAMF7)) by microarray analysis using an Affymetrix U133 Plus 2.0 array. A significantly (p = 0.0045) higher level of SLAMF7mRNA expression (A.U.) was observed in primary MM plasma cells as compared to normal plasma cells.
Fig. 1b. CS1 surface protein expression on primary CD138+ MM cells
Primary CD138+ cells obtained from MM patients were analysed for surface expression of CS1 protein by flow cytometry. Primary CD138+ cells from MM patients (n=6) are positive for CS1 protein expression.
Fig. 1c. HLA-A2 and CS1 protein expression on MM cell lines
Three MM cell lines, McCAR, U266 and MM1S, were evaluated for their surface expression of HLA-A2 and CS1 antigen. McCAR and U266 cells were found to express HLA-A2, but MM1S cells do not express HLA-A2. All three MM cell lines are positive for surface expression of CS1 antigen.
CS1239–247 peptide (SLFVLGLFL) demonstrates high HLA-A2 specific binding affinity/stability and induced CTL with a high level of IFN-γ secretion
The full length CS1 protein sequence was screened and evaluated by the search software RANKPEP, BIMAS and NetMHC to predict peptides that bind to HLA-A2 clefts with extended half-time disassociation rates and proteasomal C terminal cleavage. A total of four CS1 peptides, CS19–17 (TLIYILWQL), CS1232–240 (LLVPLLLSL), CS1236–245 (LLLSLFVLGL) and CS1239–247 (SLFVLGLFL) were selected, synthesized, and tested for their HLA-A2 binding affinities using the T2 binding assay. The affinity of CS1 peptides to HLA-A2 molecules is expressed as the Fluorescence Index (FI), which was calculated as [HLA-A2 MFI of T2 cells pulsed with the peptide plus β2-microglobulin ÷ HLA-A2 MFI of T2 cells alone plus β2-microglobulin]. A FI value >1 indicates the up-regulation of HLA-A2 molecules due to the specific binding of peptide to the molecules on the T2 cells. In this assay, the IVMP58–66 (GILGFVFTL) was used as a HLA-A2-specific positive control peptide (FI: 3.7 ± 0.06). Among the four CS1 peptides evaluated, the CS19–17 (TLIYILWQL) and CS1239–247 (SLFVLGLFL) peptides displayed the two highest (FI: 3.8 ± 0.14 and 2.95 ± 0.07, respectively) levels of HLA-A2-specific binding (Figure 2a). The CS1232–240 (LLVPLLLSL) and CS1236–245 (LLLSLFVLGL) peptides also displayed HLA-A2 specificity, but these peptides showed lower levels of HLA-A2 binding affinities, as shown by FI values of 2.30 ± 0.16 and 1.86 ± 0.1, respectively.
Figure 2. HLA-A2-affinity and immunogenicity of CS1 peptide.
Fig. 2a. CS1 peptide binding assay
The HLA-A2 binding affinity of CS1 peptides was analysed by flow cytometry. T2 cells were pulsed with the respective CS1 peptide (50 μg/ml) or influenza virus matrix protein58–66 peptide (IVMP; 30 μg/ml) plus human β2-microglobulin (3 μg/ml) in AIM-V serum-free media. After overnight incubation, the cells were washed to remove unbound peptides and stained with HLA-A2-FITC mAb. HLA-A2-specific peptide binding is shown as an increase in HLA-A2 Fluorescence Index (FI). Among the CS1 peptides tested, CS19–17 (TLIYILWQL)and CS1239–247 (SLFVLGLFL) peptides showed the highest HLA-A2 binding affinity. The values represent the mean ± SE of three separate experiments.
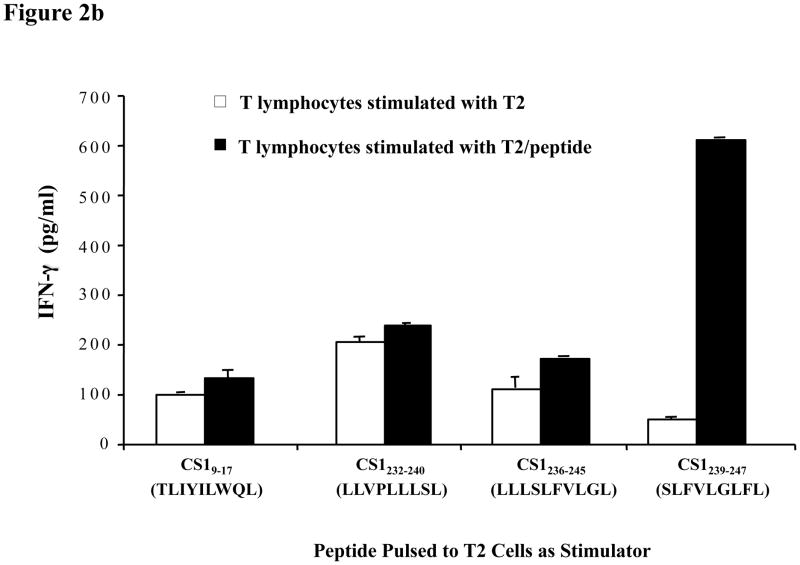
Fig. 2b. IFN-γ secretion by CS1-CTL in response to T2 cells pulsed with appropriate CS1 peptide.
IFN-γ secretion by CTL generated with CS19–17 (TLIYILWQL), CS1232–240 (LLVPLLLSL), CS1236–245 (LLLSLFVLGL) or CS1239–247 (SLFVLGLFL) peptide was measured by ELISA in the culture supernatants collected 72 h post-stimulation with T2 cells presenting the appropriate CS1 peptide (■). Among the four CS1 peptides tested, the CS1239–247 peptide (SLFVLGLFL) induced the highest level of IFN-γ (*p < 0.05) in the T cell culture in response to its specific peptide. The T lymphocytes stimulated with T2 cells alone secreted a baseline level of IFN-γ (□).
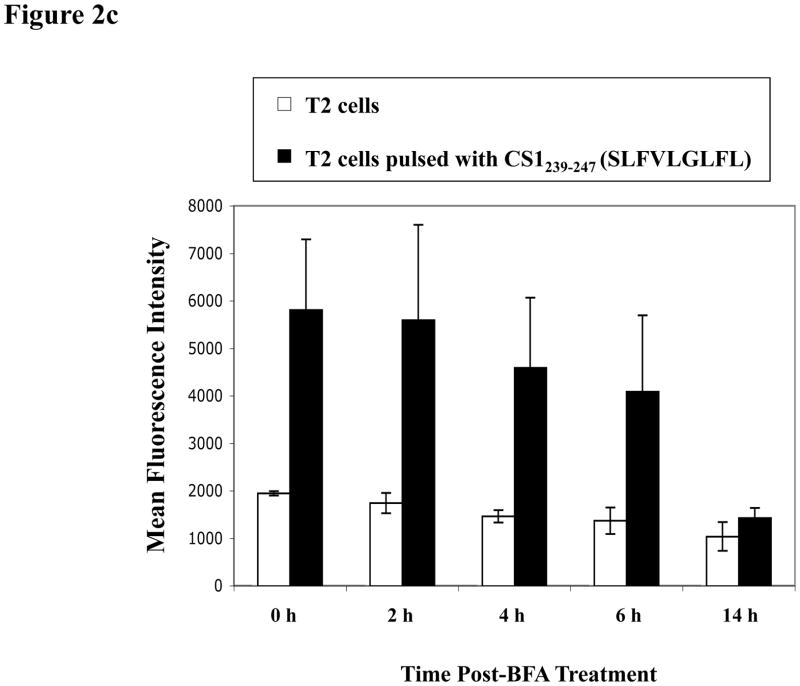
Fig. 2c. HLA-A2 binding stability of CS1239–247 (SLFVLGLFL) peptide.
The HLA-A2 binding stability of CS1239–247 peptide (SLFVLGLFL) was evaluated by flow cytometry. Either the peptide-pulsed (■) or non-pulsed (□) T2 cells were washed and incubated with Brefeldin A followed by staining with HLA-A2 FITC mAb at 0, 2, 4, 6 or 14 h incubation. Peptide binding stability was measured as HLA-A2 MFI (mean fluorescence intensity) on the T2 cells. High level of CS1239–247 peptide binding affinity was observed on T2 cells over time and the peptide stability was detected until 6 h post-Brefeldin A treatment. The HLA-A2 MFI values represent the mean ± SE of three separate experiments.
Given that the peptides all displayed HLA-A2 binding specificity (FI > 1), we generated CTL using each peptide from an HLA-A2+ normal donor as an additional screening measure to identify the peptide with the highest immunogenic potential. The respective CS1 peptide-stimulated CTL were evaluated for their ability to release IFN-γ upon stimulation with T2 cells pulsed with the appropriate CS1 peptide. The T lymphocytes stimulated with T2 cells alone were used as a negative control in these assays. The results, obtained by ELISA on the culture supernatants, showed that the CS1239–247 (SLFVLGLFL) peptide stimulated T lymphocytes secreted a significantly higher level of IFN-γ than those stimulated with each of the other CS1 peptides (Figure 2b). Based on these initial screening results, the CS1239–247 (SLFVLGLFL) peptide was selected for further evaluation of HLA-A2-specific binding-stability.
Next, we assessed the stability of the CS1239–247 peptide binding to HLA-A2 molecules. CS1239–247 peptide-pulsed T2 cells were washed and treated with Brefeldin A to block cell surface expression of newly synthesized HLA-A2 molecules, and then examined for HLA-A2 MFI at 0, 2, 4, 6 and 14 h by flow cytometry. A high level of CS1239–247 peptide binding stability to HLA-A2 was observed for up to 6 h (HLA-A2 MFI: 0 h = 5,805 ± 1,488, 2 h = 5,589 ± 2,009, 4 h = 4,586 ± 1,483, 6 hr= 4,081 ± 1,610, 14 h = 1,427 ± 207) (Figure 2c). The CS1239–247 peptide was shown to have high HLA-A2 specific affinity and was chosen for further characterization of its immunogenic potential in generating MM-specific CTL.
CS1239–247-CTL display a distinct phenotype
CS1 peptide-specific CTL were generated by repeated stimulation of HLA-A2+ normal donor CD3+ T cells weekly with APC pulsed with 50 μg/ml CS1239–247 peptide. One week after the fourth peptide stimulation, the resulting CS1-CTL were evaluated for their phenotypic profile by flow cytometry. The CS1-CTL contained a higher proportion of CD3+CD8+ T cells (Donor A: 84%, Donor B: 75%) compared to control T cells (no peptide stimulated) from the same donors (Donor A: 24%, Donor B: 23%) (Figure 3). Next, we analysed the CS1-CTL for naive (CD45RO−CCR7+), effector memory (EM) (CD45RO+CCR7−) and activated (CD69+) CD3+CD8+ T cell subsets. The CS1-CTL showed an increased frequency of EM CD3+CD8+ T cells as compared to the control T cells (Donor A: Control 5% vs. CS1-CTL 26%, Donor B: Control 4% vs. CS1-CTL 38%). A corresponding decrease was observed in the frequency of the naive CD3+CD8+ T cells (Donor A: Control 74% vs. CS1-CTL 8%, Donor B: Control 60% vs. CS1-CTL 6%) within the CS1-CTL as compared to the control T cells. In addition, an increased frequency of activated CD3+CD8+ T cells (Donor A: Control 3% vs. CS1-CTL 23%, Donor B: Control 5% vs. CS1-CTL 11%) was detected in the T cells stimulated with the CS1239–247 (SLFVLGLFL) peptide (Figure 3).
Fig. 3. Phenotype of CS1-CTL induced by CS1239–247 (SLFVLGLFL) peptide.<.

br>CS1-CTL were generated from HLA-A2+ normal donors’ CD3+ T cells by repeated stimulation with irradiated antigen-presenting cells pulsed with the CS1239–247 (SLFVLGLFL) peptide. One week after their fourth stimulation, the CTL were evaluated for their phenotype by flow cytometry. Compared to unstimulated control T cells (□), the CS1-CTL (■) generated from two different donors (Donor A, Donor B) showed enrichment of total CD3+CD8+ T cells. Further analysis of the CD3+CD8+ T cells shows that the CS1-CTL contain higher frequencies of effector memory (CD3+CD8+CD45RO+CCR7−) and activated (CD3+CD8+CD69+) T cells and a lower percentage of naive T cells (CD3+CD8+ CD45RO−CCR7+) as compared to the control unstimulated T cells.
CS1239–247 peptide-specific CD8+ T cells were detected by DimerX analyses and demonstrate functional activity specific to the peptide
DimerX technology (BD) was used to determine the frequency of the CS1239–247 peptide-specific cell population within the CS1-CTL. It was evaluated by incubating CS1-CTL with HLA-A2 dimerX loaded with CS1239–247 (SLFVLGLFL) peptide, HLA-A2-specific irrelevant CMV pp65 (NLVPMVATV) peptide or no peptide, and detected by flow cytometry. The CS1-CTL contained a distinct population of CS1239–247 peptide-specific CD8+ T cells, as shown by an increase in the frequency of cells expressing the HLA-A2 dimeric complexes loaded with the CS1239–247 peptide (Donor A CS1-CTL: 3.2%, Donor B CS1-CTL: 2.1%, Donor C CS1-CTL: 2.5%) (Figure 4a). In contrast, CS1-CTL incubated with the irrelevant HLA-A2 specific CMV pp65 (NLVPMVATV) peptide (Donor A CS1-CTL: 0.8%, Donor B CS1-CTL: 0.9%, Donor C CS1-CTL: 0.6%) or HLA-A2 dimeric complexes alone (Donor A CS1-CTL: 0.6%, Donor B CS1-CTL: 0.8%, Donor C CS1-CTL: 0.7%) displayed only background staining.
Figure 4. Detection of CS1239–247 peptide-specific CD8+ T cells in CS1-CTL and thepeptide-specific functional activity.
Fig. 4a. CS1 Peptide-specific CD8+ T cells: DimerX Technology
The frequency of CS1239–247 peptide-specific CD8+ T cell population was determined in CS1-CTL generated from three different HLA-A2+ donors using DimerX technology. CS1-CTL generated from each donor displayed a distinct population of CS1239–247 peptide-specific CD8+ T cells, as shown by an increase in the frequency of cells recognizing the HLA-A2 dimeric complex presenting the CS1239–247 peptide, but not the dimerX alone or dimeric complex presenting an irrelevant HLA-A2 specific CMV pp65 peptide.
Fig. 4b. CD107a degranulation, IFN-γ production or cellular activation of CS1-CTL from Donor A in response to CS1239–247 peptide
Peptide-specific responses of CS1-CTL generated from Donor A T lymphocytes were measured following stimulation with K562-A*0201 cells loaded with HLA-A2-specific peptides. CS1-CTL demonstrated peptide-specific responses to their cognitive CS1239–247 peptide presented by K562-A*0201 cells as shown by an increased frequency of CD107a+, IFN-γ+ or CD69+ cells within the CD3+CD8+ CTL population. CS1-CTL did not respond to irrelevant HLA-A2-specific MAGE3 or CMV pp65 peptides.
Fig. 4c. CD107a degranulation, IFN-γ production or cellular activation of CS1-CTL from Donor B in response to CS1239–247 peptide
Peptide-specific responses by Donor B CS1-CTL were measured in response to K562-A*0201 cells presenting HLA-A2-specific peptide. Compared to the control unstimulated T cells, CS1-CTL demonstrate a high level of IFN-γ production/cell activation (IFN-&+/CD69+) and degranulation/cell activation (CD107a+/CD69+) in response to CS1239–247 peptide presented by K562-A*0201 cells. The CS1-CTL showed no specific response against irrelevant HLA-A2-specific MAGE3 or CMV pp65 peptides.
We further examined CS1239–247 peptide specificity of the CS1-CTL using K562-A*0201 cells loaded with HLA-A2-specific peptides. In the assay, CS1-CTL generated from T lymphocytes of two different HLA-A2+ donors were analysed for the percentage of CD3+CD8+ T cells expressing IFN-g/CD69 or CD107/CD69 markers following stimulation with K562-A*0201 cells loaded with HLA-A2 specific peptides. Donor A CS1-CTL showed an increase in CD107a degranulation (CD107+ of CD3+CD8+ T cells: 18.3%), IFN-γ production (IFN-γ+ of CD3+CD8+ T cells: 3.6%) or cell activation (CD69+ of CD3+CD8+ T cells: 1.8%) when stimulated with CS1239–247 peptide-presenting K562-A*0201 cells. The CS1-CTL did not respond to irrelevant HLA-A2-specific peptides MAGE3 (FLWGPRALV) peptide- (CD107+ cells: 4.9%, IFN-γ+ cells: 0.5%, CD69+ cells: 0.3%) or CMV pp65 (NLVPMVATV) peptide- (CD107+ cells: 4.3%, IFN-γ+ cells: 0.5%, CD69+ cells: 0.2%) presenting K562-A*0201 cells (Figure 4b).
Donor B CS1-CTL demonstrated an increase of IFN-γ production and cell activation (IFN-γ+CD69+/CD3+CD8+ T cells) in response to CS1239–247 peptide-presenting K562-A*0201 cells (15.0%), but not to the irrelevant HLA-A2-specific peptides MAGE3 peptide- (3.6%) or CMV pp65 peptide- (4.8%) presenting K562-A*0201 cells (Figure 4c). Control K562-A*0201 alone did not induce IFN-γ production and cell activation by the CS1-CTL (2.7%). In addition, CS1239–247 peptide-specific responses were demonstrated by an increase in degranulation and cell activation (CD107+CD69+/CD3+CD8+ T cells) by the CS1-CTL upon stimulation with CS1239–247 peptide- (15.5%), but not with MAGE3 peptide- (4.3%) or CMV pp65 peptide- (4.6%) presenting K562-A*0201 cells (Figure 4c). Taken together, these results demonstrate that CS1-CTL contain a population of cells specific to the CS1239–247 peptide, which have distinct functional activities, including IFN-γ production, cell activation, and CD107a degranulation in response to the CS1239–247 peptide, which can be presented on MM cells.
CS1239–247-CTL display HLA-A2-restricted IFN-γ secretion, CD107a degranulation, cytotoxicity, and cell proliferation in response to MM cell lines
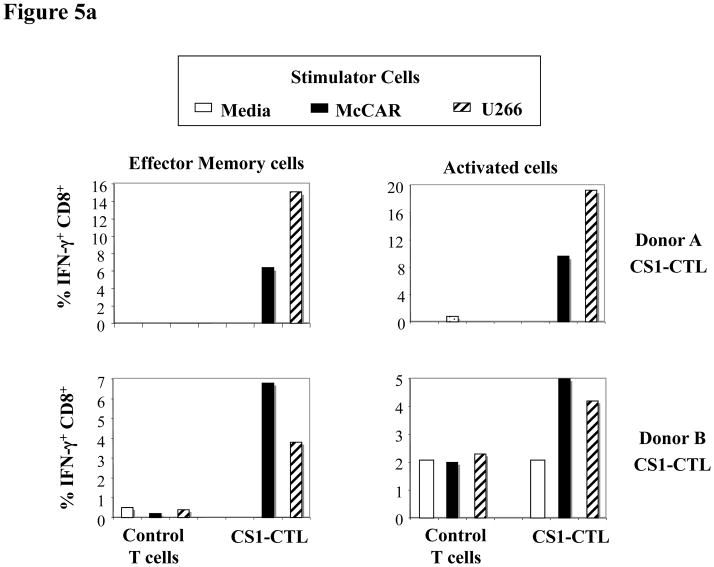
The frequency of IFN-γ production was measured within a subgroup of effector memory or activated cells of the CD3+CD8+ CS1-CTL. An increase of IFN-γ production was detected in CS1-CTL to HLA-A2+ MM cells in EM (CD45RO+CCR7-) CTL (Donor A CS1-CTL: media = 0%, McCAR stimulation = 6.4%, U266 stimulation = 15.1%; Donor B CS1-CTL: media = 0.3%, McCAR stimulation = 6.8%, U266 stimulation = 3.8%) or activated (CD69+) CTL (Donor A CS1-CTL: media = 0.4%, McCAR stimulation = 9.6%, U266 stimulation = 19.3%; Donor B CS1-CTL: media = 2.1%, McCAR stimulation = 5%, U266 stimulation = 4.2%) (Figure 5a). The level of response to each HLA-A2+ MM cell line showed a variation by the CTL generated from different individuals. No significant changes were observed in the control T cells upon stimulation with HLA-A2+ McCAR or U266 cells.
Figure 5. Functional activity of CS1-CTL in response to HLA-A2+ MM cell lines.
Fig. 5a. High level of IFN-γ production by CS1-CTL to HLA-A2+ MM cells
Intracellular IFN-γ production was measured by flow cytometry upon the stimulation of CS1-CTL with McCAR or U266 HLA-A2+ MM cells. Intracellular IFN-γ production was detected in both the effector memory (CD45RO+CCR7−/CD3+CD8+) and activated (CD69+/CD3+CD8+) T cell population to each MM cell line. Control unstimulated T cells produced no or dramatically lower level of IFN-γ in response the HLA-A2+ MM cells.
Fig. 5b. CD107a degranulation and IFN-γ production by Effector Memory or Effector cell subsets of CS1-CTL in response to MM cells
CS1-CTL generated from HLA-A2+ donors (n = 3) were evaluated by flow cytometry analyses for CD107a degranulation and IFN-γ production within the Effector Memory (CD45RO+CCR7−) and Effector (CD45RO−CCR7−) CD3+CD8+ T cell subsets following stimulation with McCAR or U266 HLA-A2+ MM cells. Both subsets of CS1-CTL display high degranulation and IFN-γ production to the MM cells. The level of response was greater within the Effector Memory subset as compared to Effector CD3+CD8+ T cell subset.
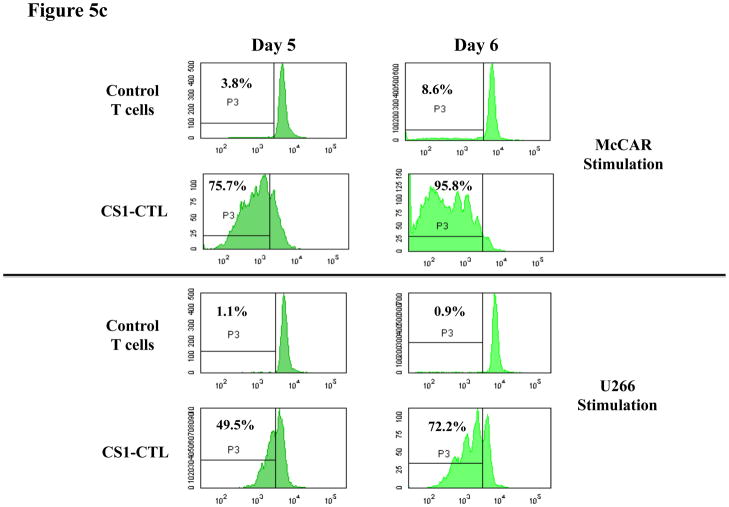
Fig. 5c. Cell proliferation in response to HLA-A2+ MM cells
Specific cell proliferation was examined by flow cytometry after stimulating CFSE-labelled CS1-CTL with tumour cell lines for 5 or 6 days. The proliferating cell population was measured as the percent decrease in CFSE expression (P3-gated cells). Background proliferation was determined using CFSE-labelled CTL cultured in media alone. The CTL demonstrated an increase in cell proliferation in response to stimulation with HLA-A2+ MM cell lines.
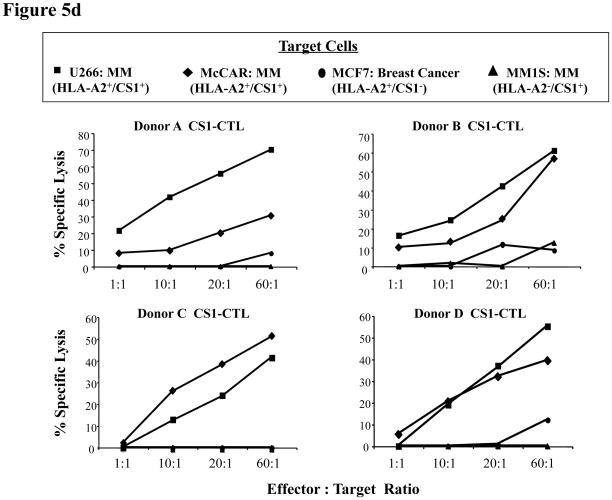
Fig. 5d: High CS1-CTL cytotoxicity against HLA-A2+ MM cell lines
Calcein-release assays were performed to measure the cytotoxic activity of CS1-CTL against different tumour cell lines. Target cells (U266, McCAR, MCF7, MM1S; 5 × 103 cells/well) were incubated in serum-free culture medium containing calcein-AM for 30 min, washed three times, and incubated with effector cells (CS1-CTL) at the various effector: target cell ratios. After 4 h incubation, the calcein-release was measured in the supernatant. Maximum release was obtained from detergent-released target cell counts and spontaneous release from target cell counts in the absence of effector cells. Cellular cytotoxicity was calculated as follows: % Specific Lysis = [(experimental release spontaneous release) ÷ (maximum release – spontaneous release)]. CS1-CTL generated from four HLA-A2+ donors (A, B, C and D) demonstrated effective lysis of both HLA-A2+ U266 and McCAR MM cell lines, but not HLA-A2+ MCF7 breast cancer cells or HLA-A2- MM1S MM cell lines.
To further characterize the immune function of CS1-CTL, we evaluated EM (CD45RO+CCR7−/CD3+CD8+) and Effector (CD45RO−CCR7−/CD3+CD8+) T cell subsets for CD107a degranulation and IFN-γ production in the CTL generated from three different donors (Figure 5b). Both the EM and Effector cell subsets demonstrated a significant increase in the frequency of cells positive for CD107a degranulation marker, a measure of cytotoxic activity, upon recognition of either the McCAR [% CD107a: EM = 13.23 ± 2.46%, Effector = 7.87 ± 2.15%] or U266 [% CD107a: EM = 8.93 ± 3.61%, Effector = 5.07 ± 2.91%] MM cell lines as compared to the CS1-CTL cultured in media alone [% CD107a: EM = 1.13 ± 0.71%, Effector = 0.83 ± 0.78%]. In addition, both the EM and Effector cell subsets demonstrated a significant increase in the frequency of cells producing IFN-γ upon recognition of either McCAR [% IFN-γ: EM = 4.97 ± 1.96%; Effector = 3.03 ± 1.10%] or U266 [% IFN-γ: EM = 4.20 ± 2.05%; Effector = 1.50 ± 0.87%] cells as compared to the CS1-CTL cultured in media alone [% IFN-γ: EM = 0.37± 0.35%; Effector = 0.20 ± 0.17%]. However, the level of immune response as measured by CD107a degranulation and IFN-γ production was higher in the EM as compared to the Effector CD3+CD8+ T cells in response to HLA-A2+ MM cell lines.
We further demonstrated CS1-CTL proliferation in response to HLA-A2+CS1+ MM cell lines. The proliferating cell population was measured as the percent decrease in CFSE expression, as defined by cells found in P3 (CFSE-low/CD8+ cells). Compared to control T cells, CS1-CTL showed a significantly higher level of cell proliferation in response to McCAR (Control T cells vs. CS1-CTL: Day 5 = 3.8% vs. 75.7%, Day 6 = 8.6% vs. 95.8%) or U266 (Control T cells vs. CS1-CTL: Day 5 = 1.1% vs. 49.5%, Day 6 = 0.9% vs. 72.2% on day 6) MM cell lines (Figure 5c).
Finally, we demonstrated the direct cytotoxic activity of CS1-CTL against various tumour cell lines using a 4-h calcein-release assay. CS1-CTL generated from four different donors’ T lymphocytes displayed high level of cytotoxic activity against HLA-A2+/CS1+ MM cells including U266 (Donor A CS1-CTL: 21.1 – 69.8%, Donor B CS1-CTL: 15.9 – 60.6%, Donor C CS1-CTL: 0 – 41.4%, Donor D CS1-CTL: 0 - 55.2%) and McCAR (Donor A CS1-CTL: 7.9 – 30.5%, Donor B CS1-CTL: 10 – 56.8%, Donor C CS1-CTL: 1.9 – 50.9%, Donor D CS1-CTL: 5.5 – 39.4%) at effector:target cell ratios between 1:1 and 60:1 (Figure 5d). However, the CS1-CTL did not demonstrate significant cytotoxicity against the antigen-mismatched HLA-A2+/CS1− breast cancer cell line MCF-7 (HLA-A2+/CS1−) (Donor A CS1-CTL: 0 – 8%, Donor B CS1-CTL: 0 – 11.1%, Donor C CS1-CTL: 0%, Donor D CS1-CTL: 0 – 12%) or the MHC-mismatched MM cell line MM1S (HLA-A2−/CS1+) (Donor A CS1-CTL: 0%, Donor B CS1-CTL: 0 – 12.4%, Donor C CS1-CTL: 0%, Donor D CS1-CTL: 0%), thereby confirming the cytotoxic activity of CS1-CTL is antigen-specific and HLA-restricted.
CS1239–247 peptide-specific CTL display anti-tumour activity to HLA-A2+ primary MM cells
The myeloma-specific functional activities of CS1-CTL were further evaluated against primary myeloma cells in CFSE-based cell proliferation assays. CS1-CTL displayed significant cell proliferation (CFSE-low/CD8+ cells; gated in Q1) after stimulation with primary CD138+ cells isolated from three different HLA-A2+ MM patients (Patient A = 24.0%, Patient B = 14.5%, Patient C = 20.2%) as compared to the CS1-CTL cultured in media alone (4%) (Figure 6a)
Figure 6. Functional anti-tumour activity of CS1-CTL to primary cells obtained from HLA-A2+ MM patients.
Figure 6a. High CS1-CTL proliferation in response to primary HLA-A2+ MM cells
Specific cell proliferation was examined by flow cytometry after stimulating CFSE-labelled CS1-CTL with primary CD138+ cells isolated from HLA-A2+ MM patients. The proliferating cell population was measured on day 5 as the percent decrease in CFSE expression (Q1-gated cells). CS1-CTL stimulated with HLA-A2+ primary MM cells show increased cell proliferation as compared to the baseline cell proliferation of the CTL cultured in media alone.
Figure 6b. CD107a degranulation of CS1-CTL to primary MM cells in HLA-A2-restricted manner
CS1-CTL degranulation (CD107a) was analysed in response of primary cells isolated from MM patients by flow cytometry. CS1-CTL showed an increased proportion of CD8+ cells expressing the CD107a degranulation marker upon recognition of primary cells from HLA-A2+ MM patients (Patients 1, 2), but not from HLA-A2− MM patients (Patients 3, 4), demonstrating HLA-restricted degranulation of the CTL. Control CS1-CTL cultured in media alone did not upregulate the expression of CD107a degranulation marker. The results are shown as the percentage of CD3+CD8+ CTL expressing surface CD107a marker.
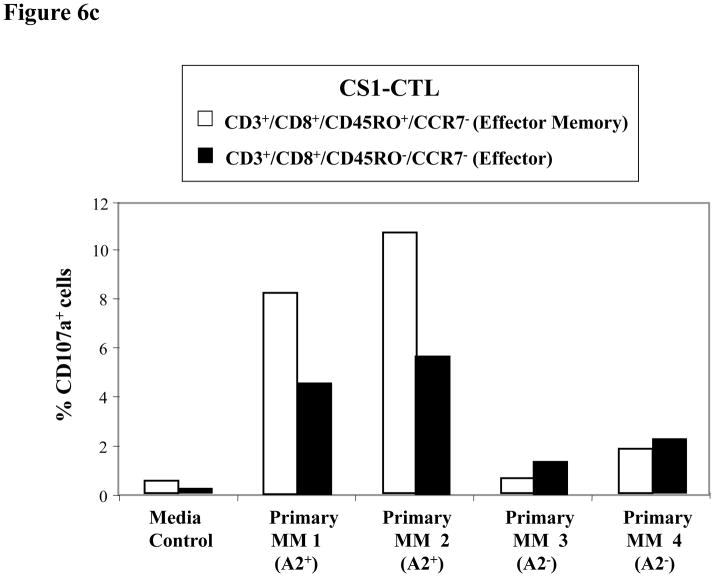
Figure 6c. Higher CD107a degranulation by Effector Memory CS1-CTL subset in response to HLA-A2+ primary MM cells
The Effector Memory (CD45RO+CCR7−/CD3+CD8+) and Effector (CD45RO-CCR7−/CD3+CD8+) CS1-CTL populations were evaluated in parallel for their upregulation of CD107a in response to primary MM cells. Between the CTL subsets, Effector Memory cells demonstrate a higher level of degranulation (CD107a) than the Effector cells to HLA-A2+ primary MM cells (Patients 1,2). The CS1-CTL CD107a degranulation response to HLA-A2− primary MM cells (Patients 3, 4) was similar to that of the background level.
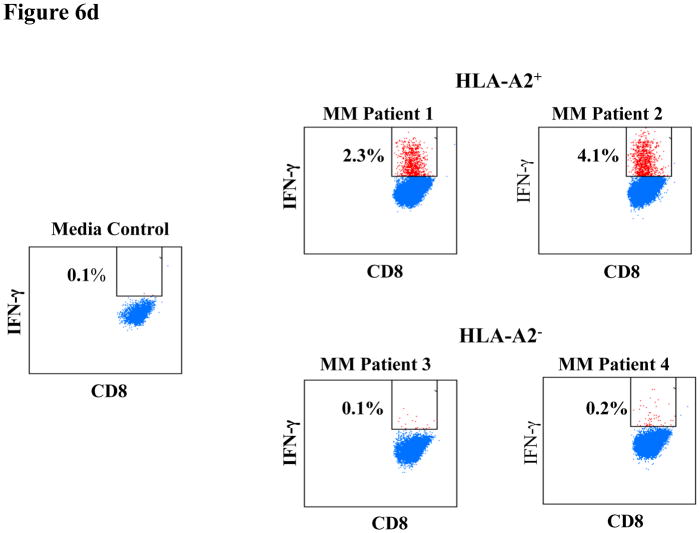
Figure 6d. IFN-γ production by CS1-CTL to primary MM cells in HLA-A2-restricted manner
CS1-CTL have increased intracellular IFN-γ production in response to primary cells from HLA-A2+ MM patients (Patients 1, 2), but not against primary cells from HLA-A2− MM patients (Patients 3, 4), thereby demonstrating an HLA-restricted IFN-γ production by the CTL. The results are shown as the percentage of CD8+ CTL producing IFN-γ against the specific stimulation.
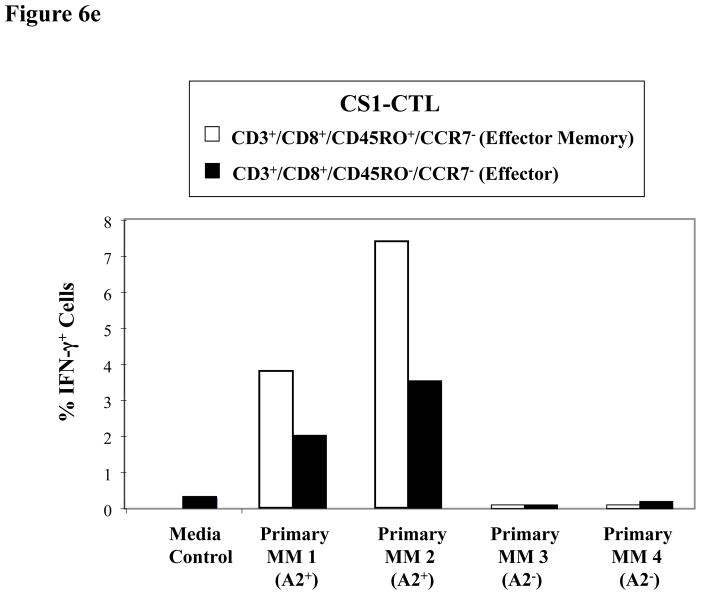
Figure 6e. Higher IFN-γ production by Effector Memory CS1-CTL subset inresponse to HLA-A2+ primary MM cells
The CS1-CTL Effector Memory (CD45RO+CCR7−/CD3+CD8+) T cell subset has a higher frequency of IFN-γ producing cells as compared to the Effector (CD45RO−CCR7−/CD3+CD8+) T cell subset in response to HLA-A2+ primary MM cells (Patients 1, 2). The CS1-CTL subsets did not produce IFN-γ in response to HLA-A2− primary MM cells (Patients 3, 4).
Next, CS1-CTL were analysed for CD107a degranulation in response to primary MM cells. The CD3+CD8+ CS1-CTL showed a significant increase in the frequency of CD107a+ cells upon stimulation with primary CD138+ cells isolated from HLA-A2+ MM patients (Patient 1: 6.0%, Patient 2: 7.9%), but not against HLA-A2−/CD138+ MM patients’ cells (Patient 3: 1.4%, Patient 4: 2.4%) (Figure 6b). Control CS1-CTL in media alone (0.6%) did not display CD107a expression. CS1-CTL CD107a+ degranulation was further characterized within the EM CTL (CD45RO+CCR7−/CD3+CD8+) or Effector (CD45RO−CCR7−/CD3+CD8+) CTL subsets (Figure 6c). The EM CTL subset (Patient 1: 8.2%, Patient 2: 10.6%) showed a higher level of degranulation than the Effector CTL subset (Patient 1: 4.5%, Patient 2: 5.6%) in response to HLA-A2+ primary MM cells. However, HLA-A2− primary MM cells did not induce CD107a degranulation in either of the CS1-CTL subsets (EM - Patient 3: 0.6%, Patient 4: 1.8%, Effector - Patient 3: 1.8%, Patient 4: 2.2%), thereby demonstrating their HLA-A2 restricted cytotoxic activity against primary MM cells.
IFN-γ production was also detected in CS1-CTL in response to CD138+ primary cells from HLA-A2+ MM patients (Patient 1: 2.3%, Patient 2: 4.1%), but not the cells from HLA-A2− MM patients (Patient 3: 0.1%, Patient 4: 0.2%) (Figure 6d). As with CD107a degranulation, the CS1-CTL demonstrated a higher level of IFN-γ production in the EM cell subset as compared to the Effector cell subset in response to HLA-A2+ MM primary cells (EM vs. Effector CTL; Patient 1: 3.8% vs. 2.0%, Patient 2: 7.4% vs. 3.5%). In addition, the CS1-CTL EM and Effector cell subsets did not produce IFN-γ in response to primary cells from HLA-A2− MM patients (EM vs. Effector CTL; Patient 3: 0.0% vs. 0.3%, Patient 4: 0.2% vs. 0.7%) (Figure 6e). Taken together, these results confirm that the anti-tumour activities of CS1-CTL against primary MM cells is HLA-A2 restricted and highlights the potential of maintaining long-term anti-MM activities through the development of CS1 peptide-specific CTL with an EM cell phenotype.
DISCUSSION
Various tumour-associated antigens (TAAs) have been identified in MM as potential targets for immunotherapeutic strategies. In addition to idiotype protein specific to MM cells, other TAAs are abundantly expressed on tumour cells with only limited or no expression in normal tissues. These TAAs include MUC1, an epithelial cell glycoprotein that has an oncogenic effect through the interaction of its cytoplasmic tail with various growth factor receptors and signalling molecules including ErbB and ERK (Tang & Apostolopoulos 2008, Bafna, et al 2010). Cancer-testis antigen members, MAGE, BAGE, GAGE, PRAME, NY-ESO-1 and Sperm protein17, are involved in key mechanisms for epigenetic regulation (DNA methylation and covalent histone modifications) of transcription (Karpf, 2006, Berger, et al 2009). The Wilms tumor gene (WT1) is involved in oncogenic functions and regulation of cell growth and differentiation (Vicent, et al 2010, Scholz & Kirschner, et al 2011). hTERT, the catalytic subunit of telomerase that is important for leukemogenesis, whose expression, along with telomere length, correlates with the occurrence of chromosomal abnormalities (Warner, et al 2005, Hartmann, et al 2005). Vascular endothelial growth factor (VEGF) promotes angiogenesis in tumour cells and facilitates improved blood flow and MM cell survival (Anderson, 2001, Podar & Anderson 2007). CD40, a tumour necrosis factor receptor superfamily member, has a costimulatory role in MM cell proliferation and differentiation (Tong, et al 2000, Hussein, et al 2010). Dickkopf-1 is a secreted protein that specifically inhibits the Wnt/β-catenin signalling and facilitates the development of lytic bone lesions in MM (Mao, et al 2001, Tian, et al 2003).
Additional MM-associated antigens and potential immunotherapeutic targets include XBP1, a critical transcription factor required for terminal differentiation to plasma cells (Reimold, et al 2001, Shaffer, et al 2002); and CD138 (Syndecan-1), which regulates the activity of many growth factors and cytokines that can influence MM cells growth (Bharti, et al 2004, Wolowiec, et al 2006). In previous studies, we have demonstrated the immunotherapeutic potential of HLA-A2-specific heteroclitic XBP1 US184–192 (YISPWILAV), heteroclitic XBP1 SP367–375 (YLFPQLISV), and native CD138260–268 (GLVGLIFAV) peptides to induce antigen-specific CTL with HLA-A2-restricted anti-tumour activities against both primary MM cells and MM cell lines (Bae, et al 2011a, b).
SLAMF7, a gene encoding the CS1 cell-surface protein (also known as SLAMF7), which was previously associated with NK cells (Boles & Mathew 2001, Bouchon, et al 2001), is highly expressed in plasma cells. In agreement with previous reports (Hsi, et al 2008, Tai, et al 2008), our microarray analyses showed higher SLAMF7 mRNA expression in plasma cells from MM patients (n = 154) compared to those from normal donors (n = 9). Importantly, using DNA microarrays, SLAMF7 gene expression was not found in 332 normal adult tissue samples representing 86 different organs (Hsi, et al 2008). Since SLAMF7 expression has been detected in normal plasma cells, NK cells, a subset of T cells, activated monocytes, and activated dendritic cells, careful monitoring of these normal cell populations will be required when developing an immunotherapy targeting the CS1 antigen. In these studies, we demonstrated CS1 protein expression on primary CD138+ MM patient cells and MM cell lines by flow cytometric analyses. Hsi et al (2008) also showed the specificity of CS1 protein expression on MM cells by demonstrating the expression of CD138 on the majority of CS1-positive infiltrating cells in MM, while finding no expression in normal tissue parenchyma. Interestingly, their analyses showed the absence of CS1 expression in most solid tumours and other haematological malignancies, including the vast majority of acute leukaemias, B-cell lymphomas and Hodgkin lymphomas. Therefore, the highly uniform expression of CS1 in MM cells, along with a restricted expression profile in normal cells and tissues, suggests that CS1 can be a potential selective therapeutic target for treatment of MM.
A humanized anti-CS1 mAb (HuLuc63) has demonstrated both ex vivo and in vivo anti-myeloma activity. This CS1-specific antibody inhibited MM cell adhesion, induced antibody-mediated cellular cytotoxicity in the bone marrow milieu (van Rhee, et al 2009, Tai, et al 2008), and is currently showing great promise in combination with lenalidomide and dexamethasone for the treatment of relapsed MM. To further expand on the therapeutic application of CS1 as a target antigen in MM, we here identified a novel CS1-specific HLA-A2 peptide and evaluated its ability to generate peptide-specific CTL to MM cells ex vivo. Initially, specific nonamers were selected by screening the full-length CS1 protein sequence (335 amino acids), using the search software BIMAS along with the algorithm RANKPEP and NetMHC programs, for peptides that bind to HLA-A2 molecules with extended half-time disassociation rates and proper proteasomal C terminal cleavage (Seyed, et al 2011, Strothmeyer, et al 2010, Lundegaard, et al 2008). Among the four CS1 peptides initially evaluated, CS1239–247 (SLFVLGLFL) showed the highest level of HLA-A2 binding/stability and induction of functional CTL; therefore, this peptide was chosen for further evaluation. In our ex vivo studies, the CS1239–247 (SLFVLGLFL) peptide-specific CTL possessed phenotypic characteristics similar to CTL described previously from MM patients (Barber, et al 2008, Svane, et al 2007). In addition, we showed the presence of CS1-peptide-specific CD8+ T cells with functional immune activity specific to CS1239–247 (SLFVLGLFL). Within the CS1-CTL, the EM (CD45RO+CCR7−/CD3+CD8+) T cell subset had the highest level of anti-tumour activity against CS1+/HLA-A2+ primary MM cells and MM cell lines as compared to the Effector (CD45RO−CCR7−/CD3+CD8+) or naive (CD45RO−CCR7+/CD3+CD8+) T cell subsets. Taken together, our data indicates a potential induction and long-term maintenance of anti-MM activity by the CS1239–247 (SLFVLGLFL) peptide-specific CTL within an Effector Memory CD3+/CD8+ T cell subset.
A potential concern with CS1-targeted therapy is damage to normal cells, especially NK cells and a subset of T cells, which express the antigen. However, this may not be a major concern because CS1 expression levels have been shown to be substantially lower in NK and T cells compared to MM cells (Hsi et al., 2008). To evaluate the potential toxicity of targeting CS1 antigen, other investigators have studied the effects of CS1-specific mAb on leucocytes in comparison with other mAbs, including rituximab (CD20-specific mAb) and alemtuzumab (CD52-specific mAb), which deplete B cells or various lymphocytes, respectively (Vugmeyster, et al 2003, Brett, et al 1996). CS1-specific mAb treatment did not result in substantial depletion of major lymphocyte subsets (20% decrease of NK cell numbers) in comparison with rituximab (70% decrease of B cells) or alemtuzumab (65–100% depletion of all lymphocyte subsets) (Hsi et al., 2008). In addition, CS1-specific mAb exerted anti-MM activity in vivo through NK cell–mediated ADCC via binding of CD16 expressed on the surface of NK cells (Hsi, et al 2008, Tai, et al 2008). Therefore, these results, coupled with the absence of CS1 expression in normal tissue and haematopoietic CD34+ stem cells, suggest that CS1-specific immunotherapy can target neoplastic cells without inducing major damage to normal cells in MM patients.
A CS1-specific peptide vaccine has the potential to be applied successfully as a follow-up or maintenance therapy post-transplant because MM cells express CS1 antigen at the time of relapse (Barlogie, et al 2006). In addition, CS1 expression is observed in all seven molecular MM subtypes, including MM with high risk and low risk molecular profiles and with or without cytogenetic abnormalities. Furthermore, a high prevalence of SLAMF7 gene expression has been detected in patients with monoclonal gammopathy of undetermined significance (MGUS) and smoldering myeloma, suggesting that CS1 targeted therapy could potentially be applied to an even broader range of patients with plasma cell disorders.
The field of cancer vaccines has yet to establish its own specific criteria for the selection of appropriate patient populations, which is an important criterion for a successful clinical outcome. Given the diversity and complexity of immune responses in patients with cancer, it is essential to assess the status of the patients’ immune system prior to and during a vaccine clinical trial (Schlom, et al 2008, Hoos, et al 2007). For instance, patients without immunological memory against vaccine antigens may take more time to develop tumour-specific immune responses. Additionally, it may be preferable to vaccinate patients early in their disease course, when they have a more fully competent immune system. For instance, patients with smoldering myeloma who have relatively normal immune function and do not require chemotherapy may provide an optimal clinical setting to evaluate immunotherapy approach.
In summary, we here propose the CS1 antigen, expressed at high levels on malignant plasma cells and not on CD34+ stem cells and other normal cells, as an attractive therapeutic target for development of a MM-specific immunotherapy. The immunogenic CS1-specific HLA-A2 peptide CS1239–247 (SLFVLGLFL) identified in these studies induced antigen-specific CTL that specifically recognized, targeted, and lysed HLA-A2+/CS1+ primary MM cells and MM cell lines. We confirmed the HLA-restricted nature of the CS1-CTL response using both HLA-A2− primary MM cells as well as MM cell lines. Taken together, these data provide evidence for the potential of CS1-specific HLA-A2 peptide in clinical application as a peptide-based vaccine in patients with MM and related diseases.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health Grants RO1-124929 to Dr. Nikhil C. Munshi, P50-100007, PO1-78378 and PO1155258 to Dr. Nikhil C. Munshi and Dr. Kenneth C. Anderson and RO1-50947 to Dr. Kenneth C. Anderson. Dr. Anderson is an American Cancer Society Clinical Research Professor. We also acknowledge the generous support of the Stewart Nagler Research Fund.
References
- Anderson KC. Novel biologically based therapies for myeloma. The Cancer Journal. 2001;7(Suppl 1):S19–23. [PubMed] [Google Scholar]
- Anderson KC. Lenalidomide and thalidomide: mechanisms of action-similarities and differences. Seminars in Hematology. 2005;42:S3–8. doi: 10.1053/j.seminhematol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Bae J, Martinson JA, Klingemann HG. Heteroclitic CD33 peptide with enhanced anti-acute myeloid leukemic immunogenicity. Clinical Cancer Research. 2004;15:7043–7052. doi: 10.1158/1078-0432.CCR-04-0322. [DOI] [PubMed] [Google Scholar]
- Bae J, Carrasco R, Lee AH, Prabhala R, Tai YT, Anderson KC, Munshi NC. Identification of novel myeloma-specific XBP1 peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma. Leukemia. 2011a;25:1610–1619. doi: 10.1038/leu.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Tai YT, Anderson KC, Munshi NC. Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders. British Journal of Haematology. 2011b;155:349–361. doi: 10.1111/j.1365-2141.2011.08850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;20:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Experimental Hematology. 2008;36:1318–1328. doi: 10.1016/j.exphem.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, Fassas A, Zangari M, Hollmig K, Pineda-Roman M, Lee C, Talamo G, Thertulien R, Kiwan E, Krishna S, Fox M, Crowley J. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. The New England Journal of Medicine. 2006;354:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- Berger Sl, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes & Development. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, Estrov Z, Talpaz M, Aggarwal BB. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- Boles KS, Mathew PA. Molecular cloning of CS1, a novel human natural killer cell receptor belonging to the CD2 subset of the immunoglobulin superfamily. Immunogenetics. 2001;52:302–307. doi: 10.1007/s002510000274. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. The Journal of Immunology. 2001;167:5517–5521. doi: 10.4049/jimmunol.167.10.5517. [DOI] [PubMed] [Google Scholar]
- Brett S, Baxter G, Cooper H, Johnston JM, Tite J, Rapson N. Repopulation of blood lymphocyte sub-populations in rheumatoid arthritis patients treated with the depleting humanized monoclonal antibody, CAMPATH-1H. Immunology. 1996;88:13–19. doi: 10.1046/j.1365-2567.1996.d01-650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek NL, Perlmutter P, Aguilar MI, Croft NP, Purcell AW. Epitope discovery and their use in peptide based vaccines. Currebt Pharmaceutical Design. 2010;16:3149–3157. doi: 10.2174/138161210793292447. [DOI] [PubMed] [Google Scholar]
- Hartmann U, Brummendorf TH, Balabanov S, Thiede C, Illme T, Schaich M. Telomere length and hTERT expression in patients with acute myeloid leukemia correlates with chromosomal abnormalities. Haematologica. 2005;90:307–316. [PubMed] [Google Scholar]
- Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, Nichol G Cancer Vaccine Clinical Trial Working Group. A clinical development paradigm for cancer vaccines and related biologics. Journal of Immunotherapy. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, Rice AG, van Abbema A, Wong M, Liu G, Zhan F, Dillon M, Chen S, Rhodes S, Fuh F, Tsurushita N, Kumar S, Vexler V, Shaughnessy JD, Jr, Barlogie B, van Rhee F, Hussein M, Afar DE, Williams MB. Expression of CS1 (SLAMF7) in benign and neoplastic plasma cells: a potential new therapeutic target for the treatment of multiple myeloma. Clinical Cancer Research. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M, Berenson JR, Niesvizky R, Munshi N, Matous J, Sobecks R, Harrop K, Drachman JG. Whiting N. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010;95:845–848. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpf AR. A potential role for epigenetic modulatory drugs in the enhancement of cancer/germ-line antigen vaccine efficacy. Epigenetics. 2006;1:116–120. doi: 10.4161/epi.1.3.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundegaard C, Lund O, Nielsen M. Accurate approximation method for prediction of class I MHC affinities for peptides of length 8, 10 and 11 using prediction tools trained on 9mers. Bioinformatics. 2008;24:1397–1398. doi: 10.1093/bioinformatics/btn128. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, Fonseca R, Stewart AK, Harousseau JL, Dimopoulos M, Jagannath S, Hajek R, Sezer O, Kyle R, Sonneveld P, Cavo M, Rajkumar SV, San Miguel J, Crowley J, Avet-Loiseau H. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podar K, Anderson KC. Inhibition of VEGF signaling pathways in multiple myeloma and other malignancies. Cell Cycle. 2007;6:538–542. doi: 10.4161/cc.6.5.3922. [DOI] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Experimental Biology and Medicine. 2008;233:522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, Kirschner KM. Oxygen-Dependent Gene Expression in Development and Cancer: Lessons Learned from the Wilms’ Tumor Gene, WT1. Frontiers in Molecular Neuroscience. 2011;4:4. doi: 10.3389/fnmol.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyed N, Zahedifard F, Safaiyan S, Gholami E, Doustdari F, Azadmanesh K, Mirzaei M, Saeedi EN, Khadem SA, Eslami FA, Sharifi I, Rafati S. In Silico Analysis of Six Known Leishmania major Antigens and In Vitro Evaluation of Specific Epitopes Eliciting HLA-A2 Restricted CD8 T Cell Response. PLoS Neglected Tropical Diseases. 2011;5:e1295. doi: 10.1371/journal.pntd.0001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, Stewart JP, Kordsmeier B, Randolph C, Williams DR, Xiao Y, Xu H, Epstein J, Anaissie E, Krishna SG, Cottler-Fox M, Hollmig K, Mohiuddin A, Pineda-Roman M, Tricot G, van Rhee F, Sawyer J, Alsayed Y, Walker R, Zangari M, Crowley J, Barlogie B. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- Silk AW, Finn OJ. Cancer vaccines: a promising cancer therapy against all odds. Future Oncology. 2007;3:299–306. doi: 10.2217/14796694.3.3.299. [DOI] [PubMed] [Google Scholar]
- Speiser DE, Romero P. Molecularly defined vaccines for cancer immunotherapy, and protective T cell immunity. Seminars in Immunology. 2010;22:144–154. doi: 10.1016/j.smim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Strothmeyer AM, Papaioannou D, Duhren-von MM, Navarrete M, Zirlik K, Heining-Mikesch K, Veelken H. Comparative analysis of predicted HLA binding of immunoglobulin idiotype sequences indicates T cell-mediated immunosurveillance in follicular lymphoma. Blood. 2010;116:1734–1736. doi: 10.1182/blood-2010-02-270199. [DOI] [PubMed] [Google Scholar]
- Svane IM, Nikolajsen K, Johnsen HE. Antigen-specific T-cell immunity in multiple myeloma patients is restored following high-dose therapy: implications for timing of vaccination. Scandinavian Journal of Immunology. 2007;66:465–475. doi: 10.1111/j.1365-3083.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weller E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DE, Anderson KC. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CK, Apostolopoulos V. Strategies used for MUC1 immunotherapy: preclinical studies. Expert Review of Vaccines. 2008;7:951–962. doi: 10.1586/14760584.7.7.951. [DOI] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. The New England Journal of Medicine. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Tong AW, Seamour B, Chen J, Su D, Ordonez G, Frase L, Netto G, Stone MJ. CD40 ligand-induced apoptosis is Fas-independent in human multiple myeloma cells. Leukemia and Lymphoma. 2000;36:543–558. doi: 10.3109/10428190009148403. [DOI] [PubMed] [Google Scholar]
- van Rhee F, Szmania SM, Dillon M, van Abbema AM, Li X, Stone MK, Garg TK, Shi J, Moreno-Bost AM, Yun R, Balasa B, Ganguly B, Chao D, Rice AG, Zhan F, Shaughnessy JD, Jr, Barlogie B, Yaccoby S, Afar DE. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Molecular Cancer Therapeutics. 2009;8:2616–2624. doi: 10.1158/1535-7163.MCT-09-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent S, Chen R, Sayles LC, Lin C, Walker RG, Gillespie AK, Subramanian A, Hinkle G, Yang X, Saif S, Root DE, Huff V, Hahn WC, Sweet-Cordero EA. Wilms tumor 1 (WT1) regulates KRAS-driven oncogenesis and senescence in mouse and human models. The Journal of Clinical Investigation. 2010;120:3940–3952. doi: 10.1172/JCI44165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugmeyster Y, Howell K, Bakshl A, Flores C, Canova-Davis E. Effect of anti-CD20 monoclonal antibody, Rituxan, on cynomolgus monkey and human B cells in a whole blood matrix. Cytometry A. 2003;52:101–109. doi: 10.1002/cyto.a.10030. [DOI] [PubMed] [Google Scholar]
- Warner JK, Wang JC, Takenaka K, Doulatov S, McKenzie JL, Harrington L, Dick JE. Direct evidence for cooperating genetic events in the leukemic transformation of normal human hematopoietic cells. Leukemia. 2005;19:1794–1805. doi: 10.1038/sj.leu.2403917. [DOI] [PubMed] [Google Scholar]
- Westers TM, van den Ancker W, Bontkes HJ, Janssen JJ, van de Loosdrecht AA, Ossenkoppele GJ. Chronic myeloid leukemia lysate-loaded dendritic cells induce T-cell responses towards leukemia progenitor cells. Immunotherapy. 2011;3:569–576. doi: 10.2217/imt.11.3. [DOI] [PubMed] [Google Scholar]
- Wolowiec D, Dybko J, Wrobel T, Urbaniak-Kujda D, Jazwiec B, Tomaszewska-Toporska B, Kapelko-Slowik K, Potoczek S, Kuliczkowski K. Circulating sCD138 and some angiogenesis-involved cytokines help to anticipate the disease progression of early-stage B-cell chronic lymphocytic leukemia. Mediators of Inflammation. 2006;2006:42394. doi: 10.1155/MI/2006/42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B, Anaissie E, Hollmig K, Pineda-Roman M, Tricot G, van Rhee F, Walker R, Zangari M, Crowley J, Barlogie B, Shaughnessy JD., Jr The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]