Abstract
BACKGROUND
Few data exist on the treatment of peritoneal surface dissemination (PSD) from ovarian cancer (OC) with hyperthermic intraperitoneal chemotherapy (HIPEC). This work represents a review of the authors’ institution’s experience with HIPEC for PSD from OC.
METHODS
Fifty-one patients with OC treated with HIPEC between 1996 and 2009 were identified in a prospectively managed database. All patients underwent maximal tumor debulking followed by HIPEC with mitomycin C, carboplatin, or paclitaxel.
RESULTS
The median survival in this cohort was 29 months. When stratified by resection status, patients undergoing R0 and R1 resections experienced longer median survival than those who underwent R2 resections (47 vs 12 months, P = .0002). Intraoperative blood loss ≤ 400 mL resulted in greater 5-year survival than blood loss > 400 mL (60% vs 15%, P = .025).
CONCLUSIONS
This experience demonstrates that long-term survival is anticipated in patients who undergo complete cytoreduction followed by HIPEC for PSD from OC. These findings not only highlight the potential utility of HIPEC in the treatment of OC but also underscore the importance of maximal cytoreduction followed by HIPEC in this cohort of patients.
Keywords: Ovarian cancer, Peritoneal surface malignancy, Hyperthermic intraperitoneal chemotherapy, Mitomycin C, Carboplatin, Paclitaxel
Ovarian neoplasms are the ninth most common cancer in women and the fifth leading cause of cancer death in women. It is estimated that >21,000 new cases of ovarian cancer (OC) are diagnosed annually. An additional 14,500 patients will die in the same year.1,2 A majority of patients will present with advanced disease, because of the vague symptoms of abdominal bloating, distention, weight loss, and fatigue.2,3 Despite advances in the treatment of OC, including surgery and systemic chemotherapy, the 5-year survival rate for stage III and IV OC with peritoneal surface dissemination (PSD) is <25%.2,4
The standard therapy of PSD from ovarian tumors is exploratory laparotomy and maximal cytoreductive surgery (CS), followed by platinum-based chemotherapy.3,5 Although combination platinum and taxane chemotherapy has a response rate of 70% to 80% in clinical trials, 75% of patients experience recurrent disease, with a median disease-free survival of only 18 to 28 months.4,6 These patients will ultimately suffer from malignant bowel obstruction and death, therefore highlighting the potential utility of additional therapies coupled with maximal cytoreduction for locoregional control of advanced OC.2,7,8 Randomized trials of intraperitoneal chemotherapy versus systemic chemotherapy after maximal CS have shown improved survival in patients receiving intraperitoneal chemotherapy. An additional approach to treatment to PSD from ovarian tumors has been maximum debulking followed by hyperthermic intraperitoneal chemotherapy (HIPEC).4,8–10 The cytoreductive procedure includes peritonectomy, complete omentectomy, and multivisceral organ resections aimed at complete removal of the tumor, followed by peritoneal perfusion with heated chemotherapy. To date, few data exist on the clinical outcomes of patients with PSD from ovarian tumors after CS and HIPEC. We therefore evaluated our institution’s prospective database of patients undergoing CS and HIPEC for PSD from ovarian primary tumors to determine factors that were independent predictors for overall survival (OS).
Methods
Patients
Patients who underwent HIPEC for PSD from ovarian primary tumors at Wake Forest University School of Medicine Baptist Hospital between 1996 and 2009 were identified from a prospective database. Clinical data on all patients were recorded in a database and maintained by a dedicated data management unit. All patients provided written informed consent for inclusion in a prospective database on the management of PSD with HIPEC.
Cytoreductive surgery
CS consisted of the removal of all gross tumor and involved organs, peritoneum, or tissue deemed technically feasible and safe for the patient. Any tumors adherent or invasive to vital structures that could not be removed were cytoreduced by using a cavitational ultrasonic surgical aspirator (Valleylab, Boulder, CO). Peritonectomy procedures were performed as indicated. The resection status of patients was judged after CS by using the following classification: R0, complete removal of all visible tumor and negative cytologic findings or microscopic margins; R1, complete removal of all visible tumor and positive postperfusion cytologic findings or microscopic margins; R2a, minimal residual tumor, nodule(s) measuring <.5 cm; R2b, gross residual tumor, nodule(s) measuring >.5 but <2 cm; and R2c, extensive disease remaining, nodule(s) measuring >2 cm.
HIPEC
Patients were cooled to a core temperature of approximately 34°C to 35°C by passive measure (ie, not warming airway gases or intravenous solutions and cooling the room). After CS was completed, peritoneal perfusion inflow and outflow catheters were placed percutaneously into the abdominal cavity. Temperature probes were placed on the inflow and outflow catheter tips. The abdominal skin incision was closed temporarily with a running cutaneous suture to prevent leakage of peritoneal perfusate. A perfusion circuit was established with approximately 3 L of Ringer’s lactate. Flow rates of approximately 600 to 800 mL/min were maintained using by a roller pump managed by the pump technician. The circuit continued through a single roller pump and through a heat exchanger and then to the patient. Constant temperature monitoring was performed at all temperature probes. Once inflow temperature exceeded 38.5°C, the chemotherapeutic agent of choice was added to the perfusate. A maximum inflow temperature of 42.0°C was realized during perfusion, with a target outflow temperature at the pelvis of 40°C. The abdomen was gently massaged throughout perfusion to improve drug distribution to all peritoneal surfaces. The total planned perfusion time after the initial addition of chemotherapy was 120 minutes. In certain patients (elderly individuals, those with extensive previous chemotherapy, those with inanition or poor performance status, and those with extensive peritoneal stripping during surgery), reductions in the dose of chemotherapy or perfusion time (to 60–90 minutes) were made because of concerns about potential toxic effects.
Clinical follow-up
Clinical follow-up occurred at 1 and 3 months and then every 3 to 6 months thereafter for up to 1 year. After 1 year, follow-up was at 6-month intervals or less frequently if the patient continued to remain without evidence of disease. Abdominal and pelvic computed tomographic scans were obtained at 3, 6, and 12 months after surgery or when clinically indicated. Some patients received systemic chemotherapy at the discretion of their medical oncologists.
Statistical analysis
All data were collected prospectively. Descriptive statistics were generated for all measures, including means, ranges, and standard deviations for continuous measures and frequencies and proportions for categorical data. OS was calculated from the date of CS and HIPEC to the last known date of follow-up or the date of death. Estimates of survival were calculated by using the Kaplan-Meier (product-limit) method; analysis with Cox proportional hazards was performed on all pertinent clinicopathologic variables to determine each variable’s association with survival. Group comparisons of OS were performed by using the approximate v2 statistic for the log-rank test. Additionally, the Cox proportional-hazards regression model was used in a stepwise fashion to perform a multivariate analysis of clinicopathologic factors to determine an overall model of independent predictors of OS. Statistical significance was defined as a P value <.05.
Results
Patient characteristics
A total of 59 HIPEC procedures were performed in 51 patients with PSD from ovarian primary tumors. There were no deaths within 30 days of CS and HIPEC in this cohort of patients. Most of the patients were Caucasian. The median age of the participants was 57 years (range, 31–74 years). The vast majority of the patients had Eastern Cooperative Oncology Group statuses of 0 or 1.
Operative and perfusion data
The operative and perfusion data for patients in this cohort are summarized in Table 1. The median surgery time was approximately 480 minutes. This was dependent on the extent and ocation of disease at exploration. The goal of the laparotomy for CS was to render the patient free of disease. This required lysis of all adhesions, omentectomy, and multivisceral resections. This surgical time was consistent with our evaluations in other histologies. The median does of carboplatin was 1,000 mg, and the median dose of mitomycin C was 30 mg. The patients typically had a perfusion times ranging between 60 and 120 minutes. The inflow and outflow temperatures were fairly standard at 41°C and 42°C. Estimated blood loss ranged from 50 mL to 2 L. Patients who had R0 or R1 resections had no grossly visible tumor at the conclusion of the procedure. Approximately 40% of patients underwent R0 or R1 resections. Individuals with R2a resections at the conclusion of the cytoreductive procedure constituted 35% of this study cohort. Approximately 20% of patients in this cohort underwent R2b or R2c resections.
Table 1.
Operative and perfusion data for patients undergoing CS and HIPEC for PSD of ovarian malignancies
| Variable | n | Median | Range |
|---|---|---|---|
| Surgery time (min) | 44 | 472.5 | 270.0–1,034.0 |
| Carboplatin dose (mg) | 19 | 1,000.0 | 500.0–2,136.0 |
| Mitomycin C dose (mg) | 32 | 30.0 | 30.0–40.0 |
| Perfusion time (min) | 51 | 98.0 | 60.0–135.0 |
| Inflow temperature (°C) | 40 | 40.8 | 38.5–42.0 |
| Outflow temperature (°C) | 46 | 39.6 | 36.9–42.0 |
| Estimated blood loss (mL) | 41 | 450.0 | 50.0–2,000.0 |
CS = cytoreductive surgery; HIPEC = hyperthermic intraperitoneal chemotherapy; PSD = peritoneal surface dissemination.
Survival and follow-up
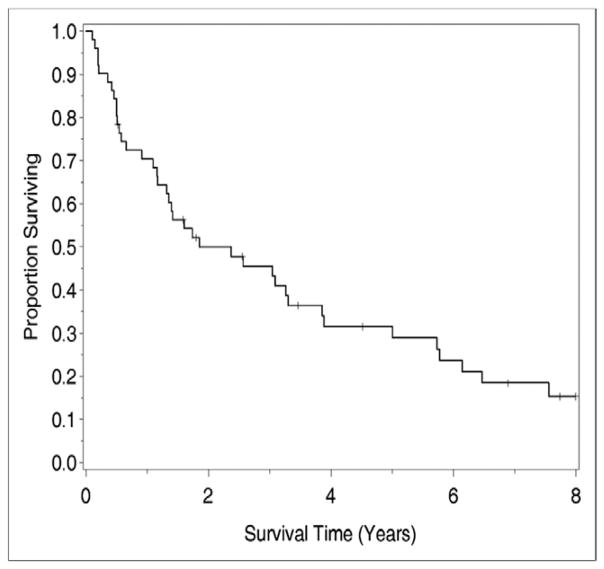
The median survival for this cohort of 51 patients was 28.5 months, with a median follow-up period of 8.2 years. The 1-year, 3-year, and 5-year survival rates for all cases were 73 ± 6%, 48 ± 7%, and 28 ± 7%, respectively (Fig. 1).
Figure 1.
OS after CS and HIPEC for ovarian cancer.
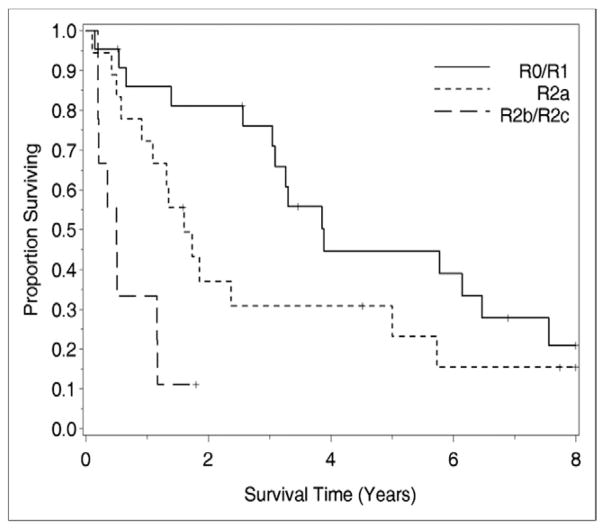
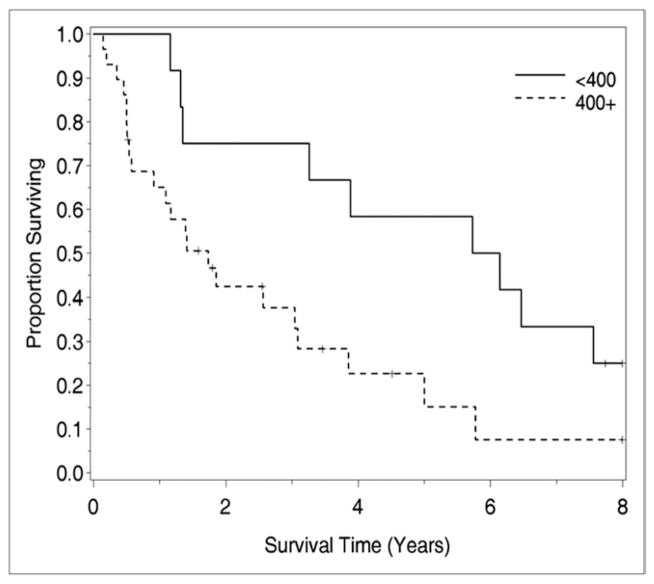
Univariate analysis of clinicopathologic factors was performed to identify singularly significant prognostic factors associated with OS after CS and HIPEC for peritoneal surface malignancy from ovarian primary tumors. As demonstrated in Table 2, these prognostic factors included performance status, resections status, and estimated blood loss. Multivariate analysis of factors affecting survival was performed via a stepwise regression technique. This analysis, which allowed for all variables regardless of their level of significance in the univariate analysis, revealed that resection status was the primary determinant of survival in this group of patients. When stratified by resection status, patients undergoing R0 and R1 resections experienced a median survival of 47 months, while those undergoing R2a, R2b, and R2c resections experienced median survival of 9, 6, and 11 months, respectively (P = .0002; Fig. 2). Estimated blood loss also correlated with survival in the multivariate analysis. As demonstrated in Figure 3, patients with estimated blood loss <400 mL had 5-year survival of 60%, while those with blood loss > 400 mL had 5-year survival of 15% (P = .025).
Table 2.
Univariate analysis of patients undergoing CS and HIPEC for PSD of ovarian malignancies
| Clinicopathologic variable | P |
|---|---|
| Eastern Cooperative Oncology Group status | .043 |
| Resection status | .0001 |
| Estimated blood loss | .03 |
| Surgery time | .87 |
| Age | .90 |
| Inflow temperature | .99 |
| Outflow temperature | .59 |
CS = cytoreductive surgery; HIPEC = hyperthermic intraperitoneal chemotherapy; PSD = peritoneal surface dissemination.
Figure 2.
The impact of resection status on survival in patients treated with CS and HIPEC. Patients undergoing R0 and R1 resections experienced longer median survival than those who underwent R2 resections (47 vs 12 months, P = .0002).
Figure 3.
The impact of intraoperative blood loss on survival in patients undergoing CS and HIPEC. Patients experiencing intra-operative blood loss <400 mL had significantly improved OS than those with blood loss >400 mL at 5 years (60% vs 15%, P = .025).
Comments
The standard care for women with PSD of OC is initial CS followed by platinum-based chemotherapy. Although OC is initially very sensitive to chemotherapy compared with many other types of cancer, it has a 5-year survival rate of just over 50%. Thus, novel approaches to the treatment of this disease are warranted. The work presented herein represents one of the largest cohorts of patients undergoing HIPEC for PSD of epithelial OC. Our data suggest that CS followed by HIPEC can provide a foundation for the treatment of peritoneal dissemination of OC.
Aggressive cytoreduction allows the chemotherapy to treat microscopic, small-volume residual disease rather than large-volume disease that is known to be relatively resistant to chemotherapy because of hypoxic environment. A recent meta-analysis by Bristow et al11 demonstrated that for every 10% increase in the percentage of patients undergoing maximal cytoreduction surgery, there is a 5.5% increase in the median duration of survival. The role of maximal cytoreduction in our cohort of patients is readily apparent when one considers that those who underwent complete cytoreduction experienced significantly improved survival relative to those who had residual disease at the conclusion of their cytoreductive procedures. Interestingly, not all patients who underwent complete cytoreduction plus HIPEC experienced long-term survival. This finding reflects the fact that adjuvants to cytoreduction are needed in the treatment in this group of patients. Further studies are needed to evaluate the role of chemoresistance in tumors that recur after maximal cytoreduction and HIPEC.
Intraperitoneal delivery of chemotherapy is largely effective because it can be used as a tool to overcome the drug resistance associated with systemic chemotherapy.12 Systemic chemotherapy for intraperitoneal disease is largely ineffective for peritoneal surface malignancy because of, at least in part, the presence of the plasma-peritoneal partition. Early studies confirm the presence of this plasma-peritoneal partition by demonstrating that drugs delivered into the peritoneal cavity have a clearance that is inversely proportional to the square root of its molecular weight.13–15 Because of this partition, drugs without lipophilic properties in high molecular weights have optimal characteristics for intraperitoneal application. The pharmacokinetic advantage of intraperitoneal perfusion can be seen by the areas under the curve of the peritoneal fluid, the plasma that favors retention of drug in the peritoneum.16–22 In addition to the pharmacokinetic advantage of intraperitoneal chemotherapy infusion after maximal tumor debulking, the addition of hyperthermia affects cell membranes, cytoskeletons, synthesis of macromolecules, and deoxyribonucleic acid repair mechanisms.23,24 The synergism between a variety of chemotherapeutic agents and hyperthermia occurs independently of the cell cycle, thus allowing for significant tumoricidal activity with brief exposures.
The Gynecologic Oncology Group (GOG) recently reported the results of a randomized study of frontline surgery plus combination intravenous cisplatin and paclitaxel versus combined intravenous and intraperitoneal chemotherapy. This study found that patients who received intraperitoneal chemotherapy had median survival of 65.6 months, while those treated with intravenous chemotherapy experienced median survival of 49.7 months.25 A subsequent meta-analysis of all randomized studies demonstrated an OS advantage for patients treated with intraperitoneal chemotherapy for PSD of OC.26 These findings prompted the National Cancer Institute to issue a clinical announcement recommending that all women with epithelial OC be offered intraperitoneal chemotherapy after frontline CS. However, these data taken in context do not suggest that normothermic intraperitoneal chemotherapy is a panacea for women with OC, as women treated with intraperitoneal therapy in the GOG-172 trial experienced a recurrence rate of 65%.25 These findings suggest that additional approaches to the treatment of PSD of OC, such as HIPEC, are warranted. Our series, which is one of the largest to date, demonstrates several important facts. First, it demonstrates that it is safe to deliver HIPEC at the time of maximal tumor debulking in patients with OC. In addition, it demonstrates that long-term survival in this group of patients is possible, as the longest survivor in this series is 9.3 years after intraperitoneal perfusion.
Our evaluation of clinical variables that affect survival confirm data from our institution and others that demonstrate a significant survival advantage for patients undergoing complete cytoreduction. We previously reported in series of patients with PSD from gastrointestinal malignancies that complete cytoreduction before HIPEC provided superior outcomes relative to incomplete cytoreduction. Individuals who underwent R0 and R1 resections experienced a median survival of 47 months, while those who received incomplete cytoreduction had median survival ranging between 6 and 11 months. Interestingly, we found that lower estimated blood loss provided some survival advantage in this population of patients. Every 100-mL increase in blood loss resulted in a 2.5 fold increased risk for death in this population of patients. These findings are not surprising given the fact that massive blood loss result in immunosuppression in these groups of patients and subsequently poor outcomes.
Several issues surround the use of HIPEC for the treatment of PSD from OC. Chief among them is establishing a collaborative group to perform a randomized phase III trial for the treatment of patients with HIPEC versus normothermic intraperitoneal chemotherapy at the time of initial operation. In addition, it is clear that we must evaluate how to standardize HIPEC in this population of patients and make it available to large numbers of patients. At present, there are approximately 45 active centers in the United States, and only half have experience of >100 cases. The operative procedures required for aggressive CS are lengthy, challenging, and morbid and use a great deal of hospital, blood bank, and house officer resources. Furthermore, the use of chemotherapy in the operating room is daunting for many centers. Additionally, great care needs to be taken in selecting patients to undergo this procedure. It is estimated that only a handful of potential candidates for this therapy actually receive it; this is underscored by the relatively small number of patients accrued in phase II studies for peritoneal carcinomatosis for gastrointestinal malignancies at large perfusion centers. It is clear that expanding the number of centers should be performed by surgical oncologists who have more than a passing knowledge of systemic chemotherapy and are comfortable with the rigors of aggressive operative procedures of the abdomen.
Footnotes
This report was presented at the 20th annual meeting of the Society of Black Academic Surgeons.
References
- 1.Chua TC, Robertson G, Liauw W, et al. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol. 2009;135:1637–45. doi: 10.1007/s00432-009-0667-4. [DOI] [PubMed] [Google Scholar]
- 2.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bookman MA, McGuire WP, III, Kilpatrick D, et al. Carboplatin and paclitaxel in ovarian carcinoma: a phase I study of the Gynecologic Oncology Group. J Clin Oncol. 1996;14:1895–902. doi: 10.1200/JCO.1996.14.6.1895. [DOI] [PubMed] [Google Scholar]
- 4.Di GA, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer. 2008;113:315–25. doi: 10.1002/cncr.23553. [DOI] [PubMed] [Google Scholar]
- 5.Deraco M, Rossi CR, Pennacchioli E, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion in the treatment of recurrent epithelial ovarian cancer: a phase II clinical study. Tumori. 2001;87:120–6. doi: 10.1177/030089160108700302. [DOI] [PubMed] [Google Scholar]
- 6.Bae JH, Lee JM, Ryu KS, et al. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol. 2007;106:193–200. doi: 10.1016/j.ygyno.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002;7(suppl):20–8. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 8.Gori J, Castano R, Toziano M, et al. Intraperitoneal hyperthermic chemotherapy in ovarian cancer. Int J Gynecol Cancer. 2005;15:233–9. doi: 10.1111/j.1525-1438.2005.15209.x. [DOI] [PubMed] [Google Scholar]
- 9.Cotte E, Glehen O, Mohamed F, et al. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg. 2007;31:1813–20. doi: 10.1007/s00268-007-9146-8. [DOI] [PubMed] [Google Scholar]
- 10.Pomel C, Ferron G, Lorimier G, et al. Hyperthermic intra-peritoneal chemotherapy using oxaliplatin as consolidation therapy for advanced epithelial ovarian carcinoma. Results of a phase II prospective multi-centre trial. CHIPOVAC study. Eur J Surg Oncol. 2010;36:589–93. doi: 10.1016/j.ejso.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Bristow RE, Gossett DR, Shook DR, et al. Recurrent micropapillary serous ovarian carcinoma. Cancer. 2002;95:791–800. doi: 10.1002/cncr.10789. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JH, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy: an evolving paradigm for the treatment of peritoneal surface malignancies. Exp Rev Anticancer Ther. 2008;8:1809–18. doi: 10.1586/14737140.8.11.1809. [DOI] [PubMed] [Google Scholar]
- 13.Dedrick RL, Flessner MF. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst. 1997;89:480–7. doi: 10.1093/jnci/89.7.480. [DOI] [PubMed] [Google Scholar]
- 14.Dedrick RL. Interspecies scaling of regional drug delivery. J Pharm Sci. 1986;75:1047–52. doi: 10.1002/jps.2600751106. [DOI] [PubMed] [Google Scholar]
- 15.Flessner MF, Dedrick RL, Schultz JS. A distributed model of peritoneal-plasma transport: theoretical considerations. Am J Physiol. 1984;246:R597–607. doi: 10.1152/ajpregu.1984.246.4.R597. [DOI] [PubMed] [Google Scholar]
- 16.Kuzuya T, Yamauchi M, Ito A, et al. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J Pharm Pharmacol. 1994;46:685–9. doi: 10.1111/j.2042-7158.1994.tb03883.x. [DOI] [PubMed] [Google Scholar]
- 17.Speyer JL, Collins JM, Dedrick RL, et al. Phase I and pharmacological studies of 5-fluorouracil administered intraperitoneally. Cancer Res. 1980;40:567–72. [PubMed] [Google Scholar]
- 18.Israel VK, Jiang C, Muggia FM, et al. Intraperitoneal 5-fluoro-2′-deoxyuridine (FUDR) and (S)-leucovorin for disease predominantly confined to the peritoneal cavity: a pharmacokinetic and toxicity study. Cancer Chemother Pharmacol. 1995;37:32–8. doi: 10.1007/BF00685626. [DOI] [PubMed] [Google Scholar]
- 19.Ozols RF, Young RC, Speyer JL, et al. Phase I and pharmacological studies of adriamycin administered intraperitoneally to patients with ovarian cancer. Cancer Res. 1982;42:4265–9. [PubMed] [Google Scholar]
- 20.Bartlett DL, Buell JF, Libutti SK, et al. A phase I trial of continuous hyperthermic peritoneal perfusion with tumor necrosis factor and cis-platin in the treatment of peritoneal carcinomatosis. Cancer. 1998;83:1251–61. [PubMed] [Google Scholar]
- 21.Markman M, Kelsen D. Efficacy of cisplatin-based intraperitoneal chemotherapy as treatment of malignant peritoneal mesothelioma. J Cancer Res Clin Oncol. 1992;118:547–50. doi: 10.1007/BF01225271. [DOI] [PubMed] [Google Scholar]
- 22.Markman M, Brady MF, Spirtos NM, et al. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group Study. J Clin Oncol. 1998;16:2620–4. doi: 10.1200/JCO.1998.16.8.2620. [DOI] [PubMed] [Google Scholar]
- 23.Dikomey E, Franzke J. Effect of heat on induction and repair of DNA strand breaks in X-irradiated CHO cells. Int J Radiat Biol. 1992;61:221–33. doi: 10.1080/09553009214550851. [DOI] [PubMed] [Google Scholar]
- 24.Roti Roti JL, Kampinga HH, Malyapa RS, et al. Nuclear matrix as a target for hyperthermic killing of cancer cells. Cell Stress Chaperones. 1998;3:245–55. doi: 10.1379/1466-1268(1998)003<0245:nmaatf>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 26.Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2006:CD005340. doi: 10.1002/14651858.CD005340.pub2. [DOI] [PubMed] [Google Scholar]