Abstract
Objective
To perform a systematic review and meta-analysis of the efficacy of risk reduction interventions on HIV-related risk behaviors among people living with HIV/AIDS (PLWHA)
Methods
Studies included in the meta-analysis were randomized clinical trials (RCTs) of risk reduction interventions, which targeted PLWHA aged 18 year or older and assessed the changes of number of sexual partners, drug use, needle sharing, and/or alcohol abuse between pre- and post-intervention. The standardized mean differences (SMD) between study arms as well as between baseline and post-intervention, defined as the effect sizes (ES), were calculated in random effects models. Heterogeneity of studies was estimated by the I2 statistic.
Results
Twelve RCTs involving 3993 PLWHA were included in our analysis: seven reported impacts on the number of sexual partners, and three reported impacts on drug use, needle sharing, and alcohol abuse, respectively. There were no statistically significant impacts of risk reduction interventions on the number of total sexual partners (mean ES, -0.10; 95% confidence interval [CI], -0.26, 0.06; P=0.22) or on the subset of HIV-negative or unknown-status sexual partners (mean ES, 0.003; 95% CI, -0.54, 0.54; P=0.99). Overall, risk reduction intervention studies documented a reduction of drug abuse (mean ES: -0.26; 95% CI: -0.51, -0.01; P=0.04) among HIV-infected drug users, but this impact was mainly attributable to one study. Risk reduction interventions did not show a reduction of needle sharing (mean ES, -0.15; 95% CI, -0.43, 0.13; P=0.29) or of alcohol abuse (mean ES, -0.10; 95% CI, -0.36, 0.17; P=0.47). No heterogeneity or publication bias was found across individual studies.
Conclusions
Our meta-analysis did not find a positive impacts of risk reduction interventions on number of sexual partners, drug use, needle sharing, or alcohol abuse among PLWHA, but the small number of studies meeting our review criteria limits these findings.
Keywords: People living with HIV/AIDS (PLWHA), Randomized clinical trial (RCT), Sexual partners, Positive prevention, Drug use, Alcohol abuse, Meta-analysis
Introduction
Over 33 million people are living with HIV/AIDS (PLWHA) around the world [1]. As HIV-infected individuals live longer on average, due to the use of combination antiretroviral therapy (cART) [2,3], the global number of PLWLA is unlikely to decline dramatically in the near future [1]. The large number of prevalent cases poses a major public health challenge: PLWHA may continue to transmit HIV through unprotected sex or sharing of contaminated needles. Even after knowing their HIV-positive serostatus, PLWHA may practice unprotected sex [4-6], have multiple sexual partners [7-9], use illicit drugs, share needles, and abuse alcohol [7,10-12].
“Positive prevention”, which targets HIV-infected individuals, is considered a key strategy for preventing new infections. An emerging biomedical approach is HIV treatment as prevention: both observational studies and a definitive randomized controlled trial (HIV Prevention Trials Network [HPTN] 052 study) have shown that antiretroviral therapy (ART) can reduce heterosexual HIV transmission in HIV-discordant couples [13-17]. There is no direct evidence that risk reduction interventions alone reduce HIV transmission among PLWHA; however, risk reduction intervention studies have shown efficacy in reducing risky behaviors [9,18-20]. These studies commonly assessed the impact on unprotected intercourse [8,9,20,21]; some evaluated the impact on actions other than unprotected sex that could lead to an increased risk of transmitting HIV, including multiple sexual partners and substance and alcohol abuse [9,20,22,23]. Multiple meta-analytic reviews have evaluated the efficacy on unprotected intercourse or condom use among PLWHA [24,25]; but few on number of sexual partners [25] and drug or alcohol use [24]. PLWHA with multiple sex partners may be less likely to disclose their HIV status to their sexual partners [26]. Substance abuse and needle sharing among PLWHA could facilitate HIV transmission [27]; Alcohol use is also associated with unprotected sex among PLWHA [12]. Therefore, it is interesting to know the efficacy of risk reduction interventions on these outcomes. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs), evaluating the efficacy of risk reduction interventions on number of sexual behaviors and drug and alcohol use among PLWHA.
Methods
Search strategy and study selection
A systematic literature search was conducted to identify RCTs that studied risk reduction intervention impacts on various outcomes among PLWHA. Because of the limitation of manuscript length, unprotected sex/condom use will be presented elsewhere (unpublished). In this manuscript, the interest outcomes for analysis included number of sexual partners, drug use, needle sharing, and alcohol abuse. Twelve electronic databases were searched for studies published as of February 2012, including AMED, British Library Direct, British Nursing Index, Centre for Reviews and Dissemination databases, Cochrane Library, EMBASE, EconLit, ERIC, Ovid Medline, PsycINFO, Scopus, and Web of Science. Keywords used in the database search included: (HIV-infected or HIV infections, HIV-positive, HIV seropositive, or people living with HIV or AIDS or acquired immunodeficiency syndrome) AND (behavior therapy or behavioral intervention or risk reduction intervention or clinical trial or intervention study) AND (sexual partners or drug use or needle sharing or alcohol abuse). Each title and abstract was reviewed to determine whether the paper was potentially relevant to the topic.
Study criteria and selection
Studies were selected if they met the following criteria: (1) original randomized clinical trials among PLWHA; (2) using risk reduction intervention; (3) targeting PLWHA aged 18 or older; (4) reporting outcomes of number of sexual partners, drug use, needle sharing, and/or alcohol abuse at baseline and at follow-up.
All abstracts were independently reviewed by two authors, and full-text papers were reviewed for determining the eligibility if abstracts missed key information. Papers that did not meet the above-mentioned criteria were excluded. The disagreements between the two reviewers were less than 10%, and were resolved by further discussion involving two other authors. The references from each eligible paper were also examined to supplement the literature search described above, termed cross-referencing.
Data extraction
Two authors independently extracted the following data from eligible studies in the same standardized manner: authors, publication year, study country, description of interventions in study arms, participant recruitment, population characteristics and sample sizes at baseline and follow-up assessments, duration of follow-ups, retention at the last follow-up, as well as the proportions and mean frequencies of number of sexual partners (any sexual partners and HIV-negative or unknown-status sexual partners), drug use, needle sharing and alcohol abuse in each study arm at the baseline and follow-ups [28]. Any disagreements were reviewed and discussed between two data extractors and/or two quality controllers until a consensus was reached.
Rigor scores
The quality of study design of the included studies was assessed using rigor scores, which included an 8-point scale adopted by other systematic review [29] plus an additional item of sample size >100 (as an indicator for good statistical power). The scale is additive, with 1 point awarded for each of 9 items. Therefore, the rigor score for an article may range from 0 to 9, with a higher value representing a higher rigorousness of study design.
Statistical methods
The primary outcomes of interest in this meta-analysis were number of sexual partners, drug use, needle sharing, and alcohol abuse. These outcome variables were typically measured at baseline and follow-up in each study arm (e.g., intervention and comparison arm), and some studies might have multiple measurements at different follow-up time points. In the latter case, the last follow-up measurement was used for estimating the overall effect size of intervention, while each follow-up measurement was compared with baseline measurement in subgroup analyses. As the measurements were either expressed as proportion differences or as mean differences we converted estimates to a common metric of standard mean differences (SMD) using a Cox transformation [30,31]. SMD in each study arm was calculated as a fraction of difference of means between follow-up and baseline in each study arm divided by pooled standard deviation (SD) of these two means. We attempted to contact authors when published articles did not provide sufficient information to make the calculations. As the study arms might not be comparable at baseline, even in RCT, Becker’s strategy was used to adjust for any differences between arms at baseline [32]. The difference of SMDs between study arms, defined as effect sizes (ES), were calculated for each study and then pooled across studies using meta-analysis with a random effects model [33,34]. A negative value of SMD difference indicates reduction of outcomes in the intervention arm compared to the comparison arm. When multiple intervention arms in the same study were available [35], we calculated individual effect sizes in each of the separate intervention arms with the same comparison group. Random effect estimates allows for variation of true effects across studies [36], and random effect estimates in our analyses were derived using the DerSimonian-Laird method [33, 37]. The meta-analysis results were displayed with forest plots.
Heterogeneities were assessed by I2 statistics [38], and standardized deleted residual analyses were performed to identify outliers. The funnel plot, Begg and Mazumdar rank correlation test, and Egger’s test of the intercept were employed to assess indications of publication bias [39].
The subgroup analyses were performed to examine change of durations of follow-ups (immediately after intervention, 3, 6, 9, 12, or 18 months). Meta-regression was also used to examine the relationship of between-group effects, except for duration of follow-ups (because outcomes at multiple follow-ups were often reported in individual studies). No subgroup analyses and meta-regressions were performed for drug use, needle sharing, and alcohol abuse due to the small number of studies. Sensitivity analyses were conducted to determine the stability of intervention effects by evaluating whether the overall effect size was sensitive to inclusion of any individual study [34]. All meta-analyses were performed in the R/S plus Software version 2.15.1.
Results
Results from literature searches
The initial searches in twelve individual electronic databases yielded 7181 entries. After excluding 2597 duplicates and 4492 irrelevant ones (not meeting above-mentioned inclusion criteria), 92 full-text papers were further reviewed, and 80 were excluded for the following reasons; not an original article but rather an editorial, comment, or review (k=6), lack of information on outcomes of interests (k=41), not a randomized clinical trial (k=23), including HIV-negative participants (k=8), and repeated publishing (k=2) (Figure 1). These 80 studies are listed in the Appendix. Finally, 12 studies were included in our review [8,9,20-23,35,40-44].
Figure 1.
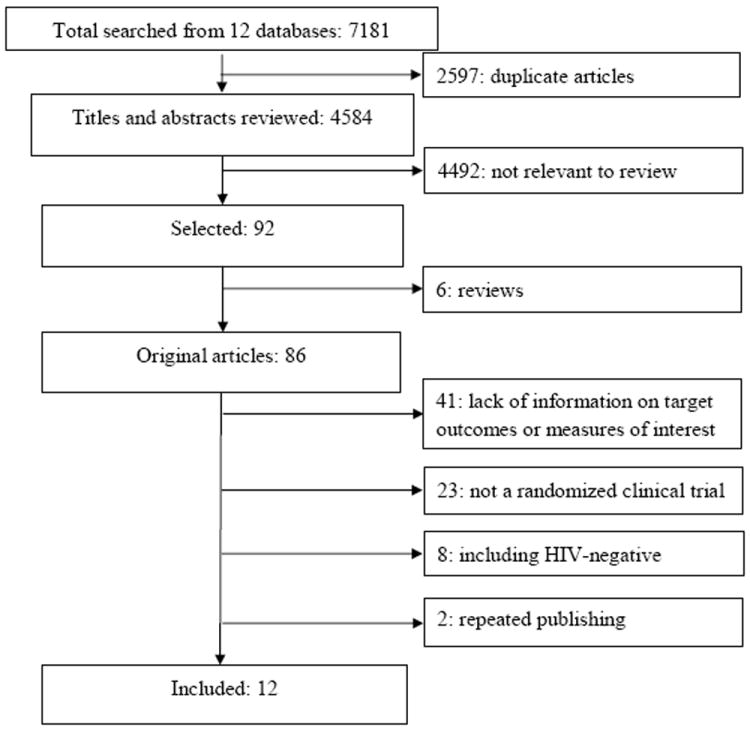
Flow diagram of the literature search process.
Description of studies
All included randomized clinical trials were conducted in the United States (Table 1). Study rigor scores ranged from 7 to 9 (mean 8.4), and six studies had a full score of 9 [8,9,20,23,42,43] (Table 2). The sample sizes at baseline ranged from 60 to 966. Ten studies recruited participants by AIDS-service-organization-based sampling (ASOB), such as hospitals, clinics, or detoxification centers [8,9,20-23,40,41,43-46], and less frequently used approaches, either combining with ASOB or not, included community-based sampling [35,42,44], paper-advertisement-based sampling [40,43], and peer-driven referrals [21]. The follow-up period of intervention ranged from 3 to 18 months, and retention rates varied from 30% to 100%.
Table 1.
Randomized clinical trials of HIV risk reduction intervention among people living with HIV/AIDS.
| Publication | Country (trial period) | Study participants | Study participants | Follow-up (months) | Retention rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Recruitment methods | Population | Sex (%) | No. of participants (age at baseline: mean and range)
|
IG | CG | |||||
| IG | CG | |||||||||
|
| ||||||||||
| Kelly et al. [32] | USA (1991-1992) | CBS | 94% MSM | 100% Male | Pre-intervention 51A or 26B | Pre-intervention 38 | A: 8-week cognitive-behavioral group intervention | No | 3 | 59 |
| Post-intervention 27 A or 14B (34, N/A) | Post-intervention 27 (34, N/A) | B: 8-week social support group intervention | ||||||||
|
| ||||||||||
| Kalichman et al. [37] | USA (N/A) | ABS, MOBS | 61% MSM | 70% Male | Pre-intervention 185 | Pre-intervention 143 | 5-session group intervention focused on strategies for practicing safer sexual behavior | 5-session contact-matched, health-maintenance support group | 6 | 78 |
| Post-intervention 150F3 or 146F6 (40, N/A) | Post-intervention 121F3 or 110F6 (40, N/A) | |||||||||
|
| ||||||||||
| Margolin et al. [23] | USA (1997-2001) | MOBS | IDU | 70% Male | Pre-intervention 45 | Pre-intervention 45 | Manual-Guided HIV+ harm reduction program | Enhanced methadone maintenance program | 3 | 70 |
| Post-intervention 32F0 or 34F3 (41, N/A) | Post-intervention 32F0 or 29F3 (41, N/A) | |||||||||
|
| ||||||||||
| Sorensen et al. [38] | USA (1994-1998) | MOBS | DU | 73% Male | Pre-intervention 92 | Pre-intervention 98 | Case management delivered by paraprofessionals | A brief contact condition | 18 | 79 |
| Post-intervention 77F6, 76F12, 71F18 (39, N/A) | Post-intervention 83F6, 74F12, 80F18 (38, N/A) | |||||||||
|
| ||||||||||
| Purcell et al.[39] | USA (2001-2005) | CBS | IDU | 61% Male | Pre-intervention 486 | Pre-intervention 480 | 10-session peer mentoring intervention | 8-session video discussion intervention | 12 | 85 |
| Post-intervention 419F3, 402F6, 417F12 (42, 22-60) | Post-intervention 421F3, 404F6, 404F12 (42, 22-60) | |||||||||
|
| ||||||||||
| Gilbert et al. [24] | USA (2003-2006) | MOBS | 51% MSM | 79% Male | Pre-intervention 240 | intervention 231 | Tailored risk-Reduction counseling via “Video Doctor” on laptop computer &printed Educational Worksheet | Usual care | 6 | 83 |
| Post-intervention 182F3, 200F6 (43, ≥18) | Post-intervention 188F3, 193F6 (44, ≥18) | |||||||||
|
| ||||||||||
| Williams et al. [40] | USA (2003-2006) | ABS, MOBS | Male | MSM | Pre-intervention 75 | Pre-intervention 62 | Sexual health intervention for men guided by cognitive-behavioral approaches | Attention- control standard health promotion comparison | 6 | 100 |
| Post-intervention 75 (43, ≥18) | Post-intervention 62 (43, ≥18) | |||||||||
|
| ||||||||||
| Coleman et al. [41] | USA (2006-2007) | MOBS, CBS | Male | MSM | Pre-intervention 30 | Pre-intervention 30 | Four 120-min sessions HIV risk reduction intervention | Four 120-min sessions (health condition) | 3 | 100 |
| Post-intervention 30 (51, 50-59) | Post-intervention 30 (51, 50-72) | |||||||||
|
| ||||||||||
| Rose et al. [8] | USA (2004-2006) | MOBS | 69% Male | SAA | Pre-intervention 181 | Pre-intervention 205 | Clinician-delivered HIV risk-reduction intervention | Standard care | 6 | 85 |
| Post-intervention 161 (43, N/A) | Post-intervention 167 (43, N/A) | |||||||||
|
| ||||||||||
| Teti et al. [22] | USA (2004-2007) | MOBS, PRS, ABS | Female | 86% Black | Pre-intervention 92 | Pre-intervention 92 | Received messages, group-level, peer-led support intervention; | Received brief messages | 18 | 30 |
| Post-intervention 61F6, 46F12, 28F18 (40, 20-70) | Post-intervention 70F6, 52F12, 27F18 (38, 20-70) | |||||||||
|
| ||||||||||
| Wolitski et al. [9] | USA (2004-2007) | MOBS | 70% Male | Homeless and unstably housed adults | Pre-intervention 315 | Pre-intervention 315 | Immediate housing opportunities for people with AIDS rental assistance | Customary housing service | 18 | 85 |
| Post-intervention 301F6, 284F12, 274F18 (N/A, 18-50+) | Post-intervention 275F6,266F12,259F18 (N/A, 18-50+) | |||||||||
|
| ||||||||||
| Kalichman et al. [20] | USA (2005-2009) | MOBS | 78% Male | 91% African American | Pre-intervention 217 | Pre-intervention 219 | Theory-based integrated behavioral intervention | Attention control | 9 | 92 |
| Post-intervention 201F3,193F6,192F9 (44, ≥18) | Post-intervention 201F3,202F6,210F9 (44, ≥18) | |||||||||
Note: IG: intervention group; CG: control group; ABS: advertisement-based sampling; CBS: community-based sampling; MOBS: medical-organization-based sampling; PRS: peer-referral sampling; MSM: men who have sex with men; DU: drug users; IDU: injection drug users; SAA: sexually active adults; N/A: not available; F0Immediately after intervention; F3At 3-month follow-up; F6At 6-month follow-up; F9At 9-month follow-up; F12At 12-month follow-up; F18At 18-month follow-up.
Table 2.
Quality assessment of study design for 12 randomized clinical trials (rigor scores*).
| Publication | Cohort (a) | With control group (b) | Pre/post intervention (c) | Random assignment (d) | Random selection for assessment (e) | Sample size >100 (f) | Follow-up ≥80% (g) | Comparable socio-demographics between study groups (h) | Comparable outcome measures at baseline (i) | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Kelly et al. [32] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Kalichman et al.[37] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Margolin et al. [23] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 7 |
| Sorensen et al. [38] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Purcell et al. [39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Gilbert et al. [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Williams et al. [40] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Coleman et al. [41] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Rose et al. [8] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Teti et al. [22] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Wolitski et al. [9] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kalichman et al. [20] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
One point score for meeting each of the following items: (a) was a prospective cohort, (b) used a comparison arm, (c) collected pre and post intervention data, (d) used random assignment of participants to study arms, (e) did random sampling for assessments, (f) sample size > 100, (g) follow-up rate ≥ 80%, (h) had a comparison group with comparable socio-demographics such as age, education, race, employment, income, marital status and others [score “1” if > 50% variables were comparable between study arms, and ‘0’ if not], and (i) had a comparison arm with comparable outcome measures at baseline between study arms.
Impact on number of sexual partners
Table 3 presents the findings in changes of the number of sexual partners due to intervention. Most studies reported a mean number of any sexual partners while two studies presented a proportion of multiple sexual partners [9,44]. All outcomes, either measured in mean or in proportion, were transferred to SMD between baseline and follow-up in each study arm, and the difference of SMD between intervention and comparison groups was used for meta-analysis. Figure 2 shows the overall efficacy. Of seven studies reporting the number of any sexual partners in post-intervention assessment, only one was statistically significant [43]. The combined efficacy from these studies was not statistically significant (mean ES: -0.10; 95% CI: -0.26, 0.06; P=0.22). Small heterogeneity was shown among these seven interventions (I2=12.9%; P=0.33). Funnel plot analysis showed no evidence of publication bias (Kendall tau=0.14, P=0.77; Egger’s t value=-1.09; P=0.27). Further subgroup analyses were performed, but no significant effect was detected in any duration of follow-up (P>0.05). With the above noted, it is important to point out that in meta-regression, no factor statistically modified the overall effect size of the number of sexual partners (P>0.05).
Table 3.
Efficacy of risk reduction interventions on number of sexual partners and drug and alcohol abuse among persons living with HIV/AIDS.
| Publication | No. of sexual partners (Mean(SD)) or proportion of multiple sexual partners (%) | Drug use or needle sharing (% or Mean(SD)) | Alcohol abuse * (% or Mean(SD)) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| IG | CG | IG | CG | IG | CG | |
|
| ||||||
| Kelly et al. [32] | N/A | N/A | Drug use | Drug use | 85.2→85.2 PM3,F3 | |
| I1: 70.4→55.6 PM3,F3 | 63.0→63.0 PM3,F3 | I1: 88.9→88.9PM3,F3 | ||||
| I2: 64.3→64.3 PM3,F3 | I2: 92.9→78.6PM3,F3 | |||||
|
| ||||||
| Kalichman et al. [37] | APPM3 | APPM3 | N/A | N/A | N/A | N/A |
| 1.6(2.4)→1.3(2.3)PM3,F3 | 1.7(3.3)→1.2(1.5)PM3,F3 | |||||
| 1.6(2.4)→1.2(1.9)PM3,F6 | 1.7(3.3)→1.3(1.9)PM3,F6 | |||||
|
| ||||||
| Margolin et al. [23] | N/A | N/A | Needle sharing | Needle sharing | N/A | N/A |
| 44.4→12.9 PM1,F0 | 53.3→22.6 PM1,F0 | |||||
| 44.4→11.8 PM1,F3 | 53.3→10.7 PM1,F3 | |||||
|
| ||||||
| Sorensen et al.[38] | N/A | N/A | Drug use | Drug use | ||
| 0.2(0.13)→0.2(0.14)PM1,F6 | 0.2(0.13)→0.2(0.13)PM1,F6 | |||||
| 0.2(0.13)→0.2(0.13)PM1,F12 | 0.2(0.13)→0.1(0.12)PM1,F12 | |||||
| 0.2(0.13)→0.1(0.10)PM1,F18 | 0.2(0.13)→0.1(0.10)PM1,F18 | 0.2(0.25)→0.2(0.22)PM1,F6 | 0.2(0.26)→0.2(0.22)PM1,F6 | |||
| Needle sharing | Needle sharing | 0.2(0.25)→0.1(0.18)PM1,F12 | 0.2(0.26)→0.2(0.23)PM1,F12 | |||
| 1.2(0.99)→0.6(0.84) PM1,F6 | 1.2(1.06)→0.7(0.98) PM1,F6 | 0.2(0.25)→0.1(0.16)PM1,F18 | 0.2(0.26)→0.1(0.19)PM1,F18 | |||
| 1.2(0.99)→0.7(0.90) PM1,F12 | 1.2(1.06)→0.8(1.04) PM1,F12 | |||||
| 1.2(0.99)→0.3(0.65) PM1,F18 | 1.2(1.06)→0.5(0.89) M1,F18 | |||||
|
| ||||||
| Purcell et al. [39] | N/A | N/A | Needle sharing for HNUP | Needle sharing for HNUP | N/A | N/A |
| 28.6→9.7 PM3,F3 | 28.9→12.0 PM3,F3 | |||||
| 28.6→7.3 PM3,F6 | 28.9→10.6 PM3,F6 | |||||
| 28.6→5.8 PM3,F12 | 28.9→6.6 PM3,F12 | |||||
|
| ||||||
| Gilbert et al. [24] | CPPM3 | CPPM3 | Drug use | Drug use | Any risk drinking2 | Any risk drinking2 |
| -2.3(9.2)F3 | -1.4(7.9)F3 | 43.8→38.5PM3,F3 | 41.1→41.5PM3,F3 | 38.3→26.4 PM3,F3 | 39.0→29.8PM3,F3 | |
| -2.7(8.4)F6 | -0.6(5.6)F6 | 43.8→29.5PM3,F6 | 41.1→42.5PM3,F6 | 38.3→23.5 PM3,F6 | 39.0→27.5PM3,F6 | |
|
| ||||||
| Williams et al. [40] | APPM6 | APPM6 | N/A | N/A | N/A | N/A |
| 6.69(1.43)-->3.46(0.51)F0 | 5.49(1.32)-->3.49(0.47)F0 | |||||
| 6.69(1.43)-->2.34(0.47)F3 | 5.49(1.32)-->3.12(0.43)F3 | |||||
| 6.69(1.43)-->1.69(0.19)F6 | 5.49(1.32)-->1.71(0.17)F6 | |||||
|
| ||||||
| Coleman et al. [41] | APPM3 | APPM3 78.6→53.9F3 | N/A | N/A | N/A | N/A |
| 81.8→45.0F3 | ||||||
|
| ||||||
| Rose et al. [8] | APPM6 | APPM6 | N/A | N/A | N/A | N/A |
| 5.2(16.3)→ 4.3(9.9)F6 | 4.6(16.0)→ 8.1(67.3)F6 | |||||
| HNUPPM6 | HNUPPM6 | |||||
| 3.1(3.8)→1.5(2.0)F6 | 2.3(2.3)→0.8(1.2)F6 | |||||
|
| ||||||
| Teti et al. [22] | APPM6 | APPM6 | N/A | N/A | N/A | N/A |
| 2.50(4.36)→4.57(16.66)F6 | 2.24(3.52)→5.15(15.91)F6 | |||||
| 2.50(4.36)→1.50(0.81)F12 | 2.24(3.52)→2.77(4.39)F12 | |||||
| 2.50(4.36)→1.40(0.84)F18 | 2.24(3.52)→1.40(1.30)F18 | |||||
|
| ||||||
| Wolitski et al. 9] | APPM3 | APPM3 | N/A | N/A | N/A | N/A |
| 27.2→22.6F6 | 29.2→20.9F6 | |||||
| 27.2→20.6F12 | 29.2→21.1F12 | |||||
| 27.2→24.3F18 | 29.2→21.1F18 | |||||
|
| ||||||
| Kalichman et al. [20] | HNUPPM3 | HNUPPM3 | N/A | N/A | N/A | N/A |
| 0.6(1.8)→0.5(1.4)F3 | 0.7(3.1)→0.6(1.3)F3 | |||||
| 0.6(1.8)→0.6(1.1)F6 | 0.7(3.1)→0.9(5.1)F6 | |||||
| 0.6(1.8)→0.04(0.6)F9 | 0.7(3.1)→0.4(0.6)F9 | |||||
Note: IG: intervention group; CG: control groups; SD: standard deviation; AP: any partners; HNUP: HIV-negative or unknown partners; IDU: injection drug use; DU: drug users; I1: cognitive-behavioral group intervention; I2: social support group intervention; N/A: not available;
Alcohol abuse was defined as exceeding the US National Institute on Alcoholism and Alcohol Abuse’s recommended numbers of drinks per week (14 or fewer for men; 7 or fewer for women) and or 3 or more binge drinking episodes (5 or more drinks on 1 occasion for men; 4 or more drinks on 1 occasion for women).
PM1: In the past month; PM3: In the past 3 months; PM6: In the past 6 months;
F0: Immediately after intervention; F3: At 3-month follow-up; F6: At 6-month follow-up;F9: At 9-month follow-up; F12: At 12-month follow-up; F18: At 18-month follow-up.
Figure 2.
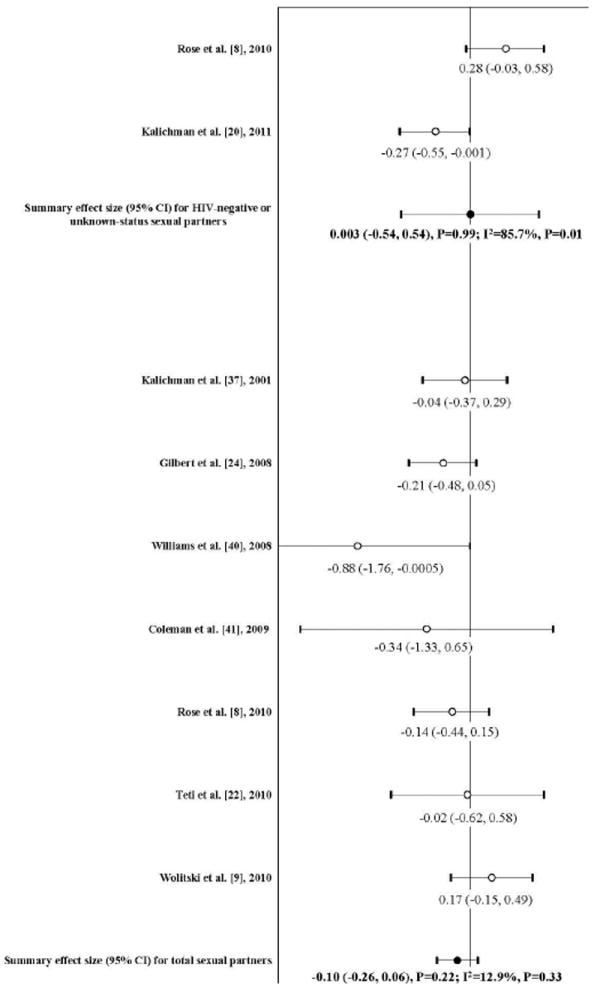
Forest plot of effect sizes: the impact of risk reduction intervention on the number of any sexual partners and HIV-negative or unknown-status sexual partners among people living with HIV/AIDS (Note: A negative ES value indicates reduction of the outcome after the intervention)
In standardized deleted residual analysis, no individual study was identified as an outlier. Sensitivity analyses were used to evaluate the stability of the summary effect size in meta-analysis by excluding the study by Gilbert et al. [23], because it only reported number of casual sexual partners, but the summary effect size did not change correspondingly (mean ES: -0.06; 95% CI: -0.25, 0.13; P=0.53).
Two randomized clinical trials were included in the meta-analysis of the efficacy on number of HIV-negative or unknown-status sexual partners [8,20], and the combined effect was null (mean ES: 0.003; 95% CI: -0.54, 0.54; P=0.99). Large heterogeneity was observed in these two studies (I2=85.7%; P=0.01) (Figure 2).
Impact on drug use
Among three studies reported the outcome of drug use, one showed a significant impact [23]. Meta-analysis found that there was statistically significant association between risk reduction intervention and reduction of drug use (mean ES:-0.26; 95% CI:-0.51, -0.01; P=0.04), which was largely attributable to one study [23] (Figure 3). Null heterogeneity was shown across these three studies (I2=0%; P=0.48). The funnel plot did not detect publication bias (Kendall tau=0.33, P=0.75; Egger’s t value=0.47, P=0.64).
Figure 3.
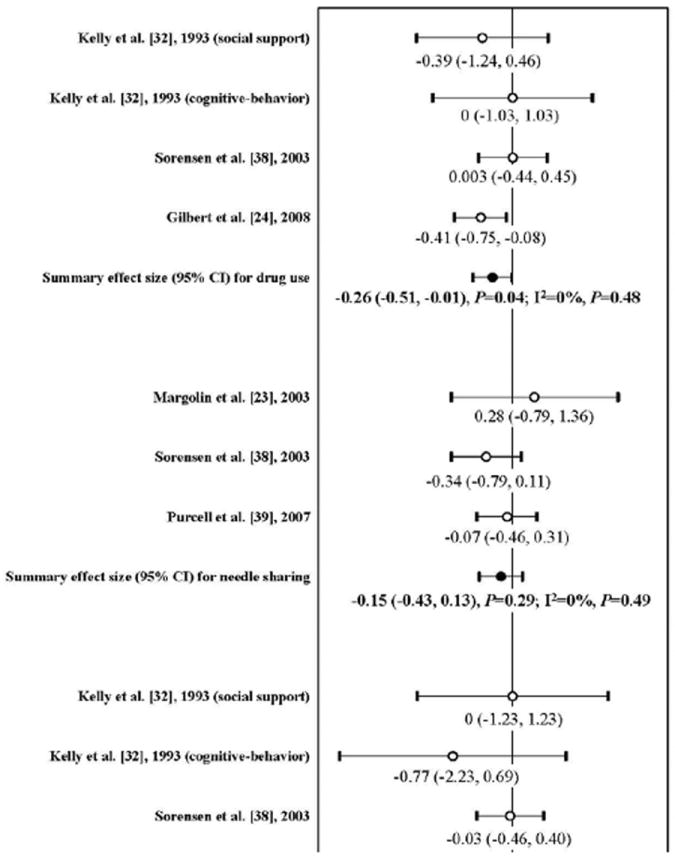
Forest plot of effect sizes: the impact of risk reduction intervention on drug use, needle sharing, and alcohol abuse among people living with HIV/AIDS (Note: A negative ES value indicates reduction of the outcome after the intervention)
Impact on needle sharing
Of three studies assessing the outcome of needle sharing among HIV-positive drug users, two showed a positive impact[41,42] while the other one did not [22]; however, no difference was statistically significant, nor was the pooled effect size(mean ES:-0.15; 95% CI:-0.43, 0.13; P=0.29) (Figure 3). Neither heterogeneity (I2=0%; P=0.49) nor publication bias were detected (Kendall tau=0.33; P=1; Egger’s t value=0.65). Further sensitivity analysis by removing the study assessing needle sharing with HIV-negative or unknown-status sexual partners did not change the conclusion [42] (mean ES: -0.23; 95% CI: -0.69, 0.23; P=0.33).
Impact on alcohol abuse
Of three studies measuring the outcome of alcohol abuse among HIV-infected persons [23,35,41], none showed a significant impact. Their pooled effect size was also non-significant (mean ES:-0.10; 95% CI:-0.36, 0.17; P=0.47) (Figure 3). There was no heterogeneity across these studies (I2=0%; P=0.82). Publication bias was not found (Kendall tau=-0.33, P=0.75; Egger’s t value=-0.53, P=0.59).
Discussion
Our meta-analysis of 12-risk reduction intervention RCTs involving 3993 PLWHA failed to show significant impacts on reduction of sexual partners, drug use, needle sharing, or alcohol abuse among PLWHA. A previous meta-analytic review also did not show efficacy in reducing the number of sexual partners, but it included studies involving both HIV-positive and negative participants [25]. Our study focused on well-designed RCTs in which all participants were HIV-positive.
HIV-infected individuals may reduce their sexual partners or practice partner serosorting after knowing their HIV status in order to reduce the risk of transmission to others [47-50]. However, it is difficult to detect a significant reduction of sexual partners between study arms if the average number of sexual partners at recruitment is low. Participants in RCTs, even in the comparison arm, may also modify their sexual behaviors during the trial as trial participants are typically offered education and risk reduction counseling for ethical reasons; this could lead to reduction in the magnitude of the intervention effect in individual studies. These are among the possible explanation of the null synthesized efficacy found in this meta-analysis.
Only two studies measured the impact on number of HIV-negative or unknown-status sexual partners; they had contradictory results [8,20]. Subgroup and sensitivity analyses did not find a significant effect on the number of any sexual partners in any subgroup.
We also analyzed the impact on reduction of drug use among HIV-infected drug users, but only three individual clinical trials were available in our analysis [23,35,41]. The synthesized efficacy was statistically significant, primarily due to one study [23]. Though risk reduction interventions studies among drug users have shown reduction of drug injection [51] as well as risky sexual behaviors [52,53], the evidence available from studies among HIV-infected drug users was too sparse for drawing a conclusion of efficacy of interventions to reduce drug use.
Sharing of contaminated needles is the primary driver of the HIV epidemic among injection drug users. There were only three RCTs estimating the efficacy of interventions on needle sharing among HIV-positive drug users [22,41,42]. Compared to a previous meta-analysis, our review added one recent RCT [42], but excluded a quasi-experimental study [54]. Both our meta-analysis and the previous one found no significant effect of interventions on needle sharing.
A previous systematic review of 27 observational studies found that any alcohol consumption was significantly associated with an increase of unprotected sex among PLWHA [12]. None of three risk reduction intervention RCTs among PLWHA showed a significant intervention effect in reducing alcohol abuse, though all demonstrated statistically significant reduction of unprotected sex [23,35,41]. The synthesized result in our meta-analysis failed to show a relationship between risk reduction interventions and reduction of alcohol abuse. As alcohol abuse among PLWHA may increase risky sexual behaviors and reduce adherence to HIV antiretroviral therapy [55,56], effective interventions for alcohol abuse among HIV-infected individuals are needed.
Our meta-analysis has several limitations. Firstly, outcomes were based on self-report and might be subject to social desirability bias. For example, if participants in the intervention arm underreported a risk activity post-intervention in order to please the researchers, this may bias the study conclusion towards the null hypothesis. Secondly, the number of RCT studies was small. Thirdly, we found English-language publications only; studies published in other languages, if any, may have different study findings. Thirdly, even though twelve international databases were explored, all included RCTs were conducted in the USA; three RCTs in Africa were excluded because no target outcomes were reported or there were not enough data available for calculation. Therefore, more trials are needed from regions other than the United States. Finally, although twelve databases were searched for, the reviews and we deployed extensive checks for completeness by cross-referencing; we cannot exclude having missed a relevant study.
In conclusion, our meta-analysis suggested that the available RCTs for risk reduction among PLWHA did not have significant impacts on reducing number of sexual partners, and substance and alcohol abuse. Studies of more promising behavioral, community, or structural interventions are needed, properly designed and powered that target “positive prevention” strategies for PLWHA.
Acknowledgments
Research reported in this publication was supported by the National Natural Science Foundation of China under Award Numbers 81273188, and by the grants from U.S. National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant # R01AI09462 and R34AI091446). This research is also partially supported by grants from the Ministry of Science and Technology of China (2012ZX10001-002) and Chinese State Key Laboratory for Infectious Disease Development Grant (2012SKLID103).
Footnotes
Publisher's Disclaimer: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Chen HT, Liang S, Liao Q, Wang S, Schumacher JE, et al. HIV voluntary counseling and testing among injection drug users in south China: a study of a non-government organization based program. AIDS Behav. 2007;11:778–788. doi: 10.1007/s10461-007-9215-x. [DOI] [PubMed] [Google Scholar]
- 2.Loutfy MR, Walmsley SL. Salvage antiretroviral therapy in HIV infection. Expert Opin Pharmacother. 2002;3:81–90. doi: 10.1517/14656566.3.2.81. [DOI] [PubMed] [Google Scholar]
- 3.Jensen-Fangel S. The effectiveness of highly active antiretroviral therapy in HIV-infected patients. Dan Med Bull. 2004;51:371–392. [PubMed] [Google Scholar]
- 4.Crepaz N, Marks G. Towards an understanding of sexual risk behavior in people living with HIV: a review of social, psychological, and medical findings. AIDS. 2002;16:135–149. doi: 10.1097/00002030-200201250-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ncube NM, Akunna J, Babatunde F, Nyarko A, Yatich NJ, et al. Sexual risk behaviour among HIV-positive persons in Kumasi, Ghana. Ghana Med J. 2012;46:27–33. [PMC free article] [PubMed] [Google Scholar]
- 6.Sarna A, Luchters S, Pickett M, Chersich M, Okal J, et al. Sexual behavior of HIV-positive adults not accessing HIV treatment in Mombasa, Kenya: Defining their prevention needs. AIDS Res Ther. 2012;9:9. doi: 10.1186/1742-6405-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinhardt LS, Kelly JA, Brondino MJ, Rotheram-Borus MJ, Kirshenbaum SB, et al. HIV transmission risk behavior among men and women living with HIV in 4 cities in the United States. J Acquir Immune Defic Syndr. 2004;36:1057–1066. doi: 10.1097/00126334-200408150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Rose CD, Courtenay-Quirk C, Knight K, Shade SB, Vittinghoff E, et al. HIV intervention for providers study: a randomized controlled trial of a clinician-delivered HIV risk-reduction intervention for HIV-positive people. J Acquir Immune Defic Syndr. 2010;55:572–581. doi: 10.1097/QAI.0b013e3181ee4c62. [DOI] [PubMed] [Google Scholar]
- 9.Wolitski RJ, Kidder DP, Pals SL, Royal S, Aidala A, et al. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14:493–503. doi: 10.1007/s10461-009-9643-x. [DOI] [PubMed] [Google Scholar]
- 10.Gerbi GB, Habtemariam T, Tameru B, Nganwa D, Robnett V. The correlation between alcohol consumption and risky sexual behaviors among people living with HIV/AIDS. J Subst Use. 2009;14:90–100. doi: 10.1080/14659890802624261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerbi GB, Habtemariam T, Tameru B, Nganwa D, Robnett V. A comparative study of substance use before and after establishing HIV infection status among people living with HIV/AIDS. J Subst Use. 2011;16:464–475. doi: 10.3109/14659891.2010.495820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuper PA, Joharchi N, Irving H, Rehm J. Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: review and meta-analysis. AIDS Behav. 2009;13:1021–1036. doi: 10.1007/s10461-009-9589-z. [DOI] [PubMed] [Google Scholar]
- 13.Bunnell R, Ekwaru JP, Solberg P, Wamai N, Bikaako-Kajura W, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20:85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 14.Del Romero J, Castilla J, Hernando V, Rodríguez C, García S. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ. 2010;340:c2205. doi: 10.1136/bmj.c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan P. Reduction of HIV transmission risk while prescribed antiretroviral therapy (ARVT): Misclassification of ARVT status as a methodological issue. AIDS Res Hum Retroviruses; AIDS Vaccine 2010; 28 Sept–1 Oct 2010; Atlanta Georgia, US. 2010. abstract. [Google Scholar]
- 17.Lu W, Zeng G, Luo J, Duo S, Xing G, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr. 2010;55:232–238. doi: 10.1097/QAI.0b013e3181e9b6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman SM, Rajabiun S, Cabral HJ, Bradford JB, Tobias CR. Sexual risk behavior and behavior change among persons newly diagnosed with HIV: the impact of targeted outreach interventions among hard-to-reach populations. AIDS Patient Care STDS. 2009;23:639–645. doi: 10.1089/apc.2008.0092. [DOI] [PubMed] [Google Scholar]
- 19.Barta WD, Tennen H, Kiene SM. Alcohol-involved sexual risk behavior among heavy drinkers living with HIV/AIDS: negative affect, self-efficacy, and sexual craving. Psychol Addict Behav. 2010;24:563–570. doi: 10.1037/a0021414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalichman SC, Cherry C, Kalichman MO, Amaral CM, White D, et al. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. Am J Public Health. 2011;101:531–538. doi: 10.2105/AJPH.2010.197608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teti M, Bowleg L, Cole R, Lloyd L, Rubinstein S, et al. A mixed methods evaluation of the effect of the protect and respect intervention on the condom use and disclosure practices of women living with HIV/AIDS. AIDS Behav. 2010;14:567–579. doi: 10.1007/s10461-009-9562-x. [DOI] [PubMed] [Google Scholar]
- 22.Margolin A, Avants SK, Warburton LA, Hawkins KA, Shi J. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psychol. 2003;22:223–228. [PubMed] [Google Scholar]
- 23.Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, et al. Interactive “Video Doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3:e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crepaz N, Lyles CM, Wolitski RJ, Passin WF, Rama SM, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20:143–157. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BT, Carey MP, Chaudoir SR, Reid AE. Sexual risk reduction for persons living with HIV: research synthesis of randomized controlled trials, 1993 to 2004. J Acquir Immune Defic Syndr. 2006;41:642–650. doi: 10.1097/01.qai.0000194495.15309.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalichman SC, Ntseane D, Nthomang K, Segwabe M, Phorano O, et al. Recent multiple sexual partners and HIV transmission risks among people living with HIV/AIDS in Botswana. Sex Transm Infect. 2007;83:371–375. doi: 10.1136/sti.2006.023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerbi GB, Habtemariam T, Tameru B, Nganwa D, Robnett V. A comparative study of substance use before and after establishing HIV infection status among people living with HIV/AIDS. J Subst Use. 2011;16:464–475. doi: 10.3109/14659891.2010.495820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA National Institute of Mental Health Healthy Living Project Team. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007;46:574–580. doi: 10.1097/qai.0b013e318158a474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta-analysis. AIDS Educ Prev. 2009;21:181–206. doi: 10.1521/aeap.2009.21.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox DR. Analysis of binary data1970. New York: Chapman & Hall/CRC; 1970. [Google Scholar]
- 31.Sánchez-Meca J, Marín-Martínez F, Chacón-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8:448–467. doi: 10.1037/1082-989X.8.4.448. [DOI] [PubMed] [Google Scholar]
- 32.Becker BJ. Synthesizing standardized mean-change measures. Br J Math Stat Psychol. 1988;41:257–78. [Google Scholar]
- 33.Lipsey M, Wilson D. Practical meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 34.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 35.Kelly JA, Murphy DA, Bahr GR, Kalichman SC, Morgan MG, et al. Outcome of cognitive-behavioral and support group brief therapies for depressed, HIV-infected persons. Am J Psychiatry. 1993;150:1679–1686. doi: 10.1176/ajp.150.11.1679. [DOI] [PubMed] [Google Scholar]
- 36.Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Deeks J, Altman D, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in a meta-analysis. In: Egger M, Davey Smith G, Altman D, editors. Systematic reviews in health care: meta-analysis in context. Statistical methods for examining heterogeneity and combining results from several studies in a meta-analysis. BMJ Publications; London: 2002. pp. 285–312. [Google Scholar]
- 39.Rothstein HR, Sutton AJ, Borenstein M. Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Wiley; Chichester, England: 2005. [Google Scholar]
- 40.Kalichman SC, Rompa D, Cage M, DiFonzo K, Simpson D, et al. Effectiveness of an intervention to reduce HIV transmission risks in HIV-positive people. Am J Prev Med. 2001;21:84–92. doi: 10.1016/s0749-3797(01)00324-5. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen JL, Dilley J, London J, Okin RL, Delucchi KL, et al. Case management for substance abusers with HIV/AIDS: a randomized clinical trial. Am J Drug Alcohol Abuse. 2003;29:133–150. doi: 10.1081/ada-120018843. [DOI] [PubMed] [Google Scholar]
- 42.Purcell DW, Latka MH, Metsch LR, Latkin CA, Gómez CA, et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46(Suppl 2):S35–47. doi: 10.1097/QAI.0b013e31815767c4. [DOI] [PubMed] [Google Scholar]
- 43.Williams JK, Wyatt GE, Rivkin I, Ramamurthi HC, Li X, et al. Risk reduction for HIV-positive African American and Latino men with histories of childhood sexual abuse. Arch Sex Behav. 2008;37:763–772. doi: 10.1007/s10508-008-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman CL, Jemmott L, Jemmott JB, Strumpf N, Ratcliffe S. Development of an HIV risk reduction intervention for older seropositive African American men. AIDS Patient Care STDS. 2009;23:647–655. doi: 10.1089/apc.2008.0276. [DOI] [PubMed] [Google Scholar]
- 45.MacNeil JM, Mberesero F, Kilonzo G. Is care and support associated with preventive behaviour among people with HIV? AIDS Care. 1999;11:537–546. doi: 10.1080/09540129947695. [DOI] [PubMed] [Google Scholar]
- 46.McKirnan DJ, Tolou-Shams M, Courtenay-Quirk C. The Treatment Advocacy Program: a randomized controlled trial of a peer-led safer sex intervention for HIV-infected men who have sex with men. J Consult Clin Psychol. 2010;78:952–963. doi: 10.1037/a0020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gómez CA, Mason B, Alvarado NJ. Culture matters: The role of race and ethnicity in the sexual lives of HIV-positive gay and bisexual men. American Psychological Association; Washington DC, USA: 2005. [Google Scholar]
- 48.Weinhardt L. HIV Diagnosis and Risk Behavior Positive Prevention. 2005:29–63. doi: 10.1007/0-306-48700-4_2. [DOI] [Google Scholar]
- 49.Morin SF, et al. A behavioral intervention reduces HIV transmission risk by promoting sustained serosorting practices among HIV-infected men who have sex with men. J Acquir Immune Defic Syndr. 2008;49(5):544–51. doi: 10.1097/QAI.0b013e31818d5def. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassels S. HIV serosorting as a harm reduction strategy: evidence from Seattle, Washington. AIDS. 2009:2497–506. doi: 10.1097/QAD.0b013e328330ed8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robles RR. Effects of combined counseling and case management to reduce HIV risk behaviors among Hispanic drug injectors in Puerto Rico: a randomized controlled study. J Subst Abuse Treat. 2004:145–52. doi: 10.1016/j.jsat.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Sterk CE. The Health Intervention Project: HIV risk reduction among African American women drug users. Public Health Rep. 2002;117(Suppl 1):S88–95. [PMC free article] [PubMed] [Google Scholar]
- 53.Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychol. 2003;22:332–339. doi: 10.1037/0278-6133.22.4.332. [DOI] [PubMed] [Google Scholar]
- 54.Grinstead O, Zack B, Faigeles B. Reducing postrelease risk behavior among HIV seropositive prison inmates: the health promotion program. AIDS Educ Prev. 2001;13:109–119. doi: 10.1521/aeap.13.2.109.19737. [DOI] [PubMed] [Google Scholar]
- 55.Falang KD, Akubaka P, Jimam NS. Patient factors impacting antiretroviral drug adherence in a Nigerian tertiary hospital. J Pharmacol Pharmacother. 2012;3:138–142. doi: 10.4103/0976-500X.95511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kenya S, Chida N, Jones J, Alvarez G, Symes S, et al. Weekending in PLWH: alcohol use and ART adherence, a pilot study. AIDS Behav. 2013;17:61–67. doi: 10.1007/s10461-012-0307-x. [DOI] [PubMed] [Google Scholar]


