Summary
Dopamine is thought to play a major role in learning. However, while dopamine D1 receptors (D1Rs) in the prefrontal cortex (PFC) have been shown to modulate working memory-related neural activity, their role in the cellular basis of learning is unknown. We recorded activity from multiple electrodes while injecting the D1R antagonist SCH23390 in the lateral PFC as monkeys learned visuomotor associations. Blocking D1Rs impaired learning of novel associations and decreased cognitive flexibility, but spared performance of already familiar associations. This suggests a greater role for prefrontal D1Rs in learning new, than performing familiar, associations. There was a corresponding greater decrease in neural selectivity and increase in alpha and beta oscillations in local field potentials for novel than familiar associations. Our results suggest that weak stimulation of D1Rs observed in aging and psychiatric disorders may impair learning and PFC function by reducing neural selectivity and exacerbating neural oscillations associated with inattention and cognitive deficits.
Introduction
Learning and memory are foundations of advanced cognition. Their impairment is found, for example, in Parkinson’s disease and schizophrenia (Owen et al., 1992; Park and Holzman, 1992; Elvevåq and Goldberg, 2000; Lewis et al., 2003; Jankovic, 2008; Wang et al., 2011). These disorders also impact the prefrontal cortex (PFC), a cortical region associated with executive functions and critical for normal learning (Miller and Cohen, 2001). Profound learning and other cognitive deficits typically follow PFC damage (Godefroy, 2003; Robbins, 2007; Kehagia et al., 2010), and neurophysiological studies show learning-related changes in PFC neural activity (Asaad et al., 1998; Pasupathy and Miller, 2005; Benchenane et al., 2010; Antzoulatos and Miller, 2011). The widespread inputs the PFC receives from dopamine axons originating in the ventral tegmental area and the substantia nigra pars compacta (Williams and Goldman-Rakic, 1998) are likely to be important. Dopamine neurons fire and release dopamine into the PFC to sensory cues that predict reward (Schultz et al., 1993), and thus provide the reward-prediction error signals needed for guiding reward-based learning (Schultz, 2007), and for gating reward-related information in and out of active working memory (Cohen et al., 2002; O'Reilly, 2006). In addition, a subset of dopamine neurons is activated by aversive events. Because these events are non-rewarded, some dopamine neurons may encode the stimulus salience rather than its positive value (Matsumoto and Hikosaka, 2009; Bromberg-Martin et al., 2010). Thus, dopamine signals in the PFC could play a role in adapting cognitive function to different arousal states (e.g. stress or fatigue) (Arnsten et al., 2010).
Neurons in the PFC densely express the dopamine D1-like family of receptors (D1Rs) (Lidow et al., 1991; de Almeida et al., 2008; Santana et al., 2009). In monkeys, D1Rs have been shown to modulate neural activity related to spatial working memory (Sawaguchi and Goldman-Rakic, 1991; Sawaguchi and Goldman-Rakic, 1994; Williams and Goldman-Rakic, 1995; Vijayraghavan et al., 2007). Too much or too little D1R activation induces a decrease in spatial tuning of sustained PFC activity during a short memory delay (Williams and Goldman-Rakic, 1995; Vijayraghavan et al., 2007). Deficits in working memory following D1R manipulation have been shown in rodents as well (Zahrt et al., 1997; Seamans et al., 1998; Chudasama and Robbins, 2004; Floresco and Magyar, 2006), along with deficits in attention (Granon et al., 2000; Chudasama and Robbins, 2004) and cognitive flexibility (Ragozzino, 2002; Floresco et al., 2006; Floresco and Magyar, 2006). However, despite the central role dopamine is thought to play in learning, its involvement in modulating neural correlates of learning in the PFC is largely unknown.
In addition to understanding D1R function at the single neuron level, additional insight can be gained from the next level up: interactions between networks of neurons. This is often studied by examining oscillations in the local field potentials (LFPs) and coherence in neural activity, that are thought to reflect communication and interactions between neuron populations. In the cortex, oscillations at alpha, beta and gamma frequencies have been associated with attention and memory (Engel et al., 2001; Fries et al., 2001; Jensen et al., 2002; Buschman and Miller, 2007; Fries et al., 2008; Schroeder and Lakatos, 2009; Siegel et al., 2009; Benchenane et al., 2011; Bollimunta et al., 2011). Importantly, altered oscillations have been observed in normal and pathological aging (Lizio et al., 2011), and in a number of neurological and psychiatric disorders, notably Parkinson's disease and schizophrenia (Spencer et al., 2003; Cho et al., 2006; Uhlhaas and Singer, 2006; Basar and Güntekin, 2008; Wang, 2010). Because patients with these disorders also show both cognitive deficits associated with PFC function (Elvevåq and Goldberg, 2000; Lewis et al., 2003) and altered prefrontal dopamine neurotransmission (Knable and Weinberger, 1997; Kulisevsky, 2000; Abi-Dargham et al., 2002), it seems likely that D1Rs might also modulate PFC oscillatory activity during learning.
To address these issues, we trained two monkeys in a delayed associative learning task and blocked D1Rs pharmacologically while recording populations of neurons and neural oscillations in the lateral PFC. We have previously shown that during associative learning neurons in the monkey lateral PFC build up neural information reflecting the acquisition between visual cues and saccades (Asaad et al., 1998; Pasupathy and Miller, 2005; Antzoulatos and Miller, 2011). In this study, we report that learning of new associations and its neural correlates, but not familiar associations, are impaired by D1R blockade.
Results
Prefrontal Dopamine D1 Receptors are More Important for Learning New Associations than Performance of Familiar Associations
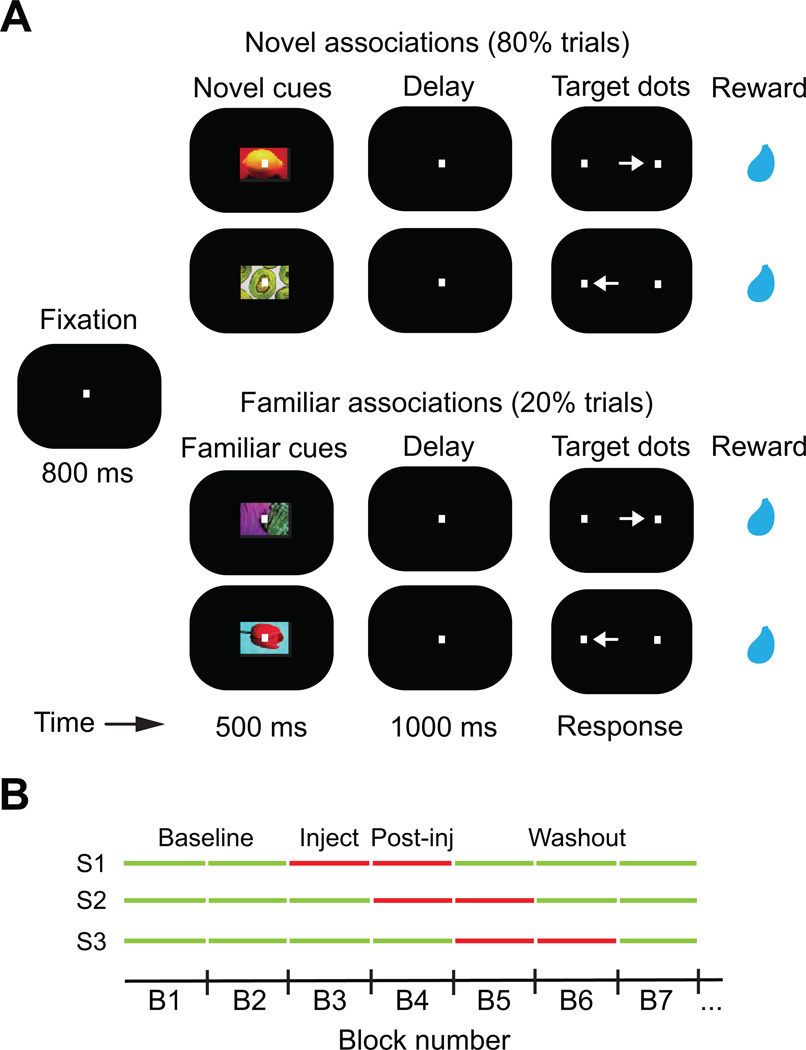
Two monkeys learned associations between visual cues presented at the center of gaze and saccades to the right or left by trial and error (Figure 1A). Cue and saccade were separated by a short (1000 ms) memory delay. To compare new learning with well-learned associations, trials were blocked with pairs of novel cues on 80% of the trials (learning trials), and pairs of highly familiar cues on 20% of the trials (familiar trials). Once a pair of new associations was learned (at least 80% correct for each novel cue; see Experimental Procedures), two new cues replaced the previously novel cues and a new block started. Familiar cues remained unchanged for the entire session. Monkeys completed 8 to 12 blocks per session during training. In each session, monkeys first completed several pre-injection (baseline) blocks (Figure 1B). Then, 3 µl of either saline or the D1R antagonist SCH23390 (30 µg) were pressure-injected into the dorsolateral or ventrolateral PFC (dlPFC and vlPFC, respectively) through a metal cannula at 0.3 µl/min (see Experimental Procedures). Injections started at the beginning of a block (injection block), and different numbers of baseline blocks were used in different sessions (Figure 1B) to avoid any confounds related to systematic changes in monkeys' behavior with block. The animals never stopped working during the session.
Figure 1. Delayed Associative Learning Task and Pharmacology.
(A) Monkeys performed a delayed associative learning task, similar to that described previously (Asaad et al., 1998; Pasupathy and Miller, 2005). Animals fixated to start trial. A cue object was followed by a brief memory delay and presentation of two target dots. Saccade to the target associated with the cue was rewarded with juice drops. Trials were blocked in pairs of novel cues (80% of trials), and pairs of highly familiar cues (20%). When performance of learning trials reached 80%, novel cues were replaced and a new block started.
(B) Monkeys first completed several baseline blocks (green lines before injections). Then, 3 µl of saline or SCH23390 (30 µg diluted in saline) were pressure-injected in the left PFC (injection block, first red line). Blocks affected by the treatments (post-injection blocks; second red line) were followed by blocks where behavior recovered (washout blocks; green lines after injections). Drugs were injected after different numbers of baseline blocks (2–4; B1–B4) in different sessions (S1–S3).
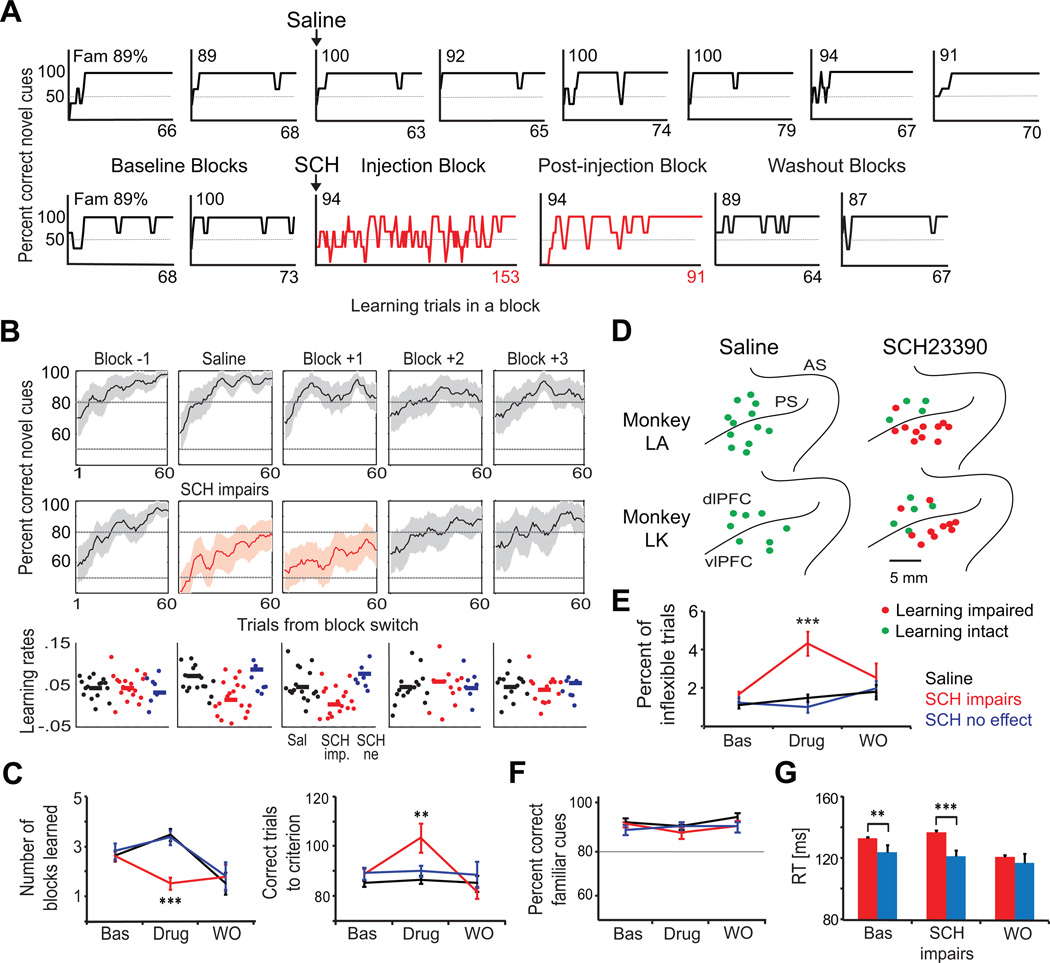
We first determined whether the monkeys' performance showed any post-injection learning deficit. A distribution of monkeys’ error rates during learning trials was generated by fitting a sigmoid curve to the trial-by-trial performance (Williams and Eskandar, 2006; see Experimental Procedures). The average distribution across the baseline blocks was compared to the distribution from each block after the injection using a Kolmogorov-Smirnov (KS) test. In saline sessions (n = 20), we did not observe any post-injection deficit (p > 0.05; first 60 trials/block, the minimum block length). In 21 of the 30 sessions where SCH23390 was injected, there were significantly worse learning performances on the injection block and/or the next block relative to baseline blocks (KS, p < 0.05). In fact, for all affected sessions, learning was impaired only on the first two post-injection blocks, even though an affected session was defined as an effect on any post-injection block. Representative examples are shown in Figure 2A.
Figure 2. Dopamine D1 Receptors in the Lateral PFC are More Involved in Learning New Associations than Performance of Familiar Associations.
(A) Representative examples of the performance (average of three trials) of two different sessions where saline (top) and SCH23390 (SCH; bottom) were injected. Each plot corresponds to an individual block. Saline did not affect learning, whereas SCH23390 altered the learning curve immediately. The performance of familiar associations remained intact (Fam, percent correct shown on top left of each block).
(B) Average percent correct performance across sessions during learning for the pre-injection baseline block (Block −1), the block during which saline or SCH23390 was injected, and the first (Block +1), second (Block +2) and third (Block +3) post-injection blocks. Also shown are the learning rates. Note that during the injection block and first post-injection block learning curves are slower (shallower) and learning rates are smaller. Saline (Sal), n = 20 sessions; SCH23390 impairs (SCH imp), n = 21 sessions; SCH23390 no effect (SCH ne), n = 9 sessions.
(C) Number of blocks learned and average number of correct trials to criterion for baseline (Bas), drug and washout (WO) blocks.
(D) Map of the injections. Injection sites where the learning performance was affected (red dots) and unaffected (green dots) by saline or SCH23390 are shown. The exact location of the injections was determined by magnetic resonance imaging (MRI). PS, Principal sulcus. AS, Arcuate sulcus.
(E) Perseverative errors increased significantly after the injection of SCH23390 (T-test).
(F) The performance of familiar associations was not affected by the treatments in any session.
(G) Reaction times (RT) during familiar associations (blue bars) were smaller than during novel associations (red bars). This difference was enhanced after the injection of SCH23390 in D1R-modulated sites. Shown is the mean and SEM. **, p < 0.01; ***, p < 0.001, T-test. See also Figure S1.
Learning rate was defined as the slope of the sigmoid curve fitted to the trial-by-trial performance; high rates indicating rapid learning. Figure 2B shows the average learning curves and learning rates across saline and affected SCH23390 sessions. The learning rates of the first two blocks following SCH23390 in significantly affected sessions were smaller than the baseline learning rates (logistic regression of the first 60 trials/block in 50 baseline blocks, mean slope = 0.05 ± 0.008 vs. 38 post-injection blocks, mean slope = 0.017 ± 0.007; mean ± standard error of the mean or SEM; Wilcoxon test, p = 0.005). These post-injection learning rates were also smaller than that of the first two blocks following saline injections (40 post-saline injection blocks, mean = 0.06 ± 0.007; p = 1×10−4), and smaller than the learning rates of the first two blocks following SCH23390 in unaffected sessions (18 post-injection blocks, mean = 0.12 ± 0.04; p = 3×10−5). On average, the impairment lasted one hour (59 ± 5 minutes), during which monkeys completed fewer numbers of blocks because they needed more correct trials to learn the associations (Figure 2C). There was an anatomical dissociation between affected and unaffected sites: most (18 of 21) sessions with a learning deficit followed vlPFC injections, whereas the sites unaffected by SCH23390 were mainly in the dlPFC (Figure 2D, proportion of vlPFC vs. dlPFC affected sites; Chi-square, p = 9×10−5). We did not observe any anterior vs. posterior trend for the location of affected sites. Performance for each of the two novel cues was similarly impaired in both animals (Figure S1).
The learning impairment was not due to altered eye movements. We did not observe any major changes in the trajectories or accuracy of the saccades after the injection of SCH23390. The vast majority of saccades during error trials ended within the target window around the incorrect target (< 4.0 degrees). In fact, if anything, saccade accuracy somewhat improved following SCH23390: there was an increase in error trial saccades ending within the incorrect target window (88 ± 4 % to 95 ± 5 % of error trials; T-test, p = 0.02). The average eye movement velocities (deg/s) also increased after injection of SCH23390 (from 401 ± 3 to 422 ± 5 deg/s; p = 0.0004) perhaps due to frustration from the learning impairment and reduction in reward. Errors were not caused by increased impulsivity, a premature saccade towards the correct target before the “go” cue (baseline, 7.4 ± 0.5 % of trials; SCH23390, 7.7 ± 0.6 %; Wilcoxon test, p = 0.62 vs. baseline, p = 0.75 vs. saline). But there was a modest increase in perseveration (the average number of consecutive repeats of an error), from 1.6 ± 0.2 % of trials during baseline to 4.3 ± 0.6 % during the first hour post-injection in affected sites (Wilcoxon test, p = 4×10−5), but not after saline (mean = 1.47 %, p = 2×10−5 vs. affected sites) or SCH23390 in unaffected sites (mean = 1 %, p = 4×10−5 vs. affected sites; Figure 2E).
In contrast to new learning, performance of familiar associations was unimpaired in all sessions (Figures 2F and S1), and the proportion of perseverative errors was not different from baseline (Wilcoxon test, p = 0.59). Reaction times were shorter for familiar associations than for novel associations during the baseline blocks (122 ± 5 ms vs. 133 ± 1 ms, Wilcoxon test, p = 0.003), an effect also observed after the injection of SCH23390 in behaviorally sensitive sites (121 ± 4 ms vs. 137 ± 1 ms, p = 6×10−4; Figure 2G).
Prefrontal Dopamine D1 Receptors and Neural Selectivity during Novel and Familiar Associations
We recorded the activity of individual prefrontal neurons from 7–15 electrodes located 1 or 2 mm away from the injection site. Typically, 15 to 30 isolated neurons could be recorded in each session. Only neurons that were well isolated before and after the injections were included in the analyses (see Experimental Procedures). The injections of saline and SCH23390 induced a small, slow, and steady increase in neuronal activity. The firing rate across all recorded neurons in saline sessions increased from 2.36 ± 0.2 to 2.57 ± 0.21 spikes/s from baseline to post-injection epochs (omitting the first 20 min after the injections; n = 286 neurons; T-test, p = 0.07). During SCH23390 sessions, there was an increase from 2.06 ± 0.13 to 2.56 ± 0.2 spikes/s from baseline to post-injection epochs (n = 279 neurons; p = 0.002), which was not different from that during post-saline injection epochs (saline: 2.57 ± 0.21 vs. SCH23390: 2.56 ± 0.2 spikes/s, p = 0.96). The small increases in firing rate are often seen and likely due to a mechanical stimulation of the neural tissue. All effects reported below are above and beyond these modest increases in firing rate.
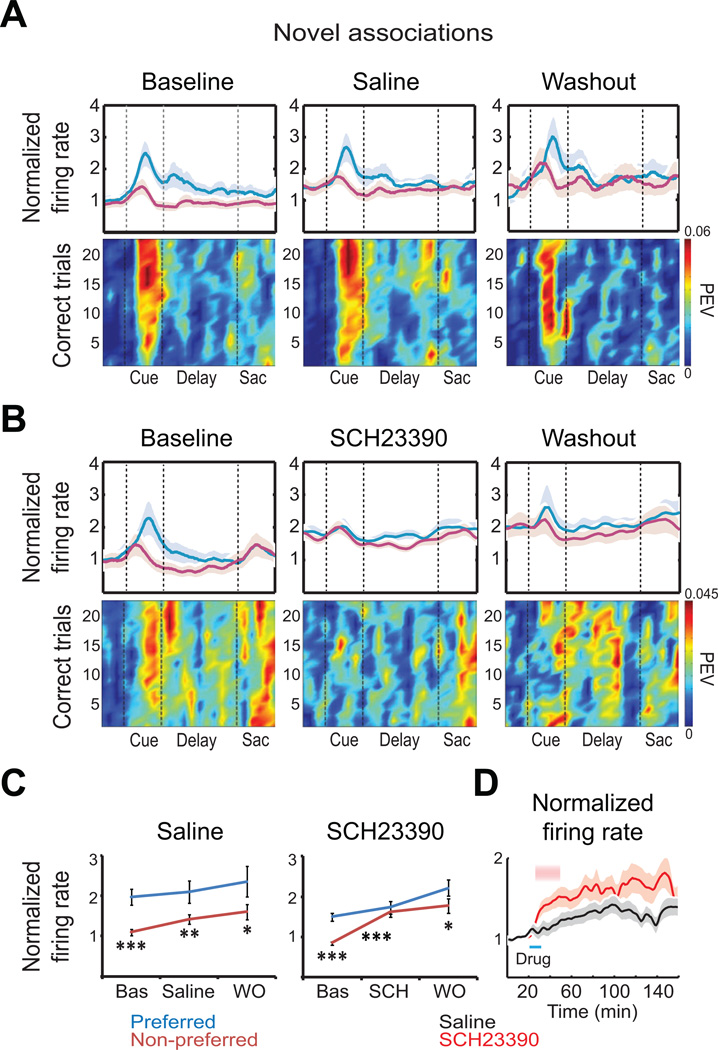
With learning, the monkeys were increasingly able to predict which saccade would be required at the end of the trial as soon as the cue appeared early in the trial. As in previous studies, there was a corresponding increase in early trial saccade-predicting activity, especially during cue presentation. By the end of the baseline blocks (last 20 correct trials), almost 30% of randomly selected neurons showed a significant difference in firing rate between the preferred and non-preferred associated directions during the cue presentation and/or the memory delay (Figure 3A and 3B top panels; saline, 81 of 286 neurons [28.3 %]; SCH23390, 78 of 279 neurons [28 %]; ANOVA during cue and/or memory delay, p < 0.05; Pasupathy and Miller, 2005). The analyses below will focus on this early trial activity. During baseline blocks, a higher proportion of neurons showing such selectivity was observed near sites behaviorally sensitive to SCH23390 than insensitive sites (28.6 % vs. 9.4 %, Chi-square, p = 8×10−7), suggesting that SCH23390 was most impairing at sites more involved in the task. As the analyses below will show, after SCH23390, but not saline, this neural selectivity was reduced (Figure 3B; see an example neuron in Figure S2).
Figure 3. Selectivity of Prefrontal Neurons is D1R-Dependent During Learning of Novel Associations.
(A–B) Normalized firing rate after learning (last 20 trials/block; correct trials only) for preferred (blue traces) and non-preferred (magenta traces) saccade directions, in (A) saline sessions and (B) SCH23390 sessions. Cell activity was normalized by the mean firing rate during the 200 ms before cue presentation in baseline blocks. Color scale shows strength of direction selectivity (PEV, proportion of explainable variance by direction factor, shuffle corrected).
(C) Relative changes in normalized firing rate between preferred and non-preferred directions (cue epoch). Note the remarkable increase in spiking for non-preferred directions after SCH23390. Two-way ANOVA with Bonferroni post-hoc test.
(D) Average normalized firing rates of neurons selective to novel associations in saline and SCH23390 sessions. Shaded area indicates significantly higher firing rate after SCH23390 relative to saline (Wilcoxon test, p < 0.05). Saline, n = 81 neurons; SCH23390, n = 78 neurons. See also Figure S2.
We quantified the neural information about saccade direction on correctly performed trials for each neuron using the percent explained variance (PEV) statistic (Siegel et al., 2009; Buschman et al., 2011). PEV reflects the amount of variance in each neuron’s trial-by-trial firing rate that is explained by saccade direction. Higher PEV indicates more selectivity for the predicted saccade (the one associated with the current cue). It was calculated over an eight-correct-trial window stepped by one trial (see Experimental Procedures; Pasupathy and Miller, 2005). We compared PEV early in learning of the novel associations (first 10 correct trials per block) vs. late in learning (last 10 correct trials per block) for the population of selective neurons (as above). During baseline and after saline injection blocks, the average PEV increased with learning (Figure 3A and 3B; cue period, first vs. last 10 correct trials on saline baseline blocks: 0.02 ± 0.002 vs. 0.04 ± 0.002, T-test, p = 1×10−6; on saline post-injection blocks: 0.029 ± 0.001 vs. 0.047 ± 0.001, p = 4×10−6; on SCH23390 baseline blocks: 0.02 ± 0.002 vs. 0.033 ± 0.001, p = 4×10−5). However, after the injection of SCH23390, there was no corresponding increase in average PEV in the first two post-injection blocks, when there was a learning impairment (first vs. last 10 correct trials per block, 0.009 ± 0.003 vs. 0.007 ± 0.001, p = 0.78; Figure 3 B lower panels). Also, average PEV during the last 10 correct trials of these blocks and sessions was reduced compared to baseline blocks (SCH23390 during the first two post-injection blocks: mean = 0.007 ± 0.001 vs. baseline blocks from the same sessions: 0.033 ± 0.001, p =1×10−8). PEV was also reduced compared to the corresponding post-saline injection blocks (mean = 0.047 ± 0.001, p = 1×10−9). Moreover, after SCH23390, but not saline, the difference in firing rate between preferred and non-preferred directions during the cue period was reduced (Figure 3C). A two-way ANOVA of the firing rate during the cue period, with drug treatment (baseline, drug, washout) and preferred direction as factors, showed a significant effect of SCH23390 on both factors (with no interaction). A post-hoc test indicated a reduction of selectivity after SCH23390 (but not saline) via increased activity to the non-preferred direction (Bonferroni post-hoc test). During washout, neural selectivity began to recover (Figure 3B and 3C). Neural activity was more noisy than during baseline, but there was once again a significant difference between preferred vs. non-preferred directions (Figure 3C, T-test, p = 0.03). ROC analysis yielded similar results (Figure S2 and Supplemental Experimental Procedures).
As mentioned above, there was no difference in average activity across the whole neuron population following SCH23390 relative to saline. However, for SCH23390, there was a small, but significant, greater increase in overall activity of neurons that were selective during learning (see above) during the first 30 min post-injection (Figure 3D and example neuron in Figure S2; saline normalized activity raised to 1.18 ± 0.02 [5–30 min post-injection], n = 81; SCH23390 normalized activity raised to 1.41 ± 0.03, n = 78; Wilcoxon test, p = 4×10−7). Thus, task-selective neurons were more susceptible than non-task-selective neurons to D1R modulation. This increase persisted during the washout period, when behavior returned to normal.
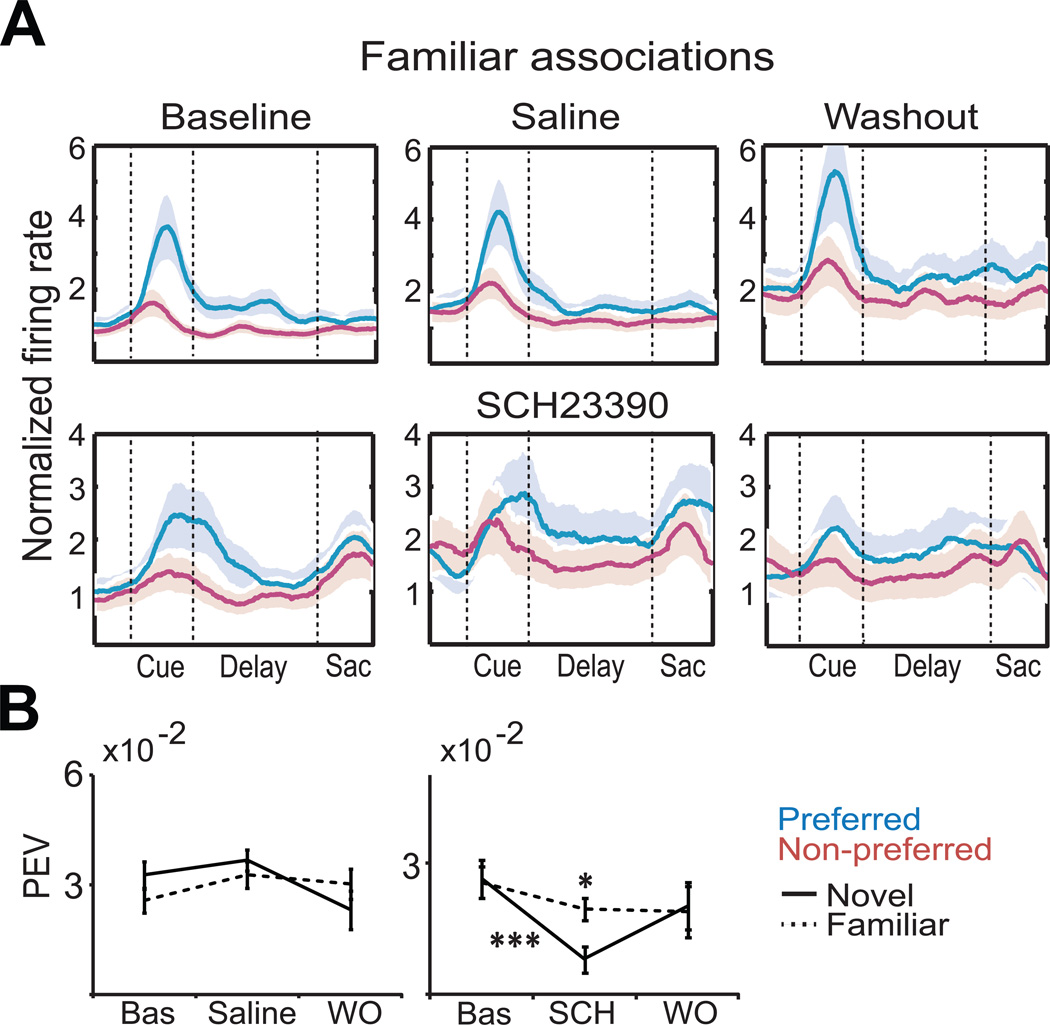
By contrast, neural selectivity to familiar associations was less affected by SCH23390. We examined the overlap of selectivity during novel and familiar associations in single neurons. We determined that ~35–40% of neurons with selectivity during learning also showed selectivity to familiar associations (Figure 4A; saline: 31 of 81 neurons [38.3 %], SCH23390: 26 of 78 neurons [33.3 %]; ANOVA during cue and/or memory delay, p < 0.05). Note that this percentage is a conservative estimate. Familiar cues were only shown to the animals on 20% of the trials, thus limiting statistical power. Thus, it is likely that many of the neurons with selectivity during learning were also selective during familiar associations. A two-way ANOVA of the PEV during the cue period, with drug treatment and novel vs. familiar association as factors, showed an effect of SCH23390 on both factors (with no interaction), indicating that blockade of D1Rs had a different effect on activity to novel vs. familiar associations. In fact, PEV was significantly larger during familiar than novel associations (Figure 4B, same neurons as in Figure 3B; PEV novel mean = 0.007 ± 0.001 vs. familiar mean = 0.017 ± 0.004; p = 0.027, Bonferroni post-hoc test). This was observed both when neurons were chosen for significant selectivity to novel associations (above) as well as when neurons were chosen for selectivity to familiar associations (Figure S3). In sum, SCH23390 reduced neural selectivity and PEV more during learning of novel associations than during performance of familiar associations.
Figure 4. Selectivity of Prefrontal Neurons is Less Dependent on D1 Receptors During Familiar Associations.
(A) Normalized activity for preferred (blue traces) and non-preferred (magenta traces) saccade directions of neurons selective to novel associations during familiar trials (same neurons as in Fig. 3).
(B) Quantification of PEV (cue epoch) of neurons selective to novel associations during familiar trials in saline and SCH23390 sessions. Two-way ANOVA with Bonferroni post-hoc test. Shown is the mean and SEM. See also Figure S3.
Dopamine D1 Receptor Blockade Increases Spike Synchronization and the Power of Alpha and Beta Oscillations
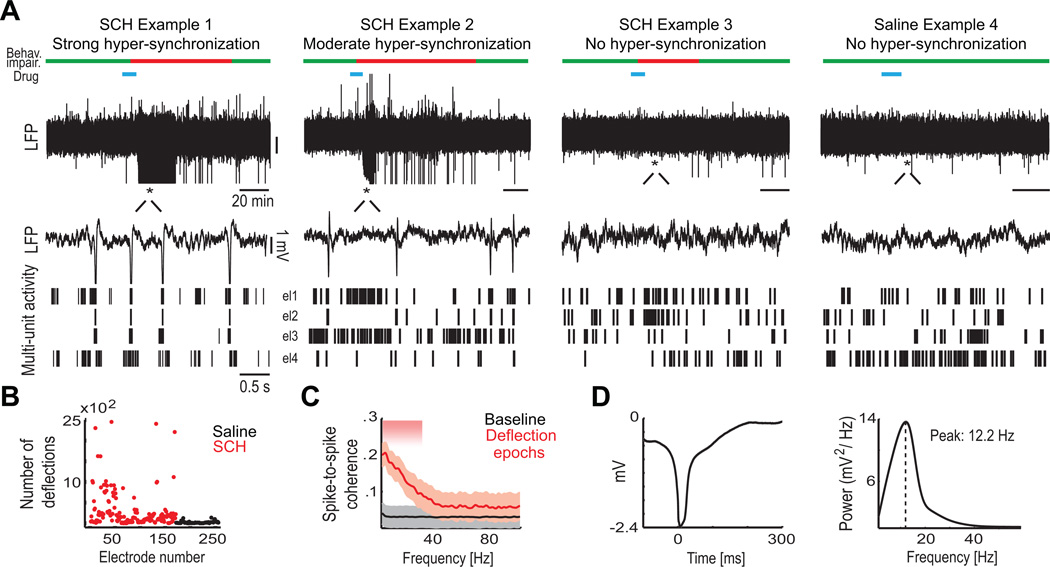
In 95 of 163 recording sites (58% of electrodes in all SCH23390 sessions), SCH23390 generated large-amplitude sharp deflections in LFPs. Monkeys never stopped working during these episodes. Deflections were downward in 72% of sites, the remainder was upward. Previous studies have shown that the polarity of LFP signals may vary as a function of cortical layer (e.g. see Kajikawa and Schroeder, 2011, in monkey; Kandel and Buzsáki, 1997, in rat).
We detected deflections with an amplitude threshold. The total number of deflections during post-SCH23390 injection periods varied across sites (Examples 1–3 in Figure 5A) but were rare after saline injections (Example 4 in Figure 5A). Typically, the largest number of deflections appeared shortly after the end of the injections and lasted on average 18 ± 5 minutes (range 10–30 min) while the learning impairment lasted about one hour (see above). The duration of the learning deficit did not correlate with the total number of deflections observed after the injections (Pearson's correlation, R-squared 0.02, p = 0.34) nor the proportion of siteswith deflections (R-squared 0.14, p = 0.09). In fact, in eight sessions without deflections on any recording site, monkeys still had severe learning deficits. Thus, the deflections per se were not sufficient for the learning impairment. As Figure 5B shows, deflections were common in the first 20 minutes after SCH23390 injection (mean total deflections per site= 394, range 0 to 3608) but were rare following saline (mean total deflections per site= 7, range 1 to 14).
Figure 5. Blockade of Prefrontal Dopamine D1 Receptors Generates Deflections in LFPs and Increases Neuron Synchronization.
(A) Top, The injection of SCH23390 in the PFC generated large amplitude deflections in 58% of the electrodes. Shown are examples of LFP traces recorded from three SCH23390 sessions and one saline session. Also shown are the time-course of the learning impairment (green lines indicate no impairment, red lines indicate impairment), and the time of the SCH23390 and saline injections (blue lines). Asterisks mark the location of the 4-second segments magnified in the bottom panels. Bottom, The deflections correlated with spike bursts of neurons recorded from multiple electrodes. Shown are 4-second segments of LFP traces and multi-unit activity from 4 electrodes (el1 to el4) from each of the examples shown in Figure 5A top panels. Spike bursts were followed by a strong rebound inhibition. Each line corresponds to a single action potential.
(B) Number of post-injection deflections in all recording sites for the first 20 minutes after saline (black) or SCH23390 (red). Deflections were detected in many electrodes after SCH23390, but were rarely observed after saline.
(C) Spike-to-spike coherence increased during epochs with deflections compared to baseline (n = 139 pairs of multi-unit activity sites, all pairs at 1 mm distance have been included, SCH23390 sessions with deflections only), indicating an augmented neuron synchronization. Shaded area indicate significance with the Wilcoxon test (p < 0.05). Confidence intervals depict a Jackknife test.
(D) Average deflection and power spectrum of 400 ms windows centered on each deflection (100 ms before to 300 ms after the initial sharp voltage change; n = 11330 deflections, 28 electrodes from 5 sessions). Dashed line marks the peak at 12.2 Hz. The power spectrum was computed by using the multi-taper method (time-bandwidth product TW = 2 and k = 3 tapers; see Experimental Procedures). See also Figure S4.
Deflections were associated with spike bursts of neurons. Figure 5A bottom panels show 4-second segments of LFP traces and multi-unit activity from 4 electrodes from each of the examples shown in Figure 5A top panels. Note that there is increased spiking activity in close temporal proximity with the deflections, suggesting that deflections might be 'population spikes' generated by the hyper-synchronization of neurons. In fact, there was an increase in spike-to-spike coherence during epochs of deflections relative to baseline epochs from the same sessions (Figure 5C; n = 139 pairs of multi-unit activity sites across electrodes pairs at 1 mm distance, SCH23390 sessions with deflections only; Wilcoxon test, p < 0.05).
Figure 5D shows an average deflection (n = 11330 deflections, 28 electrodes from 5 sessions) and the power spectrum of the LFP trace over 400 ms windows centered on each deflection (100 ms before to 300 ms after the initial sharp voltage change). It revealed a large peak at alpha frequencies (12.2 Hz) and indeed, the power spectra of the LFPs during strong deflection episodes showed a marked increase in alpha oscillations (~12 Hz) (see Figure S4 for an example).
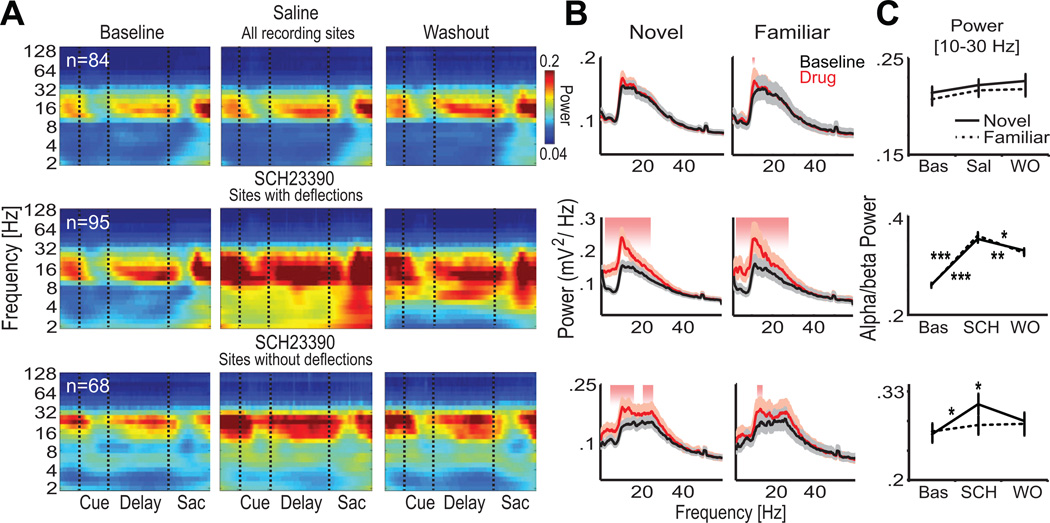
We compared LFP oscillatory power on correct trials during baseline blocks with the post-injection blocks separately for sessions or recording siteswith and without deflections. In baseline blocks, there was a prominent alpha/beta band (10–30 Hz) during the fixation, delay and saccade execution epochs (Figure 6A and 6B). During post-injection blocks following injection of SCH23390 (n = 163 electrodes), but not saline (n = 84 electrodes), there was an increase in the power of oscillations below 30 Hz compared to baseline blocks. The deflections have an alpha component (see above) so, naturally, siteswith deflections (n = 95) showed an increase in alpha band (10–14 Hz) after SCH23390 and also in beta band (14–30 Hz) for novel and familiar associations over baseline blocks (Figure 6B, Wilcoxon test, p < 0.05, shaded areas; and Figure 6C, last 20 trials/block). Importantly, this increase in low frequency power in the LFPs was still observed in siteswithout deflections (n = 68, Figure 6B) indicating that the SCH23390-induced increase in low frequency oscillations was not due to the deflections alone. This increase was more pronounced for novel than familiar associations in siteswithout deflections (Figure 6B and 6C; Wilcoxon test, p < 0.05). The increase in alpha/beta oscillations was also observed in sessions without deflections on any recording site (Figure S5), and was greater at sites where blockade of D1Rs impaired learning compared to areas where learning was intact (Figure S5).
Figure 6. Blockade of Prefrontal Dopamine D1 Receptors Increases the Power of Alpha and Beta Oscillations.
(A) Time-frequency representation of the average LFP power using wavelets for correct trials during baseline, drug and washout blocks. Separate spectrograms are shown for saline recording sites, and SCH23390 sites with and without deflections. LFP components phase-locked to the visual cues have been subtracted. The alpha and beta (10–30 Hz) bands present during baseline blocks are exaggerated after SCH23390 in all sites.
(B) The spectral analyses of entire trial epochs (2.5 s) showed that alpha and beta oscillations are greater after SCH23390, but not saline, in post-injection blocks (red) relative to baseline blocks (black). This increase occurred in sites with and without deflections. Alpha oscillations were particularly enhanced in sites with deflections, likely due to the contribution of the deflections to alpha power. Shaded areas indicate significant differences in power between post-injection and baseline blocks (Wilcoxon test, p < 0.05). Power spectra were computed by using the multi-taper method (time-bandwidth product TW = 2 and k = 3 tapers; see Experimental Procedures) and were normalized by the frequency. Confidence intervals depict a Jackknife test.
(C) Quantification of alpha and beta power during novel and familiar associations. During novel associations, alpha and beta power increased significantly after SCH23390 in sites with and without deflections. During familiar associations, this increase was only observed in sites with deflections. Wilcoxon test, *, p < 0.05; **, p < 0.01,***, p < 0.001. Shown is the mean and SEM. See also Figure S5.
Discussion
Our findings indicate that dopamine D1 receptors in the monkey lateral PFC are likely to be involved in learning new cue-response associations but less involved in performance of familiar associations. Following the injection of a D1R antagonist, especially in the ventrolateral PFC, monkeys learned new cue-response associations much more slowly, whereas performance of highly familiar associations was intact. Two not mutually exclusive possibilities may account for this dissociation: 1) familiar associations are not dependent on prefrontal D1Rs, and 2) they are dependent on another brain area, such as the striatum, where they could be encoded as habits (Graybiel, 2008).
Although we cannot conclusively say that the effects were due to a local action of SCH23390 in the lateral PFC, some evidence suggests that this is so. First, the lateral PFC is a large expanse of cortex and the drugs were injected virtually in the middle of it. Second, the effects began immediately during the long, slow, injection. If the site of action were elsewhere, it would take time for the drug to diffuse to those sites. Instead, we observed that the effects began few minutes after the start of the injection. Third, not all sites in the lateral PFC produced an effect, as might be expected if the drug had widespread actions (see Experimental Procedures); most vlPFC, but not dlPFC, sites resulted in impairment. There are similar levels of D1R expression in these areas (de Almeida et al., 2008), so this may reflect the greater number of vlPFC than dlPFC neurons with selectivity during conditional visuomotor tasks (Wilson et al., 1993), and thus suggests the vlPFC as the site of action. Finally, while SCH23390 has a preference for binding to D1Rs, it also binds to serotonin 5-HT2 and 5-HT1C receptors (Bischoff et al., 1988; Alburges et al., 1992). Thus, we cannot disregard the possibility that some of the SCH23390 effects are mediated in part by these receptors. The specificity of D1 receptors could be established by injecting several 5-HT antagonists, but conducting these experiments would be difficult in monkeys. Importantly, our neurophysiological results are in line with previous studies where D1R specificity could be established. For example, a previous study in monkeys (Sawaguchi and Goldman-Rakic, 1994) showed that local injections of ketanserin, a selective antagonist of 5-HT2 receptors, near PFC sites affected by SCH23390 failed to induce any clear changes in the monkeys' performance, suggesting that SCH23390 was acting on D1Rs.
Studies in humans indicate that prefrontal D1Rs are involved in working memory (Robbins, 2000). Muller et al., (1998) demonstrated performance-enhancing effects of a mixed D1-D2 agonist on spatial working memory, but no effect of a selective D2 agonist, suggesting a role for D1Rs. Studies in monkeys and rodents have shown that modulation by D1Rs in the dlPFC and prelimbic cortex during spatial working memory follows an inverted-U-shaped curve: too little or too much D1R stimulation causes cognitive impairment, while moderate levels of D1R stimulation strengthen and sculpt selectivity to optimize PFC function (Williams and Goldman-Rakic, 1995; Zahrt et al., 1997; Seamans et al., 1998; Granon et al., 2000; Chudasama and Robbins, 2004; Vijayraghavan et al., 2007). Our results suggest that prefrontal D1Rs in the monkey vlPFC play a relevant role in associative learning, perhaps by sculpting neural selectivity of prefrontal neurons.
D1Rs in the lateral PFC may play a role in cognitive flexibility as well. The PFC seems key for different types of behavioral flexibility and response inhibition, although the exact subregions involved have been debated (Dias et al., 1996; Dias et al., 1997; Ragozzino et al., 1999; Chudasama et al., 2003; Floresco et al., 2008; Aron, 2010; Dalley et al., 2011). In rats, local injections of SCH23390 in the medial PFC, an area that resembles in connectivity and function to the lateral PFC in monkeys, increased perseveration to the previously learned strategy (Ragozzino, 2002), similar to our finding of a moderate but significant increase in perseverative errors.
The reduction in neural selectivity induced by SCH23390 was more pronounced for novel than familiar associations in single neurons. This suggests that the synapses that modify with new learning are modulated by D1Rs and are separate from those involved in encoding of familiar associations. This supports recent in vitro work suggesting that long-term potentiation (LTP), a cellular mechanism of synaptic plasticity thought to be critical for learning and memory consolidation, is D1R-dependent (Xu and Yao, 2010). D1Rs may modulate reward-dependent plasticity of cortico-striatal synapses. Increases of dopamine release may strengthen the efficacy of cortico-striatal synapses after reward while dopamine decreases may weaken synapses for non-reward (Hikosaka et al., 2006; Hong and Hikosaka, 2011). Our results suggest this may also occur in the PFC, because during D1R blockade neurons failed to achieve the learning-induced level of selectivity seen for familiar associations (as they do without blockade). Without the influence of D1Rs, there might be no potentiation of the synaptic strength necessary for learning, and behavior might then be captured by non-D1R plasticity mechanisms that strengthen the most recently activated pathways, resulting in increased perseveration. During familiar associations, synaptic strength might be already potentiated and thus less dependent on D1Rs. It is plausible that familiar associations are encoded in structures other than the PFC. However, the fact that neural selectivity (and PEV) during familiar associations is still partly reduced by the D1R antagonist supports the co-existence of D1R-sensitive and D1R-less-sensitive sets of synapses on single prefrontal neurons.
Neural selectivity and PEV during washout periods did not return to the exact same state as the baseline before the drug was injected. Neural information returned but was more variable and neurons continued to show higher baseline firing rates. It is likely that SCH23390 had lingering effects on neural activity that could have lasted hours. However, as our analyses demonstrate, in contrast to the drug period in which neural information about the associations was virtually gone from the PFC, there was a return of neural information during the washout period that could have supported behavioral performance.
The decrease in neural selectivity seemed mostly due to an increase in activity to non-preferred saccade directions. This agrees with recent data showing that low levels of D1R stimulation enhances spatial tuning by decreasing responses to non-preferred directions during a spatial working memory task (Vijayraghavan et al., 2007). Thus, both during learning and working memory prefrontal D1Rs sculpt neural selectivity by reducing the activity for non-preferred directions, supporting a role for D1Rs in increasing signal-to-noise ratio by reducing neural noise (Arnsten, 2011). Modeling studies have proposed that these sculpting actions of D1Rs facilitate the acquisition and stabilization of memory representations by preventing responses to interfering stimuli (Durstewitz et al., 2000; Seamans and Yang, 2004; Floresco and Magyar, 2006). Indeed, we found that during D1R blockade, monkeys needed more correct trials to learn the associations, that is, they had to repeat the cue-reward contingencies more times to acquire and stabilize the new rule.
Hypostimulation of D1Rs increased spike synchronization and neural oscillations in the lateral PFC. During associative learning, alpha/beta oscillations predominated. Blockade of D1Rs increased the power of this band. In addition, shortly after SCH23390 injections, large-amplitude deflections were observed in the LFP signals in almost 60% of the recording sites, together with a strong increase in the power of alpha oscillations. The shape and irregularity of the sequences of deflections, and the long duration of deflection epochs, suggest that they were not full seizures (Steriade, 2006; Suntsova et al., 2009). In fact, a recent study has found that during seizures in epilepsy patients neural spiking activity decreases dramatically (Truccolo et al., 2011). This was not observed in our study. However, the deflections could have been a reflection of ongoing micro-seizures, recently found in epileptic patients to be associated with hyper-excitability (Schevon et al., 2008, 2011). Alpha/beta oscillations were also increased in electrodes without deflections, especially during learning of novel associations. This indicates that the SCH23390-induced increase in these oscillations was not due to the deflections alone. Increase in alpha rhythms has been associated with inattention (Fries et al., 2001; Bollimunta et al., 2011), and is thought to reflect decreased excitability to protect task-relevant information from interference (Jensen et al., 2002). Thus, it is possible that D1R blockade impairs learning by forcing the PFC into an “inattentive mode” that disrupts the development of learning related neural selectivity. The increase in beta rhythms is consistent with the aberrant hyper-synchronization proposed to underlie some neurological and psychiatric disorders such as Parkinson's disease and schizophrenia, where exacerbated beta oscillations have been observed (Uhlhaas and Singer, 2006; Wang, 2010). Further, altered dopamine neurotransmission in the PFC has been reported for these disorders (Knable and Weinberger, 1997; Okubo et al., 1997; Kulisevsky, 2000; Abi-Dargham et al., 2002; Mattay et al., 2002). Our findings demonstrate that a selective blockade of prefrontal D1Rs can generate these aberrant alpha/beta oscillations, suggesting that the cognitive deficits observed in these disorders may be partly caused by hypostimulation of prefrontal D1Rs.
In summary, blockade of D1Rs in the monkey lateral PFC impairs associative learning but not performance of familiar associations. This selective learning impairment appears to be caused by a reduction of learning-related neuron selectivity and increased alpha/beta oscillations associated with inattention and cognitive deficits. These results may have important implications for our understanding of how low stimulation of prefrontal D1Rs contributes to cognitive deficits in aging and in neurological and psychiatric disorders.
Experimental Procedures
Behavioral Task
We trained two rhesus monkeys (Macaca mulatta; LA and LK) in a delayed associative learning task (Figure 1). Animal protocols were approved by the National Institutes of Health and the Massachusetts Institute of Technology Animal Care and Use Committee. Under general anesthesia, each monkey was implanted with a head bolt to immobilize the head and a recording chamber on top of the left lateral PFC. All surgeries were performed under aseptic conditions with postoperative antibiosis and analgesia. Eye position was tracked optically with an infrared camera (EyeLink 1000 system). Stimuli were projected onto a screen 45 cm from the monkeys. Trials (Figure 1A) began when the monkey fixated at a central white dot (± 2.0 degrees). After 800 ms of fixation, one of 4 possible cues was presented centrally for 500 ms. Monkeys were required to fixate for 1000 ms until the fixation dot disappeared ('go' signal), and make a saccade to one of the two white dots positioned horizontally at ± 8.5 degrees eccentricity. Correct saccades were rewarded with drops of juice. Trials were aborted if the monkeys broke fixation before the presentation of the target dots (early trials). Impulsive trials were early trials where a saccade was implemented to the correct target. Trials were combined in blocks, each block comprised of two novel cues on 80% of the trials (learning trials) and two highly familiar cues (>1.5 years of training) on 20% of the trials (familiar trials). New cues replaced the novel ones after monkeys reached learning criterion: 30 correct trials for each novel cue and ≥ 80% performance over the last 10 consecutive trials per cue. Performance of familiar associations was not taken into consideration for this criterion. After reaching criterion, a new block started and monkeys learned a new pair of associations. Stimulus presentation and behavioral monitoring were controlled using two computers running the CORTEX real-time control system. The fixed memory delay (1000 ms) allowed monkeys to anticipate the time of target onset, and likely resulted in overall shorter reaction times than unpredictable delay.
The mean behavioral performance for each block was estimated from the monkey's binary responses (correct or incorrect) using a binomial (logistic) regression model (Williams and Eskandar, 2006). The fitted sigmoid curves were used to compare distributions with a Kolmogorov-Smirnov test. The learning rates were estimated from the slopes of the sigmoid curves. The duration of the learning impairment after SCH23390 was measured as the sum of the duration of post-injection blocks showing slower learning curves and smaller learning rates than baseline blocks.
Neural Recordings
Electrode penetration sites were determined using MRI scans obtained before surgery. The recording chamber was positioned stereotaxically over the left lateral PFC of each animal overlying the principal sulcus (i.e. the dorsolateral and ventrolateral portions of the PFC were equally accessible). The location of the principal sulcus could also be mapped out neurophysiologically (absence of cells and low amplitude LFP signals). Electrophysiological signals were recorded simultaneously from 7–15 dura-puncturing tungsten microelectrodes (FHC Instruments), located 1 or 2 mm away from the infusion cannula. Electrodes were lowered each day either independently or in pairs, and were advanced using custom-made screw-driven mini-microdrives mounted on a plastic grid (Crist Instruments) with spacing of 1 mm between adjacent locations. Neuronal activity was amplified, filtered, and stored using an integrated multichannel recording system (Plexon Neurotechnology Research Systems). To minimize any sampling bias of neuronal activity, we did not pre-screen activity for any visual responsiveness. Electrodes and cannula were advanced until the activity of neurons was isolated well from several electrodes, and then data collection began. From each electrode, we simultaneously recorded spiking activity and the local field potential (LFP). Both signals were referenced to ground. The spike signal (passband 154 Hz to 8.8 kHz) was threshold-triggered to separate neuronal spikes from background noise, and individual spike waveforms were stored at 40 kHz. LFPs (passband 0.7 Hz to 300 Hz) were recorded continuously with a sampling rate of 1 kHz.
Pharmacology
Post-injection blocks were classified as washout when learning was unimpaired (not different from baseline). We note that this was not dependent on a literal washout of the drug, nor a recovery of neural activity to the baseline state.
The dopamine D1-like receptor antagonist SCH23390 was purchased from Sigma/RBI and dissolved in commercially available sterile saline (0.9% NaCl) at 10 µg/µl under strict sterile conditions and stored at −20° C. The pH was corrected to be around 6.0. For control experiments, we used commercially available sterile saline (pH 5.5). The day of the recording, an aliquot of SCH23390 was thawed. A plastic tube (Tygon microbore) was chemically sterilized and connected to a sterile cannula that had been previously attached to a microdrive on the recording grid. Cannulas were Hamilton needles (30 GA, I.D. 0.16 mm and O.D 0.31 mm) with bevels of 45°. The grid with electrodes and cannula were then connected through the plastic tube to a 10 µl Hamilton syringe mounted on a Harvard Apparatus pump. A little air bubble was left between the tube and the syringe. The bubble was carefully monitored throughout the experiment to control for changes in pressure inside the tube or leaks. The cannula was lowered into the cortex with a microdrive that had an electrode attached at 1 mm distance. The neuronal activity recorded from this electrode was an indication that the cannula was in close proximity to active neurons. At the time of the injection the pump was manually turned on. Injections consisted of 3 µl of saline or 30 µg of SCH23390 at an infusion rate of 0.3 µl/min. The concentration and volume were chosen according to previous studies (Sawaguchi and Goldman-Rakic, 1991; Sawaguchi and Goldman-Rakic, 1994; Nakamura and Hikosaka, 2006). During the injection, we carefully monitored any changes in firing rate of the neurons. Typically, one or two neurons (of 15–30) showed changes in spiking that elapsed once the injection had finished. In a few sessions, we could not keep track of one or two neurons after the injection, and these neurons were not included in the analysis. In 20 sessions, large amplitude deflections were observed in the LFP signals shortly after the injection of SCH23390, an indication that the drug had been successfully delivered into the brain. Altogether, the consistent movement of the air bubble inside the tube, and the changes of activity and/or LFP signals during or shortly after the infusions, were good references for successful injections.
We did not perform any histology to measure the exact spread of SCH23390 inside the brain. The analysis of the data suggests that it had spread at least 2 mm (~4 mm3), since large negative deflections were observed in electrodes located 2 mm away from the injection site. A previous study in rats (Granon et al., 2000) reported that 0.5 µl of a radioligand of SCH23390 infused in the rat PFC, at a similar infusion rate as ours, spreads up to 6 mm3, but with substantial dilution. This suggests that in case SCH23390 had spread outside the lateral PFC, its effective concentration would have been compromised.
Spike Sorting Quality
We examined whether the injections altered the quality of waveform sorting. Offline Sorter (Plexon Technologies) was used to separate spikes from noise and to sort spikes from different neurons recorded from the same electrode. The waveforms of each neuron were manually classified in different clusters using principal component analysis (PCA). Sessions were then divided in segments of 15 min (roughly the duration of a block), and sorting quality statistics were performed segment by segment. The degree to which the unit clusters were separated in 2D and 3D space was determined by a Multivariate Analysis of Variance test (MANOVA) in Offline Sorter. Small p-values (<0.05) in this test indicate that each of the unit clusters has a statistically different location in 2D/3D space. We only used waveforms from electrodes where p-values were smaller than 0.05 in all the segments, i.e. the clusters were well separated before and after the injections. In general, even though some of the clusters moved slightly after the injection, p-values were very small (<10−3).
Spike-Rate Analysis
All spike-rate analyses were performed with custom software written in MATLAB (Mathworks). We note that our analyses focus on effects across neuron populations, not examples of individual neurons. As in previous studies (Asaad et al., 1998; Pasupathy and Miller, 2005), balanced analyses of variance (ANOVAs) were conducted on the spiking activity during two epochs of the trial: 'cue' (100–600 ms after cue onset) and 'delay' (100–800 ms after cue offset). Neurons displaying direction selectivity showed statistically different firing rates for preferred vs. non-preferred directions during 'cue' and/or 'delay' epochs in baseline blocks after learning (last 20 correct trials per novel association before block switch). Firing rates for preferred and non-preferred directions of all selective neurons were normalized by the mean firing rate during fixation (200 ms before the cue onset). Saccade direction selectivity was quantified as the fraction of each neuron's variance explained by saccade direction (one-way ANOVA, direction variance / [direction variance + error variance]; percent explained variance or PEV). To quantify changes during learning, PEV was calculated for each neuron across an eight-trial window (8 correct trials for right vs. 8 correct trials for left associations), slid in one-trial steps and 100 ms time window within a trial, over the first 30 correct trials per association (the minimum block length). To correct for biases in PEV values, we performed a randomization test on each neuron and time point by randomly shuffling trials between the two directions and permuting this randomization 1000 times. PEV-shuffle was then subtracted from PEV (Siegel et al., 2009; Buschman et al., 2011). We also computed the Receiver Operating Characteristic (ROC) area under the curve (Figure S2 and Supplemental Experimental Procedures) (Buschman and Miller, 2007; Histed et al., 2009). This test reflects how well one can predict the saccade direction based on the firing rate of a given neuron. ROC was shuffle-corrected in the same way as PEV.
Spectral Analysis
To investigate which LFP activity reflects signal components independent to trial events, we subtracted from each trial the LFP signal averaged across all trials. This removed stimulus-locked responses such as evoked potentials. Sixty hertz noise was digitally filtered with a Butterworth filter. Spectrograms were built using a continuous wavelet transform with a Morlet function as mother wavelet, center frequencies between 1 to 128 Hz in 0.25 octave steps (Torrence and Compo, 1998; http://paos.colorado.edu/research/wavelets). Power spectra and spike-to-spike coherence were computed using the multi-taper method described elsewhere (www.chronux.org). Fourier transform was applied to the tapered time series signal. We used an optimal family of orthogonal tapers (slepian functions). These are parameterized by their time length T and frequency bandwidth W. For chosen T and W, maximally k = 2TW-1 tapers centered in frequency are appropriate for spectral estimation. Power spectra were estimated over 0.4 s windows centered on deflections (Figure 5D) and correct trials of 2.5 s (Figure 6B) with time-bandwidth product TW = 2 and k = 3 tapers. The same parameters were used for measuring spike-to-spike coherence during baseline and epochs with the largest number of deflections. To enhance readability of the LFP power at high frequencies, which are masked by the 1/fn power-law decay, we normalized the power by the frequency.
Supplementary Material
Highlights.
Prefrontal D1 receptors are likely involved in learning but not memory
Prefrontal D1 receptors sculpt neural selectivity during learning
Neural selectivity for familiar associations is less dependent on D1 receptors
Alpha/beta oscillations are exacerbated during low prefrontal D1 receptor stimulation
Acknowledgements
We thank E. Antzoulatos, M. Bosch, S. Brincat, T. Buschman, J. Cromer, C. Diogo, M. Moazami, J. Rose, J. Roy, M. Silver, and M. Wicherski for valuable discussions on the manuscript. We also thank B. Gray, K. MacCully, M. Noble and D. Ouellette for technical assistance, and R. Marini for surgical assistance and veterinary care. This work was supported by CELEST, a National Science Foundation Science of Learning Center (NSF OMA-0835976), NIH-NINDS R01-NS035145, and the Human Frontiers Science Program Organization (to M.V.P).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
M.V.P conceived of and designed experiment. M.V.P performed (and E.K.M supervised) training, electrophysiological recording, and data analysis. M.V.P and E.K.M wrote the paper.
The authors declare no competing financial interests.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alburges ME, Hunt ME, McQuade RD, Wamsley JK. D1-receptor antagonist: comparison of [3H]SCH39166 to [3H]SCH23390. J. Chem. Neuroanat. 1992;5:357–366. doi: 10.1016/0891-0618(92)90051-q. [DOI] [PubMed] [Google Scholar]
- Antzoulatos EG, Miller EK. Differences between neural activity in prefrontal cortex and striatum during learning of novel abstract categories. Neuron. 2011;71:243–249. doi: 10.1016/j.neuron.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol. Psychiatry. 2011;69:e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic network connectivity: A new form of neuroplasticity. Trends Cogn. Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry. 2010;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Güntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr. Opin. Neurobiol. 2011;21:475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bischoff S, Heinrich M, Krauss J, Sills MA, Williams M, Vassout A. Interaction of the D1 receptor antagonist SCH 23390 with the central 5-HT system: radioligand binding studies, measurements of biochemical parameters and effects on L-5-HTP syndrome. J. Recept. Res. 1988;8:107–120. doi: 10.3109/10799898809048981. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J. Neurosci. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Siegel M, Roy JE, Miller EK. Neural substrates of cognitive capacity limitations. Proc. Nat. Acad. Sci. 2011;108:11252–11255. doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical {gamma} synchrony and cognitive control in schizophrenia. Proc. Nat. Acad. Sci. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav. Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacol. 2004;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr. Opin. Neurobiol. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Dalley J, Everitt B, Robbins T. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- de Almeida J, Palacios JM, Mengod G. Distribution of 5-HT and DA receptors in primate prefrontal cortex: implications for pathophysiology and treatment. Prog. Brain Res. 2008;172:101–115. doi: 10.1016/S0079-6123(08)00905-9. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav. Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence fom 'on-line' processing. J. Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J. Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Elvevåq B, Goldberg T. Cognitive impairment in schizophrenia is the core of the disorder. Crit. Rev. Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav. Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacol. 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacol. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J. Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy O. Frontal syndrome and disorders of executive functions. J. Neurol. 2003;250:1–6. doi: 10.1007/s00415-003-0918-2. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J. Neurosci. 2000;20:1208–115. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J. Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Histed MH, Pasupathy A, Miller EK. Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron. 2009;63:244–253. doi: 10.1016/j.neuron.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Dopamine-mediated learning and switching in cortico-striatal circuit explain behavioral changes in reinforcement learning. Front. Behav. Neurosci. 2011;5:1–17. doi: 10.3389/fnbeh.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb. Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, Schroeder CE. How local is the local field potential? Neuron. 2011;72:847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J. Neurosci. 1997;17:6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr. Opin. Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Knable MB, Weinberger DR. Dopamine, the prefrontal cortex and schizophrenia. J. Psychopharmacol. 1997;11:123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J. Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson's disease. Drugs Aging. 2000;16:365–379. doi: 10.2165/00002512-200016050-00006. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J. Neurosci. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Lizio R, Vecchio F, Frisoni GB, Ferri R, Rodriguez G, Babiloni C. Electroencephalographic rhythms in Alzheimer's disease. Int. J. Alzheimers Dis. 2011;2011:927573. doi: 10.4061/2011/927573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg T, Chase TN, Hyde TM, Weinberger DR. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann. Neurol. 2002;51:156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muller U, von Cramon DY, Pollmann S. D1- versus D2-receptor modulation of visuospatial working memory in humans. J. Neurosci. 1998;18:2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J. Neurosci. 2006;26:5360–5369. doi: 10.1523/JNEUROSCI.4853-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC. Biologically based computational models of high-level cognition. Science. 2006;314:91–94. doi: 10.1126/science.1127242. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch. Gen. Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J. Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D1 receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn. Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp. Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos. Trans. R. Soc. Lond. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;22:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J. Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann G, Waziri A, Branner A, Sosunov A, Schroeder CE, Emerson RG. Microphysiology of epileptiform activity in human neocortex. J. Clin. Neurophysiol. 2008;25:321–330. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Goodman RR, McKhann G, Emerson RG. Propagation of epileptiform activity on a submillimeter scale. J. Clin. Neurophysiol. 2011;27:406–411. doi: 10.1097/WNP.0b013e3181fdf8a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc. Nat. Acad. Sci. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J. Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Suntsova N, Kumar S, Guzman-Marin R, Alam MN, Szymusiak R, McGinty D. A role for the preoptic sleep-promoting system in absence epilepsy. Neurobiol. Dis. 2009;36:126–141. doi: 10.1016/j.nbd.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J. Neurosci. Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. A practical guide to wavelet analysis. Bull. Amer. Meteor. Soc. 1998;79:61–78. [Google Scholar]
- Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS. Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 2011;14:635–641. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AFT. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb. Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat. Neurosci. 2006;9:562–568. doi: 10.1038/nn1662. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.