Abstract
The cellular basis of brain imaging is one of the most important frontiers in current neuroscience. In this issue, Petzold et al analyze a model system, the olfactory glomerulus, showing how neurovascular coupling involves an elaborate dance between axon terminals, pre and postsynaptic dendrites, glial cells, and the capillary network.
Brain imaging has had an enormous impact on our understanding of brain function, but the mechanisms that produce the images are only starting to be addressed. Beginning a few years ago (Raichle and Mintun, 2006), the problem of neuro-vascular coupling between neural activity and the changes in blood flow that underlie functional brain imaging has become a growth industry. However, the industry has a very deep problem: what is the cellular basis of the coupling? The paper by Petzold et al in this issue is timely for highlighting the neurodrama that is being played out to answer this question.
From the introduction of 2-deoxyglucose (Kennedy et al., 1975) and the related positron emission tomography (PET), the fundamental questions were recognized: was the activity labelled by the method due to: activity in neurons or glia? axons or dendrites? action potentials or synaptic potentials? synaptic excitation or inhibition? At first it seemed that, at least in cerebral cortex, the answer would be relatively simple: synaptic potentials generate the largest ionic currents, and therefore require the most energy for pumping them back. This idea was supported by early work in various brain regions.
The olfactory bulb, with its strict lamination and separation of cellular elements, provided a model system that showed the answer was more complicated. The most intense glucose uptake was indeed in the glomerular layer, where all the afferent axons make their terminals, but there was also uptake in the olfactory nerve layer, containing millions of unmyelinated axons (Sharp et al., 1977). High resolution studies were able to reveal uptake over single presumed glial cells, indicating a glial link in the coupling of action potential traffic to glucose uptake from the vasculature ((Benson et al., 1985)).
The proximate stimulus for the Petzold study was the recent series of papers on the cortex and the olfactory bulb from several laboratories which have used local field potentials and two-photon imaging to show that local functional hyperemia is correlated with neuronal input and synaptic responses (Chaigneau et al., 2003; Chaigneau et al., 2007; Lauritzen, 2005; Logothetis and Wandell, 2004) or spiking activity of principal neurons ((Rees et al., 2000)) or both (Mukamel et al., 2005). Astrocytes entered the cast of characters with the demonstrations that they can be involved in arteriolar constriction (Mulligan and Mavicar 2004) or dilatation (the latter by mGluRs and prostaglandins: (Zonta et al., 2003) in both in vitro and in vivo preparations (Takano et al., 2006). The classical in vitro model places astrocytes in a key position for supplying glucose and oxygen from the vasculature for energy metabolism, the astrocyte-neuron lactate shuttle, and the glutamate-glutamine cycle (Pellerin et al., 2007).
Contemporaneously with these studies has been a parallel series of studies of the olfactory bulb with new methods, including intrinsic optical imaging and multiphoton laser scanning microscopy. These have had the motivation to take advantage of the unique properties of the glomerulus: the sharply defined anatomical boundaries; the high convergence of unimodal input axons; the excitatory glutamatergic actions of their terminals onto the distal dendrites of olfactory bulb neurons; and the dense synaptic neuropil of the glomerular interior, lacking in cell bodies - the same advantages that motivated the earlier studies.
With two-photon microscopy in the anesthetized rat it was established that odor stimulation evokes changes in single capillary blood flow (increases or decreases in RBC velocity and density) that are odor and glomerulus specific (Chaigneau et al, 2003). These local vascular changes appear to delimit precisely the regions of synaptic activation as determined by local field potentials.
Insight into neurovascular coupling within the glomerulus has come from a study using intrinsic optical imaging and pharmacological manipulations (Gurden et al, 2006). This provided evidence that odor-evoked intrinsic signals are tightly coupled to release of glutamate; they independent of postsynaptic glutamate responses but inhibited by blockers of astrocytic glutamate transporters. This suggested a model in which the astrocytes generate the intrinsic signals by light scattering due to cellular swelling and blood flow changes, in parallel with but independently of postsynaptic neuronal responses (Gurden et al., 2006). In further experiments with TPLSM, rats and G-Camp2 mice, Chaigneau et al (2007) recently found that blocking synaptic transmission in a single glomerulus did not affect local odor-evoked vascular responses, consistent with a presynaptic coupling to neurovascular responses.
However, local TTX, to block neural activity, did not block vascular responses either. This and related experiments led them to evidence suggesting that when odor stimulation activates many glomeruli, silencing one of them does not block neurovascular coupling in a single glomerulus. Thus, measuring the LFP at a single site may not adequately monitor neurovascular coupling within a whole system: “the role of postsynaptic activation in triggering neurovascular coupling cannot be definitely established using local blockade of synaptic transmission” (Chaigneau et al, 2007).
Petzold have carried this approach a step further by using mice in which synaptophluorin (spH) is expressed in the presynaptic olfactory sensory neurons. It thus serves as a marker for presynaptic activity in the form of release of synaptic vesicles at these glutamatergic synapses. Combined with markers for postsynaptic responses and the use of pharmacological blockers, it enabled them to discriminate between the coupling of presynaptic and postsynaptic activity to vascular changes. In a tour de force, the methods include two photon laser scanning microscopy of blood velocity, flux, and RBC flow; multiunit recordings, spH fluorescence for vesicle release, imaging of plasma flow by injections of Texas Red, individual capillary diameter, and Ca2+ imaging of postsynaptic cell responses, in the in vivo single glomerulus.
The results show that with odor stimulation, a given glomerulus is as expected differentially activated by different odors (Fig. 3). Increases in presynaptic activity lead the increases in single capillary blood flow flux and velocity (Fig. 2) by about 2 secs; the two are closely coupled. Of most interest is the fact that local injection of the glutamate receptor blockers AMP and NMDA eliminates the postsynaptic Ca2+ response, as expected, but not the presynaptic spH signal (Fig. 4). The spH signal is in fact enhanced, presumably due to loss of feedback inhibition from the blocked postsynaptic dendrites. The mechanisms acting through the astrocytes include mGluR5 and COX activation, and glutamate uptake through the transporter GLT-1.
Among the lessons learned are the following. Local field potentials are not always reliable indicators of presynaptic versus postsynaptic activity. Nor are they reliable monitors of activity in local versus spatially extended systems; this is consistent with a long tradition of analysis of field potentials in the brain. Control of a local capillary may be overridden by levels of activation of many neighboring sites; analysis of a given site must take into consideration the system as a whole. This point should always be considered in fMRI studies investigating the correlation between blood flow and neuronal activity recorded at a single site.
These results thus support the idea that astrocytes act as sensors of glutamate to regulate local capillary perfusion in relation to neuronal activity. But Petzold et al in addition point out that the level of activity may be a critical variable; they used a lower level of activation than Chaigneau et al. They note that a recent study (Nawroth et al., 2007) has calculated an energy budget for the olfactory glomerulus, which indicated that at low levels of activation (i.e. low odor concentrations near the detection threshold) the energy consumption is extremely low, whereas it increases exponentially to one-third the total budget with strong stimulation. Petzold et al speculate that presynaptic release and astrocytic sensing may prevail at low activity levels, whereas “the contribution of postsynaptic mechanisms to functional hyperemia increases with stronger activation”. The possible contrast between low and high levels of input activity is summarized in the diagram of Fig. 1. Note that during strong odor stimulation, the proportion of glutamate released from dendrites upon rises of intracellular calcium becomes particularly significant over glutamate released by olfactory terminals. Blocking dendritic release would then affect the vascular response whether it involves astrocytes or possibly interneurones (Cauli et al., 2004). A second possibility is that silencing a large volume of tissue modifies the basal level of nitric oxide (Metea and Newman, 2006) and/or the vessel resting tone to a state where it loses its ability to respond to astrocyte activation.
Figure 1.
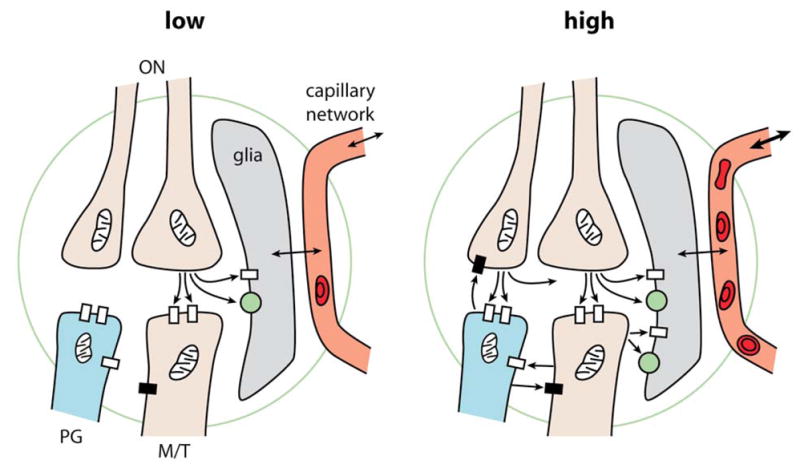
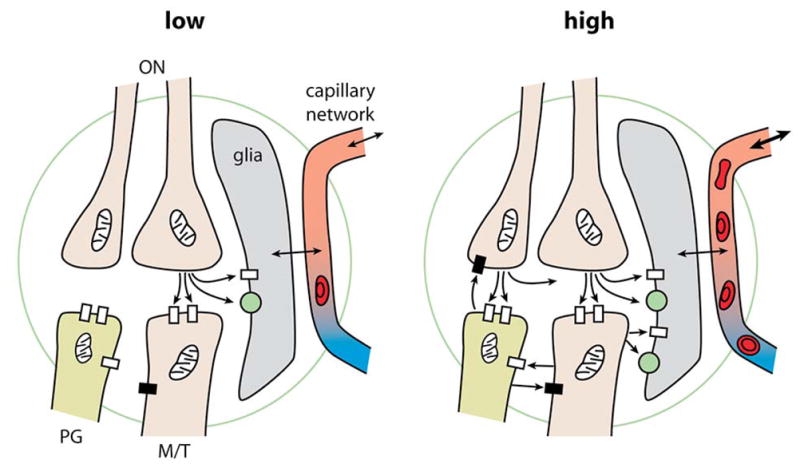
Diagrams summarizing the cellular elements taking part in neurovascular coupling in the olfactory glomerulus. Left: At low input intensities, action potentials in a few olfactory nerves (ON) cause release of glutamate from terminals, which activate glutamate receptors (open rectangles) on limited numbers of postsynaptic mitral and tufted cell (M/T) dendrites, and also glutamate receptors and transporters (green spheres) on glial cells, which in turn regulate blood flow through local capillaries. Right: At high input intensities, action potentials in many ONs cause release of Glu from many hundreds to thousands of terminals, which activate corresponding numbers of M/T dendrites and also PG cell dendrites. This strong input is restrained by feedback inhibition through GABA released from PG cell dendrites. The M/T dendrites postsynaptic to axon terminals are also presynaptic and release Glu on PG cell dendrites. It is postulated that axonal and dendritic Glu summate to mediate strong activation of the glial GluRs and glutamate transporters. The glial cells in turn exert strong regulation of local capillaries to increase local blood flow, which may also affect blood flow in the capillary network to neighboring glomeruli. Adapted from Shepherd, 2004, Gurden et al 2006 and Nawroth et al 2007.
All of these considerations provide a much richer understanding of the coupling between neural activity, pre and postsynaptic, glia, and the capillary network both local and more extensive, in the olfactory glomerulus. It provides a remarkable new level of insight with which to assess what the glomerular activity patterns representing different odor molecules are really telling us. They should enable us to compare more clearly the similarities and differences between the patterns obtained with the different methods: fMRI, intrinsic imaging, and 2 deoxyglucose.
The olfactory glomerulus is a remarkable model, the most clearly demarcated cortical module in the vertebrate brain. It seems a reasonable hypothesis that the lessons learned there may apply to other parts of the brain, but this will require careful testing and comparisons between the actual connectivity and functional states that are being compared.
Contributor Information
Gordon M. Shepherd, Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510
Serge Charpak, INSERM U603, 45 rue des Saints Peres, Paris 75270, France.
Selected Reading
- Benson TE, Burd GD, Greer CA, Landis DM, Shepherd GM. High-resolution 2-deoxyglucose autoradiography in quick-frozen slabs of neonatal rat olfactory bulb. Brain Res. 1985;339:67–78. doi: 10.1016/0006-8993(85)90622-5. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci U S A. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Tiret P, Lecoq J, Ducros M, Knopfel T, Charpak S. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci. 2007;27:6452–6460. doi: 10.1523/JNEUROSCI.3141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Uchida N, Mainen ZF. Sensory-evoked intrinsic optical signals in the olfactory bulb are coupled to glutamate release and uptake. Neuron. 2006;52:335–345. doi: 10.1016/j.neuron.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Des Rosiers MH, Jehle JW, Reivich M, Sharpe F, Sokoloff L. Mapping of functional neural pathways by autoradiographic survey of local metabolic rate with (14C)deoxyglucose. Science. 1975;187:850–853. doi: 10.1126/science.1114332. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nature reviews. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual review of physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Nawroth JC, Greer CA, Chen WR, Laughlin SB, Shepherd GM. An energy budget for the olfactory glomerulus. J Neurosci. 2007;27:9790–9800. doi: 10.1523/JNEUROSCI.1415-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nature neuroscience. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Laminar analysis of 2-deoxyglucose uptake in olfactory bulb and olfactory cortex of rabbit and rat. J Neurophysiol. 1977;40:800–813. doi: 10.1152/jn.1977.40.4.800. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Greer CA. The Synaptic Organization of the Brain. 5. New York: Oxford University Press; 2004. Olfactory Bulb; pp. 165–216. [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nature neuroscience. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature neuroscience. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


