Abstract
We describe a facile method to functionalize semiconducting polymer dots (Pdots) with polyelectrolytes. The polyelectrolyte coating dramatically improves the colloidal stability of the Pdots in solutions which are either of high ionic strength or contain bivalent metal ions: This feature allows Pdots to be used under physiologically relevant environments without losing their functionality. We conjugated the polyelectrolyte-coated Pdots with streptavidin to demonstrate their application in specific cell labeling.
Semiconducting polymer dots (Pdots) represent a new class of highly fluorescent nanoparticles with emissions tunable from the visible to the near IR region.1–10 The fluorescence intensity of a single green-emitting Pdot can be about 30 times brighter than a single quantum dot of similar emission wavelength (Qdot565) when excited with a 488-nm laser.3 In addition, most Pdots exhibit excellent photostability without blinking.3 Previous studies have also shown that Pdots have good biocompatibility.11,12 These fluorescence properties and good biocompatibility of Pdots make them excellent probes for cellular imaging and bioassays. We have focused on developing new methods to control the surface properties of Pdots for bioconjugation3–5 as well as constructing Pdot-based sensors.6–8
Functionalizing the surface of Pdots and controlling their colloidal stability are an important consideration in translating Pdots for use in biological studies.13 To prepare the surface of Pdots for bioconjugation, we recently reported a simple technique where we co-condensed a small amount of an amphiphilic polymer bearing functional groups with the semiconducting polymer into a single dot.3–5 This method is simple and versatile but the number of charges present on the surface of the Pdots can be limited. A highly charged particle is important in many cases for colloidal stability, especially under conditions of high ionic strength, which is often encountered in biological applications.
To address this issue, we describe here an approach that coats the Pdots with polyelectrolytes to control their surface and bioconjugation properties. This strategy has several advantages for Pdot functionalization. First, the Pdot surface is completely covered by polyelectrolytes, which significantly improves the colloidal stability of Pdots in high ionic-strength solutions. Second, the polyelectrolyte coating can improve the processibility of Pdots; coated Pdots can be centrifuged and re-suspended without aggregation. Third, functional groups on polyelectrolytes are readily accessible for further bioconjugation. Overall, we find this polyelectrolyte coating strategy to be facile and efficient for stabilizing the Pdot surface and subsequent bioconjugation.
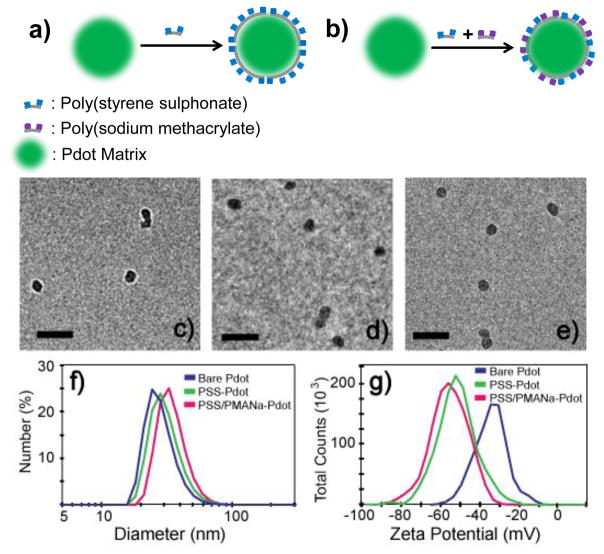
In our experiment, we used poly(styrene sulphonate) (PSS) and poly(sodium methacrylate) (PMANa) for coating the Pdot surface, but a wide range of other polyelectrolytes also can be used.14–17 Figure 1A and B depict the strategy of functionalizing Pdot with polyelectrolytes. To ensure complete surface coverage, Pdots were incubated with an excess amount of a specific polyelectrolyte in aqueous solution followed by the removal of the free polyelectrolytes from the coated Pdots by centrifugation at 80,000 rpm (Figure 1a). We also extended this strategy to introduce multiple types of polyelectrolytes onto the surface of Pdots (Figure 1b), such as PSS and PMANa; PSS serves as a stabilizer to improve the colloidal stability of the Pdots while the carboxyl groups from PMANa provide reactive sites for further bioconjugation.
Figure 1.
Functionalize Pdot with polyelectrolyte. a) Schematic of coating of Pdot with a single type of polyelectrolyte (e.g. PSS). b) coating of Pdot with multiple types of polyelectrolytes (e.g. PSS and PMANa). PSS: poly(styrene sulphonate); PMANa: poly(sodium methacrylate). Pdot characterization: TEM images of (c) bare Pdots, (d) PSS-coated Pdots, and (e) PSS/PMANa-coated Pdots. The scale bars represent 100 nm. The diameters and surface charges of bare Pdots, PSS-Pdots, and PSS/PMANa-Pdots were measured using (f) DLS and (g) zeta potential.
For many biological applications, it is beneficial to have Pdots of small sizes because they usually exhibit better colloidal stability and mass transfer properties, which are important for efficient cellular labelling and subcellular targeting.18 In this study, we used a bare green-fluorescent Pdot (poly(9,9-dioctylfluorene-co-benzothiadiazole) (PFBT)) with a diameter of 24 nm (Figure 1c, 1f). After polyelectrolyte coating, we found the thickness of the coated layer to be only 2–4 nm. The diameters of PSS-coated (PSS-Pdot) and PSS/PMANa-coated Pdots (PSS/PMANa-Pdot) were 28 nm and 32 nm, respectively (Figure 1d–1f). In contrast, the surface charge, as reflected in the zeta potential of the Pdots, was significantly altered. The values went from −35 mV for bare Pdots19 to −52 mV for PSS-coated Pdots, and −55 mV for PSS/PMANa-coated Pdots (Figure 1f).
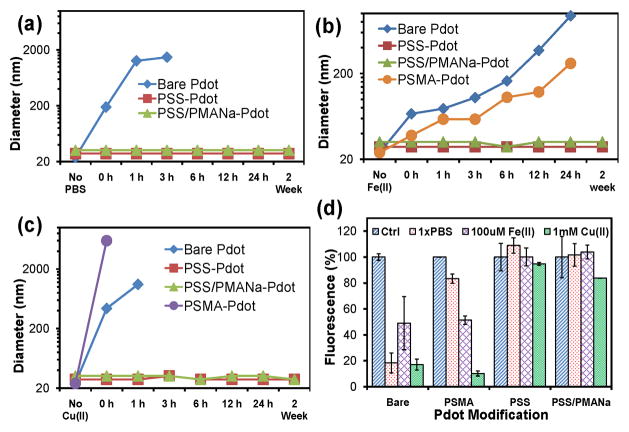
A key advantage of the polyelectrolyte-coated Pdots was their enhanced stability in high ionic strength solutions or in solutions that contain ions that tend to aggregate Pdots. To illustrate the improved colloidal stability bestowed by the PSS and PSS/PMANa polyelectrolyte coatings, we monitored the hydrodynamic size (Figure 2a–2c) and fluorescence emission (Figure 2d) from Pdots in three different solutions: Phosphate buffered saline (PBS) (Figure 2a, 2d), 0.1 mM Fe(II) solution (Figure 2b, 2d), and 1mM Cu(II) solution (Figure 2c, 2d). Both PSS-coated and PSS/PMANa-coated Pdots showed excellent colloidal stability in PBS, and their hydrodynamic diameters remained unchanged for over 2 weeks as monitored by dynamic light scattering (DLS) (Figure 2a). In sharp contrast, bare Pdots aggregated rapidly in PBS. In fact, they completely precipitated out of solution in 6 hours (Figure 2a). Pdots co-condensed with poly(styrene-co-maleic anhydride) (PSMA) produced PSMA- Pdots with carboxyl groups on the surface. These were prepared by nano-precipitation and displayed considerable improvement in colloidal stability in PBS. However, aggregation was still visually observed over 24 hours: the PSMA-Pdots could be seen to partially aggregate and adhere to the cuvette surface (Figure S2, ESI†)).
Figure 2.
Changes in the size and fluorescence of bare Pdots, PSS-coated Pdots, and PSS/PMANa-coated Pdots in three different solutions: PBS (pH 7.4), 1mM Cu(II) (CuSO4 in DI water), and 100 μM Fe(II) (FeSO4 in DI water). (a)–(c) The diameters of Pdots were monitored by DLS. Bare Pdots and Pdots formed by co-condensation with PSMA both increased in size, indicating the formation of aggregates in the three solutions; polyelectrolyte-coated Pdots showed excellent colloidal stability and no signs of aggregation. (d) The fluorescence intensities of bare Pdots and PSMA-blended Pdots also showed significant reduction in the three solutions, again indicating aggregation and self quenching; polyelectrolyte-coated Pdots showed excellent stability without a decrease in fluorescence emission. Control samples were dispersed in DI water.
Measuring the intensity of fluorescence emission from Pdots is a sensitive way to monitor aggregation because even a small amount of aggregation can decrease the measured fluorescence intensity due to self quenching. Figure 2d shows the fluorescence intensities of Pdots recorded one hour after they were dispersed in PBS, 1mM of Cu(II), or 100 μM of Fe(II). In PBS, bare Pdots lost more than 80% of their fluorescence; PSMA-Pdots also showed a nearly 20% reduction in fluorescence intensity. Both types of polyelectrolyte-coated Pdots showed stable fluorescence without a noticeable decrease in their emission intensity.
Bivalent metal ions, such as Cu(II) and Fe(II), are often present in biological buffers and solutions in the micro- to millimolar range. Both bare Pdots and PSMA-Pdots aggregated in the solutions containing Fe(II) and Cu(II) (Figure 2b, 2c). For example, DLS measurements showed that the presence of 1mM Cu(II) triggered severe and immediate aggregation of both bare and PSMA-Pdots (0 hour result, Figure 2c). However, the mechanisms of Cu(II) and Fe(II)-induced aggregation of bare Pdots and PSMA-Pdots are different. Bare Pdots precipitated in the presence of ions because of the increase in ionic strength, which destabilized the bare Pdots. PSMA-Pdots aggregated because of specific interactions between the carboxyl groups on the PSMA-Pdot surfaces and the Cu(II) and Fe(II) ions.6 Neither PSS-coated nor the PSS/PMANa-coated Pdots showed signs of aggregation in Cu(II) and Fe(II) solutions (Figure 2b, 2c) because of the improved colloidal stability of the highly negatively charged sulphonate surface. The measured fluorescence intensity from these Pdots (Figure 2d) confirmed the DLS measurements
In the case of PSS/PMANa-coated Pdots, PMANa does contain a large number of carboxyl groups, which can interact with Cu(II) and Fe(II) in a similar way as seen with PSMA-Pdots. However, when the Pdots were coated with both PSS (60% sulphonate group) and PMANa (40% carboxyl group), the sulphonate groups stabilized the Pdots and prevented aggregation of Pdots in the presence of Cu(II) and Fe(II) while presenting carboxyl groups for bioconjugation.
To demonstrate that this polyelectrolyte coating strategy is a general method for Pdot functionalization, we coated three types of Pdots. The first two were a blue (PFO, which is (Poly(9,9-dioctylfluorenyl-2,7-diyl)) and a green (PFBT) Pdot that each consisted of one type of semiconducting polymer. The third type of Pdot was a red Pdot formed from a blend of two semiconducting polymers (PFBT and PFTBT, which is Poly(9,9-dioctylfluorene)-co-(4,7-di-2-thienyl-2,1,3-benzothiadiazole)). Figure 3 shows the absorbance and fluorescence spectra of these three types of Pdots coated with PSS and dispersed in PBS.
Figure 3.
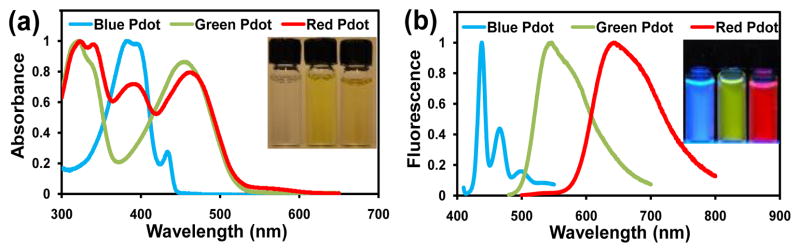
Normalized absorption and fluorescence spectra in PBS of a blue (PFO), green (PFBT), and red (PFBT blended with PFTBT) fluorescing Pdot after coating with PSS. The inserts are optical (in a) and fluorescence (in b) images of the three types of PSS-coated Pdots. These spectra illustrate PSS coating can stabilize Pdots of different types.
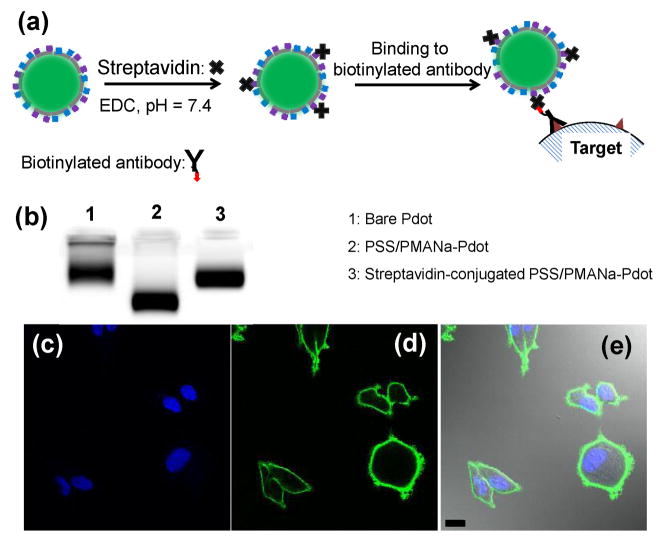
To further demonstrate the applicability of polyelectrolyte-functionalized Pdots in biological applications, we conjugated streptavidin to PSS/PMANa-Pdots through EDC reaction. (Figure 4a).3, 5, 20 We used gel electrophoresis to verify successful streptavidin conjugation (Figure 4b). PSS/PMANa-Pdots migrated faster than bare Pdots because of the increased surface charge. After conjugation to streptavidin, the resultant Pdots travelled much slower because of the slight increase in particle size and decrease in surface charge. We then used streptavidin-conjugated PSS/PMANa-Pdots for labelling MCF-7 cells, which is a breast cancer cell line that expresses the cell surface antigen EpCAM. We first labeled EpCAM with biotinylated primary antibody and then tagged the biotinylated primary antibodies with streptavidin functionalized Pdots (Figure 4c–4e). This experiment demonstrates the utility of polyelectrolyte-coated Pdots for cellular targeting.
Figure 4.
Bioconjugation of PSS/PMANa-coated Pdots with streptavidin for labeling MCF-7 cells. (a) Schematic showing the procedure for bioconjugation of PSS/PMANa-Pdots and their use in specific cellular targeting. (b) Gel electrophoresis of bare Pdots, PSS/PMANa coated Pdots, and streptavidin-conjugated PSS/PMANa-Pdots. (c–e) Confocal fluorescence images of MCF-7 cells labeled with the streptavidin-conjugated PSS/PMANa-Pdots. (c) Cell nucleus stained using Hoechst 34580 (blue fluorescence). (d) Fluorescence image of cell membrane lableled with Pdots (green fluorescence). (e) Merged image of the lableled cells. The scale bar is 30 μm.
In conclusion, we have developed a facile and effective strategy for functionalizing Pdots using polyelectrolytes. Compared with bare Pdots or those functionalized via co-precipitation with an amphiphilic polymer (e.g. PSMA), Pdots made by this method exhibited dramatically improved colloidal stability in high ionic strength solutions, such as PBS. Moreover, multiple types of functional groups could be simultaneously introduced onto the Pdot surface by co-coating with different types of polyelectrolytes (e.g. PSS and PMANa). We showed this polyelectrolyte functionalization strategy is general enough to be applied to different types of Pdots. These Pdots can then be conjugated to biologically relevant molecules for cellular targeting. We anticipate this approach will be useful in preparing robust Pdots for demanding applications that involve harsh conditions, and where enhanced colloidal stability is required.
Supplementary Material
Acknowledgments
We gratefully acknowledge support of this work by the National Institutes of Health (NS062725 and CA147831) and the National Science Foundation (CHE-0924320)
Footnotes
Electronic Supplementary Information (ESI) available: detailed experimental description. See DOI:
Notes and references
- 1.Wu C, Szymanski C, McNeill J. Langmuir. 2006;22:2956. doi: 10.1021/la060188l. [DOI] [PubMed] [Google Scholar]
- 2.Wu C, Bull B, Szymanski C, Christensen K, McNeill J. ACS Nano. 2008;2:2415. doi: 10.1021/nn800590n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Schneider T, Zeigler M, Yu J, Schiro PG, Burnham DR, McNeill JD, Chiu DT. J Am Chem Soc. 2010;132:15410. doi: 10.1021/ja107196s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C, Jin Y, Schneider T, Burnham DR, Smith PB, Chiu DT. Angew Chem Int Ed. 2010;49:1. doi: 10.1002/anie.201004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Hansen SJ, Hou Q, Yu J, Zeigler M, Jin Y, Burnham DR, McNeill JD, Olson JM, Chiu DT. Angew Chem Int Ed. 2011;50:3430. doi: 10.1002/anie.201007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan YH, Jin Y, Wu C, Chiu DT. Chem Commun. 2011;47:2820. doi: 10.1039/c0cc04929h. [DOI] [PubMed] [Google Scholar]
- 7.Chan YH, Wu C, Ye F, Jin Y, Smith PB, Chiu DT. Anal Chem. 2011;83:1448. doi: 10.1021/ac103140x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye F, Wu C, Jin Y, Chan YH, Zhang X, Chiu DT. J Am Chem Soc. 2011;133:8146. doi: 10.1021/ja202945g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y, Ye F, Zeigler M, Wu C, Chiu DT. ACS Nano. 2011;5:1468. doi: 10.1021/nn103304m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pecher J, Mecking S. Chem Rev. 2010;110:6260. doi: 10.1021/cr100132y. [DOI] [PubMed] [Google Scholar]
- 11.Pu K, Li K, Shi J, Liu B. Chem Mater. 2009;21:3816. [Google Scholar]
- 12.Fernando LP, Kandel PK, Yu J, McNeill J, Ackroyd PC, Christensen KA. Biomacromolecules. 2010;11:2675. doi: 10.1021/bm1007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howes P, Green M. Photochem Photobiol Sci. 2010;9:1159. doi: 10.1039/c0pp00106f. [DOI] [PubMed] [Google Scholar]
- 14.Caruso F. Adv Mater. 2001;13:11. [Google Scholar]
- 15.Johnston APR, Cortez C, Angelatos AS, Caruso F. Curr Opin Colloid Interface Sci. 2006;11:203. [Google Scholar]
- 16.Poon Z, Chang D, Zhao X, Hammond PT. ACS Nano. 2011;5:4284. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon Z, Lee JB, Morton SW, Hammond PT. Nano Lett. 2011;11:2096. doi: 10.1021/nl200636r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Y, Lohstreter S, Pierce DT, Parisien J, Wu M, Hall C, Zhao JX. Chem Mater. 2008;20:4411. [Google Scholar]
- 19.Clafton SN, Beattie DA, Mierczynska-Vasilev A, Acres RG, Morgan AC, Kee TW. Langmuir. 2010;26:17785. doi: 10.1021/la103063p. [DOI] [PubMed] [Google Scholar]
- 20.Hermanson GT. Bioconjugate techniques. Academic Press; San Diego: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.