Abstract
The first examples are described of catalyzed γ-additions of nitrogen nucleophiles to γ-substituted alkynoates or allenoates that proceed with good efficiency, specifically, intra- and intermolecular processes that employ distinct and useful families of nitrogen nucleophiles (anilines and 2,2,2-trifluoroacetamide), catalyzed by spirophosphine 1. Furthermore, the first demonstrations are reported of asymmetric reactions, affording interesting classes of target molecules such as enantioenriched pyrrolidines, indolines, and γ-amino-α,β-unsaturated carbonyl compounds.
Keywords: amination, asymmetric catalysis, heterocycles, organocatalysis, phosphanes
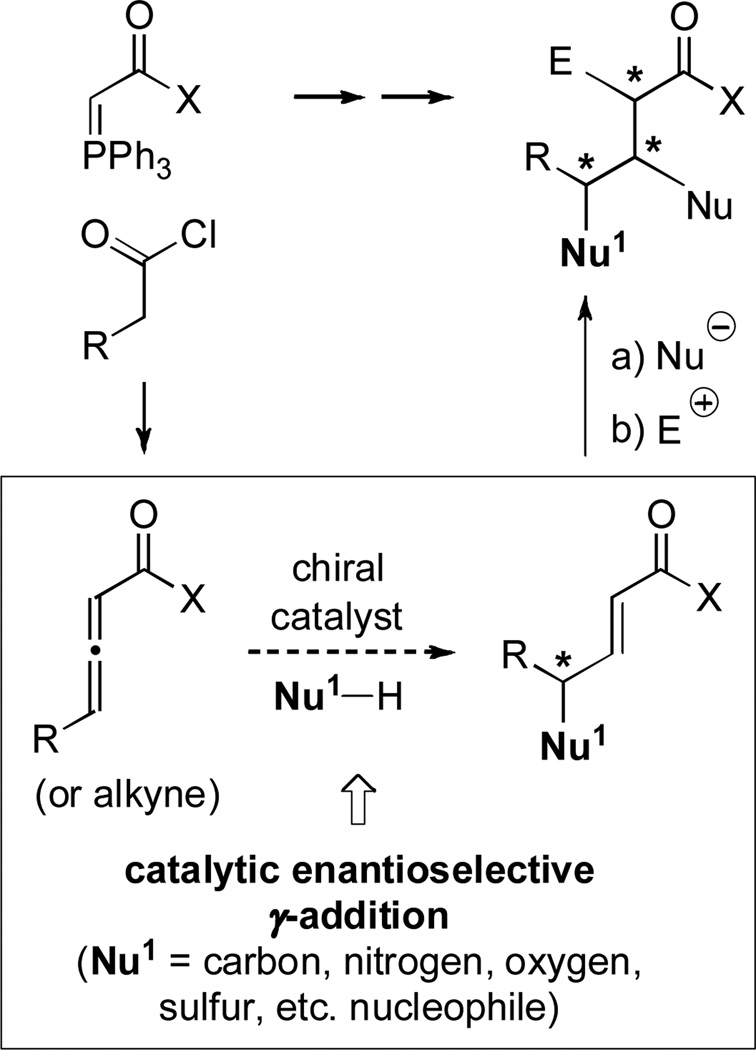
The use of chiral phosphines as nucleophilic catalysts represents an important second dimension to their utility in catalytic asymmetric synthesis,[1] in addition to their more familiar role as ligands for transition metals.[2] Cognizant of the paucity of general methods for the catalytic enantioselective α-functionalization of carbonyl compounds,[3] we have recently pursued the development of phosphine-catalyzed processes that couple nucleophiles with allenoates and related compounds in the γ-position (Figure 1).[4–6] Given the ready availability of the starting allenes, along with the plethora of methods for stereoselective α- and β-functionalization of α,β-unsaturated carbonyl compounds,[7,8] this approach should provide straightforward access to highly functionalized, stereochemically rich, target molecules (Figure 1).
Figure 1.
Catalytic enantioselective γ-additions to allenes: Efficient access to highly functionalized, stereochemically rich, carbonyl compounds.
To date, we have established the viability of this approach with oxygen (intramolecular additions to alkynes), carbon (intermolecular/allenes), and sulfur (intermolecular/allenes) nucleophiles.[4] In view of the biological significance of amines,[9,10] including γ-amino-α,β-unsaturated carbonyl compounds,[11–13] achieving catalytic enantioselective γ-additions with nitrogen nucleophiles is a particularly important objective.[14] However, attempts to effect phosphine-catalyzed γ-addition (even non-enantioselective) of nitrogen nucleophiles to γ-substituted 2,3-allenoates and 2-alkynoates (and related compounds) have been unsuccessful (≤30% yield),[15] due in part to the propensity of such electrophiles to isomerize to 1,3-dienes.[16] In this report, we demonstrate that spirophosphine 1 not only can achieve γ C–N bond formation in good yield for the first time, but it can also provide good enantioselectivity, both for intra- and for intermolecular processes [Eq. (1) and Eq. (2); CPME = cyclopentyl methyl ether; TBME = tbutyl methyl ether].
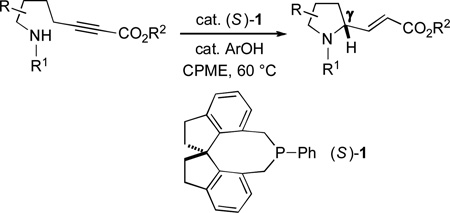 |
(1) |
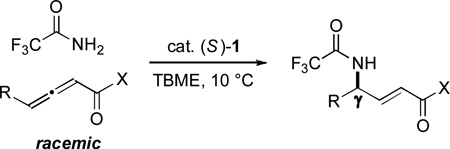 |
(2) |
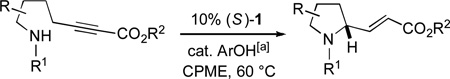
From the outset of our investigation of phosphine-catalyzed γ-additions of nitrogen nucleophiles, we decided to address simultaneously the two key challenges: accomplishing C–N bond formation and controlling the stereochemistry of the γ-carbon. Upon examining an array of conditions for the enantioselective cyclization of the amino-alkyne illustrated in entry 1 of Table 1, we developed a method whereby spirophosphine 1[17–19] catalyzes the desired intramolecular γ-addition to generate the target pyrrolidine[20,21] with very good enantioselectivity (91% ee) and acceptable yield (68%).
Table 1.
Catalytic enantioselective synthesis of pyrrolidines and indolines via intramolecular γ-additions of nitrogen nucleophiles to alkynoates.
 | |||
|---|---|---|---|
| entry | substrate | ee (%) | yield (%)[b] |
| 1 | 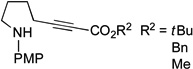 |
91 | 68 |
| 2 | 95 | 70 | |
| 3 | 94 | 78 | |
| 4 | 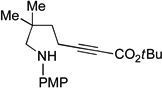 |
94 | 60 |
| 5 | 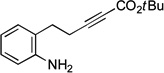 |
90 | 55 |
| 6 | 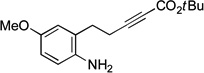 |
89 | 44 |
| 7 | 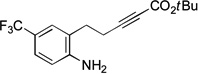 |
88 | 44 |
| 8 | 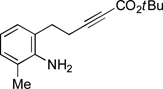 |
88 | 67 |
All data are the average of two experiments. PMP = p-methoxyphenyl.
For entries 1–4, cat. ArOH = 50% 2,4-dimethoxyphenol. For entries 5–8, cat. ArOH = 20% 2-fluoro-6-methoxyphenol.
Yield of purified product.
Spirophosphine 1 serves as an effective catalyst for the asymmetric cyclization of an array of amino-alkynes (Table 1; >95:5 E:Z for all reactions).[22] The choice of ester attached to the alkyne has only a modest impact on the efficiency of the catalytic enantioselective γ-addition process (entries 1–3). Furthermore, substitution on the alkyl chain between the nucleophilic aniline and the electrophilic alkyne is tolerated (entry 4).
If the aromatic ring of the aniline lies between the amine and the alkyne, then spirophosphine-catalyzed asymmetric intramolecular γ-addition of the amine furnishes enantioenriched indolines[23] (Table 1, entries 5–8). Relative to the parent substrate (entry 5), incorporation of an electron-donating or an electron-withdrawing group on the aromatic ring leads to cyclization with similar enantioselectivity, but somewhat lower yield (entries 6 and 7). On the other hand, the presence of a methyl substituent ortho to nitrogen results in more efficient cyclization (entry 8).
Next, we turned our attention to the challenge of also achieving the first effective phosphine-catalyzed intermolecular γ-additions of nitrogen nucleophiles to alkynes/allenes. Unfortunately, our standard conditions for intramolecular reactions of anilines (Table 1) were not useful for intermolecular additions of anilines to alkynes/allenes (<10% yield).
2,2,2-Trifluoroacetamide is a particularly attractive nitrogen nucleophile, since it can be hydrolyzed under mild conditions to liberate a free amine. Employing our published methods for enantioselective phosphine-catalyzed γ-additions of other families of nucleophiles,[4] we obtained either poor ee (<35%) or poor yield (<10%) for the catalytic asymmetric γ-addition of 2,2,2-trifluoroacetamide to ethyl 2,3-heptadienoate.
Nevertheless, upon surveying a range of parameters, we developed a new method wherein spirophosphine 1 catalyzes the desired γ-amination process with good enantioselectivity and yield, as well as excellent E/Z selectivity (≥95:5) (Table 2, entry 1); interestingly, although we have found this spirophosphine to be the catalyst of choice for intramolecular catalytic asymmetric γ-additions, it had not previously emerged as the optimal phosphine for intermolecular reactions.[4] An array of other chiral phosphine catalysts that we have found useful in other contexts furnish significantly lower ee, yield, or E/Z selectivity in this enantioselective γ-amination (entries 2–6). The amount of γ-addition product diminishes when a smaller quantity of allene (entry 7) or catalyst (entry 8) is employed, and a small erosion in ee is observed when the catalytic asymmetric γ-addition is conducted at room temperature, rather than at 10 °C (entry 9).
Table 2.
Catalytic enantioselective intermolecular γ-addition of a nitrogen nucleophile to an allene: Effect of reaction param eters.
 | |||
|---|---|---|---|
| entry | change from the "standard conditions" | ee (%) | yield (%)[a] |
| 1 | none | 87 | 90 [≥95:5] |
| 2 | 2 instead of 1 | 80 | 88 [65:35] |
| 3 | 3 instead of 1 | 9 | 52 [≥95:5] |
| 4 | 4 instead of 1 | 64 | 89 [90:10] |
| 5 | 5 instead of 1 | 84 | 64 [60:40] |
| 6 | 6 instead of 1 | 27 | 96 [85:15] |
| 7 | 1.0, instead of 2.0, equiv of allene | 88 | 52 [≥95:5] |
| 8 | 5%, instead of 10%, 1 | 88 | 39 [≥95:5] |
| 9 | room temperature instead of 10 °C | 82 | 90 [≥95:5] |
All data are the average of two experiments.
The yield was determined through 1H NMR analysis with the aid of an internal standard. The E:Z ratio, also determined through 1H NMR analysis, is provided in brackets.
![[a]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dc8c/3819219/966a5337d3df/nihms521614f6.jpg)
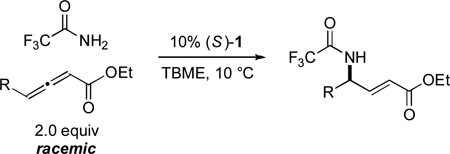
Under the standard conditions, spirophosphine 1 catalyzes the intermolecular γ-amination of an array of allenoates by 2,2,2-trifluoroacetamide in generally excellent yield, thereby furnishing ready access to γ-amino-α,β-unsaturated esters; at the same time, good ee’s are obtained (Table 3).[24] As might be anticipated on the basis of the simplicity of the method and the mild reaction temperature, a variety of functional groups are compatible with the asymmetric γ-addition process, including a terminal alkyne, a Z alkene, an ester, and a sulfur heterocycle. The method is not particularly air- or moisture-sensitive: for example, the addition of 0.5 equiv of water did not erode enantioselectivity or yield, and running the reaction in a capped vial under air had no effect on ee and only a modest impact on yield. On a gram-scale, the γ-amination illustrated in entry 4 of Table 3 proceeds with comparable results (87% ee, 95% yield, ≥95:5 E:Z). The 2,2,2-trifluoroacetyl group can be removed by hydrolysis under mild conditions.[25]
Table 3.
Catalytic enantioselective intermolecular γ-addition of 2,2,2-trifluoroacetamide to allenoates: Scope.
 | |||
|---|---|---|---|
| entry | R | ee (%) | yield (%)[a] |
| 1 | Me | 86 | 89 |
| 2 | n Pr | 87 | 90 |
| 3 | iBu | 88 | 92 |
| 4 | (CH2)2Ph | 88 | 94 |
| 5 | (CH2)4OBn | 89 | 87 |
| 6 | 89 | 86 | |
| 7 | 87 | 88 | |
| 8 | (CH2)2CO2Me | 82 | 68 |
| 9 | 86 | 87 | |
All data are the average of two experiments.
Yield of purified product (E:Z >95:5).
This catalytic asymmetric α-amination process is not limited to additions of 2,2,2-trifluoroacetamide to carbethoxy-substituted allenoates. Thus, the method can be applied to reactions with a methyl and a tbutyl ester, as well as with a Weinreb amide, in ~90% ee [Eq. (3) and Eq. (4)].
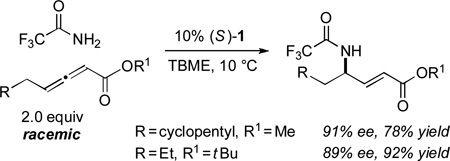 |
(3) |
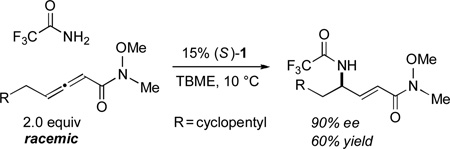 |
(4) |
A preliminary mechanistic investigation revealed that product ee correlates linearly with catalyst ee and that the rate law is positive order in allene and catalyst, but negative order in nucleophile. Although 31P NMR spectroscopy studies did not provide clear evidence for a catalyst–nucleophile adduct, several phosphorus-containing species (potentially, phosphonium intermediates) in addition to free spirophosphine 1 were observed during the course of the γ-addition process.
In this report, we have provided the first examples of catalyzed γ-additions of nitrogen nucleophiles to γ-substituted alkynoates or allenoates that proceed with good efficiency, specifically, intra- and intermolecular processes that employ distinct and useful families of nitrogen nucleophiles (anilines and 2,2,2-trifluoroacetamide), catalyzed by spirophosphine 1. Furthermore, we have furnished the first demonstration of asymmetric reactions, affording interesting classes of target molecules such as enantioenriched pyrrolidines, indolines, and γ-amino-α,β-unsaturated carbonyl compounds. This investigation thus adds an important new family of nucleophiles (nitrogen) to those (carbon, oxygen, and sulfur) that have previously been shown to engage in phosphine-catalyzed asymmetric γ-additions. Ongoing studies are directed at further expanding this strategy for the rapid generation of functionalized carbonyl compounds.
Supplementary Material
Acknowledgments
This research has been supported by the National Institutes of Health (National Institute of General Medical Sciences: R01–GM57034), the Natural Sciences and Engineering Research Council of Canada (postdoctoral fellowship for R.J.L.), the Laboratoire de Synthèse Organique, CNRS UMR 7652, Ecole Polytechnique DCSO, F91128 Palaiseau, France (fellowship for C.M.), and the Croucher Foundation (postdoctoral fellowship for Y.K.C.). We thank Shoshana Bachman for a preliminary study.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org
Contributor Information
Rylan J. Lundgren, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, California 91125 (USA)
Ashraf Wilsily, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 (USA).
Nicolas Marion, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 (USA).
Cong Ma, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 (USA).
Ying Kit Chung, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 (USA).
Gregory C. Fu, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, California 91125 (USA).
References
- 1.For reviews, see: Zhou Z, Wang Y, Tang C. Curr. Org. Chem. 2011;15:4083–4107. Marinetti A, Voituriez A. Synlett. 2010:174–194. Cowen BJ, Miller SJ. Chem. Soc. Rev. 2009;38:3102–3116. doi: 10.1039/b816700c. Gröger H, Burda E. In: Phosphorus Ligands in Asymmetric Catalysis. Börner A, editor. Vol. 3. New York: Wiley–VCH; 2008. pp. 1175–1197. Methot JL, Roush WR. Science of Synthesis. 2008;42:469–501.
- 2.Börner A, editor. Phosphorus Ligands in Asymmetric Catalysis. New York: Wiley–VCH; 2008. [Google Scholar]
- 3.For two recent examples, see: Halskov KS, Johansen TK, Davis RL, Steurer M, Jensen F, Jørgensen KA. J. Am. Chem. Soc. 2012;134:12943–12946. doi: 10.1021/ja3068269. Zultanski SL, Fu GC. J. Am. Chem. Soc. 2011;133:15362–15364. doi: 10.1021/ja2079515.
- 4.a) Intramolecular additions of oxygen nucleophiles to alkynes: Chung YK, Fu GC. Angew. Chem., Int. Ed. 2009;48:2225–2227. doi: 10.1002/anie.200805377.; b) Intermolecular additions of carbon nucleophiles to allenes: Smith SW, Fu GC. J. Am. Chem. Soc. 2009;131:14231–14233. doi: 10.1021/ja9061823. Sinisi R, Sun J, Fu GC. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20652–20654. doi: 10.1073/pnas.1003597107.; c) Intermolecular additions of sulfur nucleophiles to allenes: Sun J, Fu GC. J. Am. Chem. Soc. 2010;132:4568–4569. doi: 10.1021/ja101251d. Fujiwara Y, Sun J, Fu GC. Chem. Sci. 2011;2:2196–2198. doi: 10.1039/c1sc00414j.
- 5.For a pioneering study of a process that generates a stereocenter in the δ, not the γ, position, see: Chen Z, Zhu G, Jiang Q, Xiao D, Cao P, Zhang X. J. Org. Chem. 1998;63:5631–5635.
- 6.For key early studies of non-enantioselective processes, see: Trost BM, Li C-J. J. Am. Chem. Soc. 1994;116:3167–3168. Trost BM, Li C-J. J. Am. Chem. Soc. 1994;116:10819–10820. Zhang C, Lu X. Synlett. 1995:645–646. Trost BM, Dake GR. J. Org. Chem. 1997;62:5670–5671.
- 7.For a review and leading references on catalytic asymmetric β functionalizations of carbonyl compounds, see: Cordova A, editor. Catalytic Asymmetric Conjugate Reactions. Weinheim: Wiley–VCH; 2011.
- 8.For a review and leading references on catalytic asymmetric α functionalizations of carbonyl compounds, see: MacMillan DWC, Watson AJB. Science of Synthesis. 2011;3:675–745.
- 9.For leading references, see: Knölker H-J, editor. The Alkaloids: Chemistry and Biology. Vol. 70. San Diego: Elsevier; 2011.
- 10.For an overview of methods for the enantioselective synthesis of amines, see: Nugent TC, editor. Chiral Amine Synthesis. Weinheim: Wiley–VCH; 2010.
- 11.For an example of a bioactive natural product that includes a γ-amino-α,β-unsaturated carbonyl subunit, see: Hagihara M, Schreiber SL. J. Am. Chem. Soc. 1992;114:6570–6571.
- 12.γ-Aminobutyric acid (GABA), γ-amino-β-hydroxybutyric acid (GABOB), carnitine, statine, and pregabalin (Lyrica™) are examples of bioactive α-aminocarboxylic acids.
- 13.a) For a review of methods for the stereoselective synthesis of γ-amino acids, see: Ordonez M, Cativiela C. Tetrahedron: Asymmetry. 2007;18:3–99. doi: 10.1016/j.tetasy.2009.01.002.; b) For a review of methods for the synthesis of allylic amines, see: Feng JQ, Li C-J. Science of Synthesis. 2009;40a:587–614.
- 14.For complementary methods for catalytic asymmetric γ amination that employ a nitrogen electrophile, see: Poulsen TB, Alemparte C, Jørgensen KA. J. Am. Chem. Soc. 2005;127:11614–11615. doi: 10.1021/ja0539847. Bertelsen S, Marigo M, Brandes S, Dinér P, Jørgensen KA. J. Am. Chem. Soc. 2006;128:12973–12980. doi: 10.1021/ja064637f. Wang J, Chen J, Kee CW, Tan C-H. Angew. Chem., Int. Ed. 2012;51:2382–2386. doi: 10.1002/anie.201107317.
- 15.We are not aware of any reports of phosphine-catalyzed intramolecular γ-additions of nitrogen nucleophiles to γ-substituted 2,3-allenoates or 2-alkynoates, or of any enantioselective intra- or intermolecular processes. For reports of non-asymmetric intermolecular additions to γ-substituted electrophiles, all of which proceed in ≤30% yield, see: Trost BM, Dake GR. J. Am. Chem. Soc. 1997;119:7595–7596.: 9% yield; Liu B, Davis R, Joshi B, Reynolds DW. J. Org. Chem. 2002;67:4595–4598. doi: 10.1021/jo016154u.: 17% and 28% yield; Virieux D, Guillouzic A-F, Cristau H-J. Tetrahedron. 2006;62:3710–3720.: 30% yield.
- 16.a) Trost BM, Kazmaier U. J. Am. Chem. Soc. 1992;114:7933–7935. [Google Scholar]; b) Guo C, Lu X. J. Chem. Soc., Perkin Trans. 1993;1:1921–1923. [Google Scholar]; c) Kwong CKW, Fu MY, Lam CSL, Toy PH. Synthesis. 2008:2307–2317. [Google Scholar]
- 17.For the development of spirophosphine 1 as a chiral ligand for metal-catalyzed enantioselective processes, see: Zhu S-F, Yang Y, Wang L-X, Liu B, Zhou Q-L. Org. Lett. 2005;7:2333–2335. doi: 10.1021/ol050556x. Xie J-H, Zhou Q-L. Acc. Chem. Res. 2008;41:581–593. doi: 10.1021/ar700137z.
- 18.For the first use of spirophosphine 1 as a chiral nucleophilic catalyst, see Reference 4a. Spirophosphine 1 is not highly susceptible to oxidation: after exposure of the solid to air for three days at room temperature, no phosphine oxide is observed by NMR spectroscopy.
- 19.Spirophosphine 1 is commercially available.
- 20.For leading references to the enantioselective synthesis of pyrrolidines, see: Royer J, editor. Asymmetric Synthesis of Nitrogen Heterocycles. Weinheim: Wiley–VCH; 2009.
- 21.For a recent report of non-enantioselective phosphine-catalyzed intramolecular additions of nitrogen nucleophiles to allenoates that lack a γ-substituent, see: Andrews IP, Blank BR, Kwon O. Chem. Commun. 2012;48:5373–5375. doi: 10.1039/c2cc31347b.
- 22.Under our standard conditions: the ee of the product was constant during the course of a γ-addition process; in the absence of spirophosphine 1, no reaction was observed; the phosphine oxide of 1 does not serve as a catalyst for γ-addition; six-membered ring formation was less efficient; γ-addition to an alkyne substituted with a Weinreb amide proceeded in excellent ee but modest yield; the 2,4-dimethoxyphenol enhances the yield, not the enantioselectivity.
- 23.For a review and leading references on the synthesis of indolines, see: Liu D, Zhao G, Xiang L. Eur. J. Org. Chem. 2010:3975–3984.
- 24.(a) Under our standard conditions: the ee was constant during the course of a γ-addition; the carboxylic acid and the phenol additives that we examined were deleterious with respect to ee, yield, or E:Z selectivity; in the absence of spirophosphine 1, no reaction was observed; the phosphine oxide of 1 does not serve as a catalyst for γ-addition; an allenoate with a γ-isopropyl substituent, a 2-alkynoate, an allenyl phosphonate, and an allenyl nitrile were not suitable electrophiles; 1,3-diene is a side product. (b) In a preliminary study, we have determined that spirophosphine 1 can catalyze the γ-addition of TsNH2 to an allenoate in 68% ee and 75% yield (unoptimized); further exploration revealed that phosphepine 2 can achieve an array of γ-additions of TsNH2 with ~85% ee and ~85% yield (~85:15 E:Z; toluene, r.t.).
- 25.For leading references, see: Spencer C, Balsells J, Li H. Tetrahedron Lett. 2009;50:1010–1012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.