Abstract
Three new α-tetralone galloylglucosides (1–3), were isolated from the fresh pericarps of Juglans sigillata (Juglandaceae), together with the six known ones. The structures of the new compounds were determined as 4,6-dihydroxy-α-tetralone-4-O-[6′-O-(3″, 4″,5″-trihydroxybenzoyl)]-β-D-glucopyranoside (1), (4S)-4,5-dihydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-trihydroxybenzoyl)]-β-D-glucopyranoside (2) and (4S)-4,5,6-tri-hydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-trihydroxybenzoyl)]-β-D-glucopyranoside (3), respectively, on the basis of detailed spectroscopic analyses, and acidic and enzymatic hydrolysis. The antimicrobial activities of the isolated compounds (2, 4 and 7–9) were evaluated.
Keywords: Juglans sigillata, Juglandaceae, α-tetralone galloylglucosides, antimicrobial activity
Introduction
The genus Juglans (Juglandaceae) comprising about 20 species is widely distributed in the temperate and subtropical areas of the world [1]. The seeds of Juglans species, particularly J. regia, known as walnuts, are excellent sources of unsaturated fatty acids and polyphenols, and used as folk remedies for cancer and kidney and stomach diseases in Asia and Europe [2]. The fresh pericarp of some species, e.g. J. mandshurica and J. regia, commonly named as “Qing-Long-Yi”, have been medicinally used for thousands of years in China, Japan and Korea, owing to its anti-tumor, anti-inflammatory, antinociceptive and antioxidant effects. The roots and leaves of these plants are also used as folk medicine for the treatment of cancer, rheumatic pains and eczema [3,4]. Some naphthalene glucosides [5], α-tetralones and their derivatives [6–8] and diarylheptanoids [9] have been reported from the fresh pericarps or fruits of Juglans species.
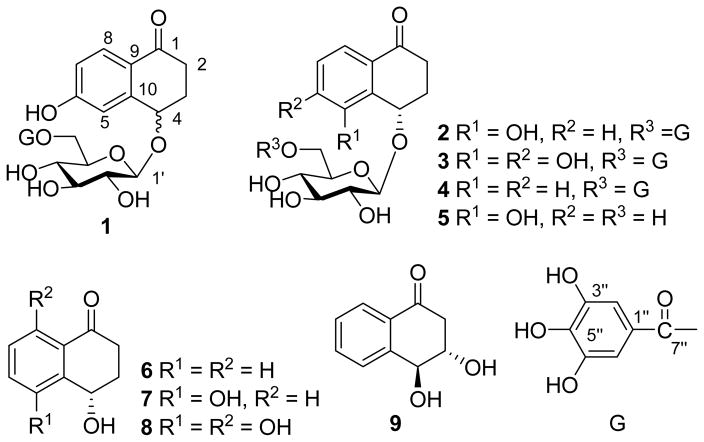
Juglans sigillata Dode, known as the iron walnut, has been widely cultivated for its edible nuts in the southwest of China. To date, no chemical work has been carried out on this species. Our detailed chemical investigation on the fresh pericarps of J. sigillata led to the isolation of three new α-tetralone galloylglucosides (1–3), together with six known ones (4–9) (Figure 1). The isolated compounds 2, 4 and 7–9 were evaluated for their antimicrobial activities.
Figure 1.
α-Tetralone Derivatives from the Fresh Pericarps of Juglans sigillata
Results and Discussion
The fresh pericarps of J. sigillata were extracted three times with 80% aqueous acetone at room temperature. After removal of the organic solvent, the aqueous fraction was extracted with CHCl3 and EtOAc, successively. The EtOAc fraction was subjected to column chromatography (CC) over Sephadex LH-20, silica gel and MCI-gel CHP20P to afford compounds 1–9. Of them, the known compounds 4–9 were identified as (4S)-4-hydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-tri-hydroxybenzoyl)]-β-D-glucopyranoside (4) [6], (4S)-4,5-dihydroxy-α-tetralone-4-O-β-D-glucopyranoside (5) [7], (4S)-4-hydroxy-α-tetralone (6) [7], (4S)-4,5-dihydroxy-α-tetralone (7) [7], (4S)-4,5,8-trihydroxy-α–tetralone (8) [7], and 3,4-dihydro-naphthalen-1(2H)-one (9) [8], respectively, by comparison of their spectroscopic and physical data with those reported previously in literatures.
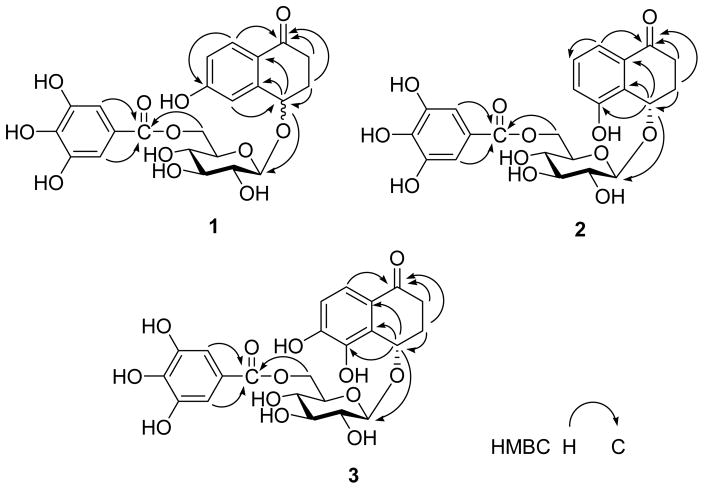
Compound 1 was obtained as a white amorphous powder. Its molecular formula C23H24O12 was determined on the basis of the HR-ESI-MS (m/z 491.1210, [M - H]−), The IR spectrum showed absorption at 3439, 1627, and 1606 cm−1, suggesting the presence of hydroxyl and carbonyl groups. Acidic hydrolysis of 1 gave D-glucose as the sole sugar residue. The 1H- and 13C-NMR data of 1 exhibited the signals arising from a carbonyl (δ(C) 197.8), a benzene ring (δ(H) 7.74, 7.08 and 6.81), a β-glucopyranosyl (anomeric proton at δ(H) 4.60 (1H, d, J = 7.9 Hz, H–C(1′)), and carbon signals at δ(C) 103.7 C(1′), 76.5 C(2′), 74.3 C(3′), 71.1 C(4′), 77.1 C(5′) and 63.4 C(6′)), a galloyl (δ(H) 7.11 (2H, s)) group, two methylenes (δ(C) 35.0 C(2) and 31.2 C(3)) and one oxygen-bearing methine (δ(C) 74.4 C(4)). These NMR characteristics resembled those observed in 4. However, instead of a 1,2-disubstituted benzene ring in 4, compound 1 had a 1,2,4-trisubstituted benzene ring in view of the coupling patterns of the aromatic protons (δ(H) 7.74 (1H, d, J = 8.6 Hz, H–C(8)), 7.08 (1H, d, J = 2.4 Hz, H–C(5)), and 6.81 (1H, dd, J = 8.6, 2.4 Hz, H–C(7))). In the HMBC spectrum of 1, correlations of δ(H) 7.74 (H–C(8)) with δ(C) 197.8 (C(1)) and δ(H) 7.08 (H–C(5)) with δ(C) 74.4 (C(4)) were observed. These HMBC correlations in conjunction with the multiplicities in the 1H NMR spectrum of 1 demonstrated that C(6) of the benzene ring was substituted with a hydroxyl group. In addition, the HMBC correlations of H–C(4) (δ(H) 4.82) with C(1′) (δ(C) 103.7, glucosyl unit) indicated the glucose moiety to be attached to C(4) of the aglycone. The location of the galloyl group at the C(6) hydroxyl group of the glucosyl unit was also determined by the HMBC experiment, in which correlations of CH2(6′) (δ(H) 4.57, 4.36, glucosyl unit) with the carboxyl carbon C(7″) (δ(C) 167.3) of the galloyl group were displayed. Other HMBC correlations (Figure 2) also confirmed the structure of 1. The configuration at C(4) could not be established due to insufficient amounts of compound 1. Accordingly, compound 1 was determined to be 4,6-dihydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-trihydroxybenzoyl)]-β-D-glucopyranoside.
Figure 2.
Key HMBC Correlations of Compounds 1–3
Compound 2 was isolated as a white amorphous powder. The molecular formula C23H24O12 was deduced from the HR-ESI-MS (m/z 491.1191, [M – H]−) data. The 1H- and 13C-NMR spectra of 2 were very similar to those of 1, except for the substitution of the benzene ring, which showed proton signals at δ(H) 7.37 (1H, dd, J = 9.5, 1.0 Hz, H–C(8)), 7.26 (1H, dd, J = 10.0, 9.5 Hz, H–C(7)), and 7.14 (1H, dd, J = 10.0, 1.5 Hz, H–C(6)). These observations indicated that the benzene ring was a 1,2,3-trisubstituted system. In the HMBC spectrum of 2 (Figure 2), correlations of H–C(4) (δ(H) 5.24) with C(5) (δ(C) 156.0), C(9) (δ(C) 133.5) and C(10) (δ(C) 128.7), and H–C(8) (δ(H) 7.37) with C(1) (δ(C) 199.7) revealed that a hydroxyl group was attached to the C(5) position. Connectivities of the sugar moiety with the 4-hydroxy-α-tetralone skeleton and the galloyl unit were confirmed by the HMBC experiment, in which correlations of H–C(4) (δ(H) 5.24) with C(1′) (δ(C) 103.5), and H–C(6′) (δ(H) 4.57, 4.35) with C(7″) (δ(C) 167.3) were observed. Furthermore, enzymatic hydrolysis of 2 gave 7 as the aglycone, whose absolute configuration was determined as 4S by the positive [α]D value [7]. Acidic hydrolysis, followed by GC analysis, revealed D-glucose as the sugar residue. Thus, the structure of 2 was established as (4S)-4,5-dihydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-trihydroxy-benzoyl)]-β-D-glucopyranoside.
Compound 3 was obtained as a white amorphous powder. Its molecular formula was determined to be C23H24O13 by HR-ESI-MS ([M – H]−, m/z 507.1118). The 1H- and 13C-NMR spectra displayed the signals due to a 4-hydroxy-α-tetralone skeleton, a glucosyl moiety and a galloyl unit, suggesting that 3 was also a 4-hydroxy-α-tetralone derivative. The ortho coupled aromatic proton signals at δ(H) 7.35 and 6.89 (each 1H, d, J = 8.4 Hz) indicated a 1,2,3,4-tetrasubstituted benzene ring in 3. Acidic hydrolysis of 3 gave D-glucose as the sole sugar residue. In the HMBC spectrum of 3 (Figure 2), correlations of H–C(8) (δ(H) 7.35) with C(1) (δ(C) 199.0) and C(4) (δ(C) 71.0) and H–C(4) (δ(H) 5.25) with C(5) (δ(C) 143.0) assigned the ortho aromatic protons as H-C(7) and H-C(8). Furthermore, HMBC correlations of H–C(4) (δ(H) 5.25) with C(1′) (δ(C) 103.2) and H–(6′) (δ(H) 4.55, 4.34) with C(7″) (δ(C) 167.4) confirmed the connectivities of the sugar moiety with the 4-hydroxy-α-tetralone aglycone and the galloyl moiety. Accordingly, the structure of 3 was established as (4S)-4,5,6-trihydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-trihydroxybenzoyl)]-β-D-glucopyranoside.
Compounds 1–4, with a 4-hydroxy-α-tetralone aglycone and 6-O-galloylglucosyl moiety in molecule, were isolated for the first time from fresh pericarps of Juglans species. The known compound 4 was reported to have strong inhibitory activity against the protein tyrosine phosphatase 1B [6], which plays a major role in the dephosphorylation of the insulin receptor. Thus, it may have a potential for the development of new pharmacological agents for the treatment of type-2 diabetes and obesity.
The isolated compounds 2, 4 and 7–9 were evaluated for in vitro antifungal (Aspergillus fumigatus, Candida albicans, C. glabrata, C. krusei, Cryptococcus neoformans) and antibacterial (Escherichia coli, Mycobacterium intracellulare, Pseudomonas aeruginosa, Staphylococcus aureus and methicillin-resistant S. aureus) activities using a modified version of the CLSI (formerly NCCLS) methods [11–13]. Only compound 8 exhibited moderate antibacterial activity against S. aureus and methicillin-resistant S. aureus with 50% inhibitory concentrations (IC50)/minimum inhibitory concentrations (MIC) of 4.96/10.0 μg/mL, and 3.46/5.0 μg/mL. The IC50/MIC values of the positive antibacterial control ciprofloxacin were 0.13/0.50 μg/mL and 0.14/0.50 μg/mL.
Expertimental Part
General
FAB-MS (negative ion mode) and HR-ESI-MS (negative ion mode) spectra were measured on VG AutoSpec 3000 and API Qstar Pulsar LC/TOF spectrometers, respectively. The matrix for FAB-MS was glycerol. Optical rotations: SEPA-3000 automatic digital polarimeter. IR Spectra: Bio-Rad FTS-135 spectrometer; in cm−1. UV Spectra: JASCO V-560 UV/VIS spectrophotometer. 1D- and 2D-NMR spectra were run on a Bruker AM-400 or a DRX-500 instruments operating at 400 or 500 MHz for 1H, 100 or 125 MHz for 13C, respectively. Coupling constants were expressed in Hertz and chemical shifts were given on a ppm scale with tetramethylsilane as internal standard. CC: Sephadex LH-20 (Pharmacia Fine Chemical Co., Ltd.); silica gel (200–300 mesh, Qingdao Makall Group Co., Ltd.); MCI gel CHP 20P (Mitsubishi Chemical Co.). TLC was carried out on silica gel H-precoated plates (Qingdao Makall Group Co., Ltd.) with chloroform/methanol/water (8:2:0.2). Spots were detected by spraying with 10% sulfuric acid reagent followed by heating. GC analysis was run on a Shimadzu GC-14C gas chromatograph.
Plant Material
The fresh pericarps of J. sigillata were collected in August 2007 in the botanical garden of Kunming Institute of Botany, Chinese Academy of Sciences, Yunnan province, China, and identified by Prof. Xiao Cheng (Botanical Garden, Kunming Institute of Botany, Chinese Academy of Sciences). The voucher specimen (KIB-ZL2007002) has been deposited in the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences. The fresh material was soaked into 80% aqueous acetone at room temperature as soon as collected.
Extraction and Isolation
The fresh pericarps of J. sigillata (37 kg) were extracted three times with 80% aqueous acetone (30 L) at room temperature (each 7 days). After removal of the organic solvent under reduced pressure, the aqueous fraction was extracted with equivoluminal CHCl3 and EtOAc, successively, to give a CHCl3 fraction (24 g) and an EtOAc fraction (240 g).
The EtOAc fraction (240 g) was subjected to CC over Sephadex LH-20, eluted with MeOH/H2O (0:1–1:0) to afford four fractions (Frs. 1–4). Further CC on MCI-gel CHP20P and Sephadex LH-20, both of which were eluted with MeOH/H2O (0:1–4:6) gave 1 (2.0 mg) and 3 (3.0 mg) from Fr. 1 (4.9 g); 2 (44 mg) from Fr. 2 (3.8 g); and 4 (114 mg) from Fr. 3 (21.8 g); respectively. Fr. 4 (3.3 g) was subjected to CC over silica gel, eluted with petroleum ether/ethyl acetate (7:3, 1:1, and 2:8 successively), and then over Sephadex LH-20 and MCI-gel CHP20P, eluted with MeOH/H2O (0:1–3:7), to afford 5 (35 mg), 6 (6 mg), 7 (48 mg), 8 (25 mg), and 9 (20 mg).
4,6-Dihydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-trihydroxybenzoyl)]-β-D-glucopyranoside (1)
White amorphous powder, [α]16D = 0 (c = 0.08, MeOH). UV (MeOH): 290 (3.69), 224 (3.86), 207 (3.85). IR (KBr) cm−1: 3439, 1627, 1606, 1042, 577. 1H- and 13C-NMR data: Table 1. FAB-MS: 491 ([M - H]−). HR-ESI-MS: 491.1210 ([M - H]−, C23H23O12−; calc. 491.1190).
Table 1.
13C- and 1H-NMR Data of 1–3 (in CD3COCD3, 500 MHz and 125 MHz for 1H and 13C NMR, respectively, δ in ppm, J values in Hz)
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| δ(C) | δ(H) | δ(C) | δ(H) | δ(C) | δ(H) | |
| 1 | 197.8 | 199.7 | 199.0 | |||
| 2 | 35.0 | 2.63 (ddd, J = 4.5, 7.0, 17.0) | 33.4 | 2.88 (dt, J = 19.0, 6.0) | 33.1 | 2.78 (ddd, J = 4.5, 9.5, 18.5) |
| 2.28–2.37 (m) | 2.36–2.44 (m) | 2.34 (ddd, J = 4.5, 8.5, 18.5 ) | ||||
| 3 | 31.2 | 2.28 (dt, J = 17.0, 5.0 ) | 29.0 | 2.33 (dt, J = 21.0, 4.7) | 29.2 | 2.28 (dt, J = 17.5, 4.5) |
| 2.08–2.13 (m) | 2.11 (dt, J = 16.0, 4.7) | 2.05–2.09 (m) | ||||
| 4 | 74.4 | 4.82 (dd, J = 10.0, 4.3) | 71.0 | 5.24 (t, J = 4.0) | 71.0 | 5.25 (t, J= 4.0) |
| 5 | 114.5 | 7.08 (d, J= 2.4) | 156.0 | 143.0 | ||
| 6 | 163.1 | 121.8 | 7.14 (d, J = 7.0) | 151.6 | ||
| 7 | 116.3 | 6.81 (dd, J = 8.6, 2.4) | 130.2 | 7.26 (t, J = 7.8) | 115.8 | 6.89 (d, J= 8.4) |
| 8 | 130.0 | 7.74 (d, J= 8.6) | 118.2 | 7.37 (d, J = 7.8) | 120.6 | 7.35 (d, J= 8.4) |
| 9 | 124.3 | 133.5 | 124.9 | |||
| 10 | 147.0 | 128.7 | 129.3 | |||
| 1′ | 103.7 | 4.60 (d, J= 7.9) | 103.5 | 4.64 (d, J = 8.0) | 103.2 | 4.64 (d, J= 7.9) |
| 2′ | 74.3 | 3.31 (dd, J = 8.9, 7.9) | 74.4 | 3.22 (dd, J = 8.0, 9.0) | 74.2 | 3.22 (t, J= 8.5, 7.9) |
| 3′ | 77.1 | 3.48 (t, J= 8.9) | 76.9 | 3.48 (t, J = 8.9) | 76.8 | 3.47 (t, J= 8.9) |
| 4′ | 71.1 | 3.42 (t, J= 9.4) | 70.7 | 3.41 (t, J = 9.4) | 71.3 | 3.43 (t, J= 9.4) |
| 5′ | 76.5 | 3.67–3.71 (m) | 74.7 | 3.68–3.70 (m) | 74.6 | 3.65–3.69 (m) |
| 6′ | 63.4 | 4.57 (dd, J = 11.8, 2.5) | 64.4 | 4.57 (dd, J = 12.0, 2.5) | 64.4 | 4.55 (dd, J = 11.8, 2.0) |
| 4.36 (dd, J = 11.8, 7.2) | 4.35 (dd, J = 12.0, 8.5) | 4.34 (dd, J = 11.8, 7.1) | ||||
| 1″ | 121.0 | 120.8 | 120.8 | |||
| 2″ | 109.7 | 7.11 (s) | 109.7 | 7.12 (s) | 109.7 | 7.10 (s) |
| 3″ | 145.8 | 145.8 | 145.6 | |||
| 4″ | 139.0 | 139.1 | 138.9 | |||
| 5″ | 145.8 | 145.8 | 145.6 | |||
| 6″ | 109.7 | 7.11 (s) | 109.7 | 7.12 (s) | 109.7 | 7.10 (s) |
| 7″ | 167.3 | 167.3 | 167.4 | |||
(4S)-4,5-Dihydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-trihydroxybenzoyl)]-β-D-glucopyrano-side (2)
White amorphous powder, [α]26D = +10.3 (c = 0.20, MeOH). UV (MeOH): 321 (3.87), 235 (4.24), 198 (4.14). IR (KBr) cm−1: 3422, 1679, 1609, 1348, 1319, 1232, 1074, 1038, 589. 1H- and 13C-NMR data: Table 1. FAB-MS: 491 ([M - H] −). HR-ESI-MS: 491.1191 ([M - H] −, C23H23O12−; calc. 491.1189).
(4S)-4,5,6-Trihydroxy-α-tetralone-4-O-[6′-O-(3″,4″,5″-trihydroxybenzoyl)]-β-D-gluco-pyranoside (3)
White amorphous powder, [α]16D = 14.2 (c = 0.11, MeOH). UV (MeOH): 321 (3.87), 220 (4.03), 203 (4.08). IR (KBr) cm−1: 3425, 1611, 1318, 1225, 1038, 592. 1H- and 13C-NMR data: Table 1. FAB-MS: 507 ([M - H] −). HR-ESI-MS: 507.1118 ([M - H]−, C23H23O13−; calc. 507.1139).
Acidic Hydrolysis of Compounds 1–3
Compounds 1 (1.5 mg), 2 (4.0 mg) and 3 (1.5 mg) were separately hydrolyzed with 2 M HCl/dioxane (1:1, 4 mL) under reflux for 6 h. The reaction mixture was extracted with EtOAc for 4 times. The aqueous layer was neutralized with 2 M NaOH and dried under reduced pressure. The residue was dissolved in pyridine (2 mL). L-cysteine methyl ester hydrochloride (about 1.5 mg) was added and the mixture was kept at 60 °C for 1 h. Next, trimethylsilylimidazole (about 1.5 mL) was added to the reaction mixture in ice water and kept at 60 °C for 30 min. Then, the mixture was subjected to GC analysis, run on a Shimadzu GC-14C gas chromatograph equipped with a 30 m × 0.32 mm i.d. 30QC2/AC-5 quartz capillary column and an H2 flame ionization detector with the following conditions: column temperature, 180–280 °C; programmed increase, 3 °C/min; carrier gas, N2 (1 mL/min); injector and detector temperature, 250 °C; injection volume, 4 μL; and split ratio, 1/50. The configuration of the sugar moiety was determined by comparing the retention time with the derivatives of authentic samples. The retention times of D-/L-glucose were 19.21/14.94 min.
Enzymatic Hydrolysis of Compound 2
A solution of 2 (10 mg) in H2O was incubated with β-glucosidase (Sigma Chemical Co., 8.92 U/mg, 8 mg) at 37 °C for 7 days. The reaction mixture was extracted with CHCl3 for 5 times. The CHCl3 fraction was evaporated to dryness and subjected to column chromatography over MCI-gel CHP20P, eluted successively with water and methanol, to afford 7 (2 mg), [α]18D = +16.7 (c = 0.03, MeOH).
Antifungal and Antibacterial Bioassays
All organisms are obtained from the American Type Culture Collection (Manassas, VA) and include the fungi Candida albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, Cryptococcus neoformans ATCC 90113, and Aspergillus fumigatus ATCC 204305 and the bacteria Staphylococcus aureus ATCC 29213, methicillin-resistant S. aureus ATCC 33591 (MRS), Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, and Mycobacterium intracellulare ATCC 23068. Susceptibility testing is performed using a modified version of the CLSI methods [11] [12]. M. intracellulare is tested using a modified method of Franzblau et al [14]. Samples (2, 4, and 7–9) are serially-diluted in 20% DMSO/saline and transferred in duplicate to 96-well flat bottom microplates. Microbial inocula are prepared by correcting the OD630 of the microbe suspensions in incubation broth to afford final target inocula after addition to the samples. All organisms are read spectrometrically prior to and after incubation. The detailed protocol has been described in a previous paper [15].
Acknowledgments
The authors are grateful to the staffs of the analytical group at State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, for measuring the spectral data. This work was supported by the grants from NSFC (U0632010), the State Key Laboratory of Phytochemistry and Plant Resources in West China, Chinese Academy of Sciences (P2008-ZZ08), the West Light Program of the Chinese Academy of Sciences, and NIH (AI027094).
References
- 1.Kuang KR, Li PQ. Flora of China Science Press. 1979;21:30. [Google Scholar]
- 2.Hideyuki I, Takahiro O, Toshiyuki F, Tsutomu H, Takashi Y. J Agric Food Chem. 2007;55:672. [Google Scholar]
- 3.Li FS, Shen J, Tan GS. Chin Tradit Pat Med. 2007;29:1490. [Google Scholar]
- 4.Erdemoglu N, Kupeli E, Yesilada E. J Ethonpharmacol. 2003;89:123. doi: 10.1016/s0378-8741(03)00282-4. [DOI] [PubMed] [Google Scholar]
- 5.Muller WU, Leistner E. Phytochemistry. 1978;17:1739. [Google Scholar]
- 6.An TY, Hu LH, Chen RM, Chen ZL, Li J, Shen Q. Chinese Chem Lett. 2003;14:489. [Google Scholar]
- 7.Liu LJ, Li W, Koike K, Zhang SJ, Nikaido T. Chem Pharm Bull. 2004;52:566. doi: 10.1248/cpb.52.566. [DOI] [PubMed] [Google Scholar]
- 8.Machida K, Matsuoka E, Kasahara T, Kikuchi M. Chem Pharm Bull. 2005;53:934. doi: 10.1248/cpb.53.934. [DOI] [PubMed] [Google Scholar]
- 9.Liu JX, Di DL, Huang XY, Li C. Chinese Chem Lett. 2007;18:943. [Google Scholar]
- 10.Zou DP, Kim IH, Kawahara N, Goda Y. Jpn J Food Chem. 2006;13:114. [Google Scholar]
- 11.NCCLS. Reference method for broth dilution antifungal susceptibility testing of yeasts. approved standard. 2002;22:M27-A2. [Google Scholar]
- 12.NCCLS. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. approved standard. 2002;22:M38-A. [Google Scholar]
- 13.NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. approved standard. 2000;20:M7-A5. [Google Scholar]
- 14.Franzblau SG, Witzig RS, Mclaughlin JC, Torres P, Madico G, Hermandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. J Clin Microbiol. 1998;36:362. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samoylenko V, Ashfaq MK, Jacob MR, Tekwani BL, Khan SI, Manly SP, Joshi VC, Walker LA, Muhammad I. J Nat Prod. 2009;72:92. doi: 10.1021/np800653z. [DOI] [PMC free article] [PubMed] [Google Scholar]