Abstract
Exopolysaccharide (EPS) of Myxococcus xanthus is a well-regulated cell surface component. In addition to its known functions for social motility and fruiting body formation on solid surfaces, EPS has also been proposed to play a role in multi-cellular clumping in liquid medium, though this phenomenon has not been well studied. In this report, we confirmed that M. xanthus clumps formed in liquid were correlated with EPS levels and demonstrated that the EPS encased cell clumps exhibited biofilm-like structures. The clumps protected the cells at physiologically relevant EPS concentrations, while cells lacking EPS exhibited significant reduction in long-term viability and resistance to stressful conditions. However, excess EPS production was counterproductive to vegetative growth and viable cell recovery declined in extended late stationary phase as cells became trapped in the matrix of clumps. Therefore, optimal EPS production by M. xanthus is important for normal physiological functions in liquid.
Keywords: Myxococcus xanthus, exopolysaccharide, vegetative growth, stress survival, stationary phase recovery
Introduction
Myxococcus is a group of Gram-negative soil bacteria with complex life styles (Reichenbach, 1993). This study focuses on exopolysaccharide (EPS), a key component of Myxococcus extracellular matrix (ECM) (Behmlander and Dworkin, 1994; Dworkin, 1993), which is distributed over the entire cell surface of wild-type cells (Merroun et al., 2003). The current understanding of Myxococcus EPS mainly comes from the studies of M. xanthus behaviors on solid surfaces, where EPS plays important roles in fruiting body formation (Lux et al., 2004; Shimkets, 1986), social (S−) motility (Li et al., 2003; Lu et al., 2005), cell-cell cohesion (Arnold and Shimkets, 1988), cell-substratum adhesion, and protection from adverse environmental factors (Dworkin, 1993; Merroun et al., 1998). It is also known that the production of EPS in M. xanthus is well regulated by different genetic loci (Yang, 2008), such as the dif chemotaxis-like operon (Yang et al., 1998; Yang et al., 2000); pilA, a gene encoding the pilus structural protein (Black et al., 2006); eps and eas regions, encoding proteins for polysaccharide biosynthesis (Lu et al., 2005); and stkA and sglK, encoding DnaK homologues (Dana and Shimkets, 1993; Yang et al., 1998).
The functions of EPS in Myxococcus liquid cultures are much less studied. Due to EPS production, many strains of Myxococcus, especially newly isolated ones from soil (Wall et al., 1999), fail to grow as dispersed cells in liquid culture, and form thin films or clumps (Schurmann, 1967), which often hinders laboratory examination or genetic manipulation. This phenomenon has proposed to be associated with the production of EPS, as wild-type M. xanthus cells stick together to form clumps in liquid medium (Kim et al., 1999), while cells defective in EPS production, such as SW504 (ΔdifA) and SW810 (ΔepsA), can stay dispersed in liquids (Lu et al., 2005; Yang et al., 1998).
In this study, we are interested in that if different level of EPS production may affect M. xanthus physiology in liquid, since some previous studies in other bacteria indicate the similarities between bacterial cells within typical biofilms and cells within the aggregates in liquid (Costerton et al., 1995; Hall-Stoodley et al., 2004), while profound difference between planktonic and biofilm cells (Bhinu, 2005). The utilization of confocal laser scanning microscopy (CLSM) (Neu et al., 2010) and conjugated lectins (Flemming and Wingender, 2010) allows for direct visualization of EPS and cells in situ (Lux et al., 2004) and avoids the collapse of EPS which frequently occurs during the fixation and dehydration procedures for electron microscopic observation (Merroun et al., 2003). Combined with other microbiological and physical chemical assays, we investigated the effects of EPS production on liquid vegetative growth, stress survival and stationary phase recovery of M. xanthus by analyzing various mutants that produce negligible or excess EPS.
Materials and methods
Bacterial strains, media and growth conditions
To test the viability phenotypes of M. xanthus cells, different strains (listed in Table 1) were grown at 32 °C in casitone-yeast extract (CYE) medium (Campos et al., 1978) at 300 rpm for 1 d. The cells were harvested and the cell clumps were dissociated by vortexing in the presence of 3 mm glass beads, resuspended to 1×109 cells/ml with fresh CYE. Subsequent culturing was performed at 32 °C on a rotary shaker at 300 rpm. At various time points, cells and clumps were taken, dispersed by voretxing with glass beads and tested for viability by counting colony-forming unit (CFU) as previously described (Nelson and Killeen, 1986). Since the EPS production in M. xanthus is stationary-phase dependent (Kim et al., 1999), in order to investigate the long-term viability of these strains, a high cell concentration (1×109 cells/ml) was used for inoculation, which allowed the different strains to enter stationary phase rapidly and eliminated possible differences in the initial growth stages.
Table 1.
M. xanthus strains used in this study
| Strain | Relevant genotype | EPS productiona | Reference |
|---|---|---|---|
| M. xanthus | |||
| DK1622 | Wild type | 100±9.3 % | (Kaiser, 1979) |
| DK10547 | DK1622, gfp-expressing derivative | 95.2±10.9 % | (Welch and Kaiser, 2001) |
| DK10410 | DK1622, ΔpilA | 21.5±5.6 % | (Wu and Kaiser, 1996) |
| DK3088 | DK1622, stk | 197.6±21.8 % | (Dana and Shimkets, 1993) |
| SW504 | DK1622, ΔdifA | 2.7±1.6 % | (Yang et al., 1998) |
| SW505 | DK1622, difA::Tn5 kan903V101 | 3.2±1.9 % | (Yang et al., 1998) |
| SW810 | DK1622, ΔepsA | 3.9±3.1 % | (Lu et al., 2005) |
The data represent triplicate experiments, and mean ± SD is presented.
For the mixed culture experiments, about 8.0×107 SW505 (difA::Tn5, Kmr) cells were inoculated into 5 ml CYE together with 4.0×108 DK1622 (Wt) cells, and incubated at 32 °C on a rotary shaker at 300 rpm for 12 d. As a negative control, 4.8×108 SW505 cells were inoculated into 5ml CYE and 4.8×108 DK1622 cells were inoculated into 5 ml CYE as a positive control. At various time points, cells of SW505/DK1622 and SW505 were assayed for viable SW505 cells by CFU counting on CYE agar containing 100 μg/ml kanamycin, and cells of DK1622 were assayed for viable DK1622 on CYE agar.
For the re-growth test, the cell clumps of DK1622 (Wt) or DK3088 (stk) were directly removed from 4 d cultures and gently washed twice in fresh CYE. Cell clumps (about 15 mg of dry biomass) were either directly inoculated into 50 ml fresh CYE medium or dispersed by vortexing with glass beads prior to inoculation. These cultures were incubated at 32 °C at 50 rpm and cell growth was monitored by determining the dry biomass. The biomass was collected by 13,000 ×g centrifugation and dried at 80 °C for 24 h.
Trypan blue binding assay
EPS production of different strains in CYE liquid was measured using a trypan blue binding assay (Black and Yang, 2004). The relative amounts of EPS were calculated relative to trypan blue bound to the wild-type DK1622 cells.
Agglutination assay
The cohesion of M. xanthus cells was measured with an agglutination assay described by Shimkets (Shimkets, 1986; Shimkets, 1986). The percentage of agglutination was calculated as the ratio of OD600nm at different time point versus initial absorbance at 600 nm.
Measurement of viscosity and rheology
Cells of different strains were harvested from 1 d liquid CYE cultures. EPS were isolated and purified from 5×1010 cells according to the protocol previously described (Chang and Dworkin, 1994; Li et al., 2003), and suspended in 2 ml 0.3 M AcOH/0.05 M AcONa/4 mM CaCl2 buffer to generate a homogeneous gel suspension. The efflux times of the EPS suspensions and solvent were measured using an Ubbelohde capillary viscometer (DI10070-75) in a constant-temperature water bath at 32 ± 0.5 °C. The specific viscosity (ηsp) was calculated as:
| [1] |
where η is the dynamic viscosity of an EPS suspension and η0 is the dynamic viscosity of buffer.
The solvent used for rheological experiments was MMC buffer (10 mM MOPS, 8 mM MgSO4, 4 mM CaCl2). The lyophilizated EPS isolated from wild-type DK1622 cells were crushed in a mortar, weighted and suspended in the buffer. Then, WT-EPS suspensions with different concentrations were incubated at 32 °C for 48 hr. The measurement of apparent viscosity of EPS suspensions was performed on a LDV-III Ultra rheometer (Brookfield, US) equipped with a LV-1 spindle and an UL-adapter. The influence of shear rate on rheological curves of EPS suspensions was determined at 32 ± 0.1 °C.
Sample preparation and staining method
At different time points, cell clumps were directly isolated from liquid cultures of EPS+ strains while the cell pellets were collected from EPS− strains following 13,000 ×g centrifugation for 5 min. The cell-membrane-permeant nucleic acid binding dyes, SYTO 9 or SYTO 82 (both at 5 μM, Molecular Probes, USA), were used to differentiate cells from debris and matrix. 5 mM 5-cyano-2,3-ditolyl tetrazolium chloride (CTC, Molecular Probes), a red fluorescent indicator dye of respiratory activity, was used to reveal metabolically active cells. Carbohydrates present in the EPS portion of the cell clumps or pellets were stained with 5 μg/ml of Alexa 633-conjugated derivatives of wheat germ agglutinin lectin (WGA, Molecular Probes) as previously described (Lux et al., 2004). Before the examination under CLSM, the specimens were incubated with dyes in MOPS buffer (10 mM MOPS, 8 mM MgSO4, pH 7.6) for 30 min in the dark. The 12 d clumps formed by EPS+ cells were incubated with 5 mM CTT in CYE medium for 6 h, then CYE medium was removed by aspiration, replenished with MOPS buffer containing SYTO 9 and Alexa 633-WGA, and incubated for 30 min in the dark.
Confocal laser scanning microscopy (CLSM)
CLSM was employed to visualize the M. xanthus clumps and pellets with various dye combinations using a PASCAL5 confocal laser scanning microscope (Zeiss, Germany). Excitation at 488 nm in combination with a 505–530 nm band-pass emission filter were used for Gfp and SYTO 9 imaging, respectively. CTC was visualized using 488 nm excitation and a 560–615 nm band-pass emission filter. SYTO 82 signals were visualized using 543 nm excitation with a helium-neon laser and a 560–615 nm band-pass emission filter. Excitation at 633 nm and a 650 nm long-pass emission filter were used to reveal Alexa 633-WGA.
Exposure to UV irradiation
All planktonic and clumped M. xanthus cells were harvested from 1 d CYE liquid cultures. The clumps in broth were removed with 500 ×g centrifugation for 10 min, and DK1622 (Wt) planktonic cells were collected by 13,000 ×g centrifugation of supernatant for 5 min. After washing two times with MMC buffer, about 5×108 cells or clumps containing equal amount of cells were suspended in 1ml MOPS buffer. The suspensions were transferred into 6-well culture plates and irradiated in a microprocessor controlled UV crosslinker (XL-1000, Spectroline, US) to achieve the inactivation effects as previously described (Sudo and Dworkin, 1969). Viable cell counts were taken before and immediately after UV exposure with the CFU assay described above, and SW505 (difA::Tn5, Kmr) viable cells were enumerated on CYE agar containing 100 μg/ml kanamycin. All dose-survival experiments with planktonic and clumped cells were done with five different dosages controlled by energy level of crosslinker, and two replicate irradiated suspensions. The UV dosage required for 99.9% inactivation of cells was determined using a method previously described (Chang et al., 1985).
Sodium dodecyl sulphate (SDS) treatment
The preparation of cells and clumps for SDS treatment was identical to that used for UV irradiation described above. After washing, the cells or clumps (about 5×108 cells) were suspended in 1 ml MMC buffer containing 0.01% SDS as previously described (Elias and Murillo, 1991), while mock treatment was done with MMC buffer. The suspensions were incubated at 32 °C for 30 min, and the cells or clumps were washed 2 times with MMC buffer to remove residual SDS. The viable cells were numerated with the CFU assay described above, and survival rate of each strain was calculated as the ratio of viable cells numbers in SDS treated samples versus that in mock treated samples. Two replicate experiments were performed.
Results
EPS production influences viability of M. xanthus cells during liquid vegetative growth
As a mixture of polysaccharides, the absolute amount of EPS in stationary broth of M. xanthus wild-type strain DK1622 was about 127 ± 9 μg of carbohydrate/mg of protein, which was determined as the amount of anthrone-reactive materials (Kim et al., 1999). A trypan blue binding assay was further developed to quantitatively measure the relative amount of EPS of different M. xanthus strains (Black and Yang, 2004). Using this assay, EPS production of four M. xanthus mutants in liquid medium was determined (Table 1). Strains SW504 (ΔdifA) and SW810 (ΔepsA) are defective in EPS production due to mutations in EPS regulatory and biogenesis genes, respectively (Lu et al., 2005; Yang et al., 2000); DK10410 (ΔpilA) produces about 20% of the amount of EPS as DK1622, because PilA positively functions upstream of Dif proteins in regulating EPS production (Black et al., 2006); and mutation of the stk locus results in cells with a higher-than-normal level of EPS (Dana and Shimkets, 1993), such as in the strain DK3088.
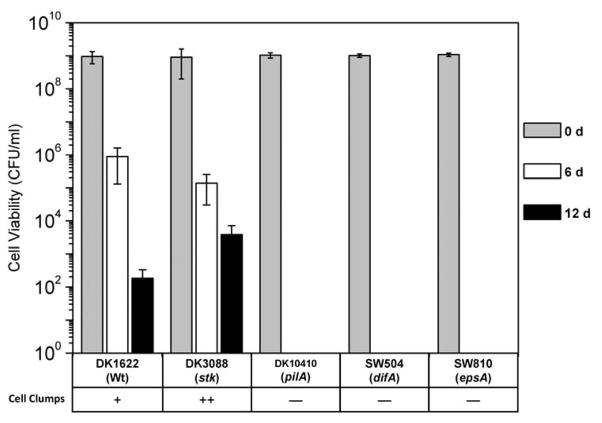
In liquid cultures (Fig. 1), cells producing negligible or much reduced EPS (SW504, SW810 and DK10410), which grew dispersed without forming aggregates, were not viable after 6 d. In strains producing sufficient EPS (DK1622 and DK3088), cell clumps were observed and viable cells were still detected after 12d. The EPS-overproducing strain DK3088 (stk) displayed enhanced survival abilities compared to wild-type strain DK1622. Microscopic examination revealed that the DK3088 cells had formed a significantly greater number of large clumps versus DK1622 cells. These results show a correlation between EPS production with both clumping and long-term viability of M. xanthus cells in liquid culture.
Fig. 1.
Viabilities of M. xanthus strains in liquid culture. Viable cells of different strains were enumerated from CFU per milliliter in broth on 0 d, 6 d and 12 d. The data represent triplicate experiments, and mean ± SD is plotted. Cell clumps in the liquid medium were examined through microscope and ‘−’ represents not detected.
Clump forming in M. xanthus broth is a consequence of EPS production
The cohesion of different cells in liquid was examined and shown in Fig. 2A. DK1622 (Wt) and DK3088 (stk) showed rapid agglutination, while SW504 (ΔdifA) and SW810 (ΔepsA) were defective in cellular cohesion, which is consistent with their EPS production (Table 1) and cell clumping (Fig. 1) phenotypes. However, about 30% agglutination (Fig. 2A) was detected in DK10410 (ΔpilA), though stable clumps were not observed in this strain under our shaken culture condition (Fig. 1).
Fig. 2.
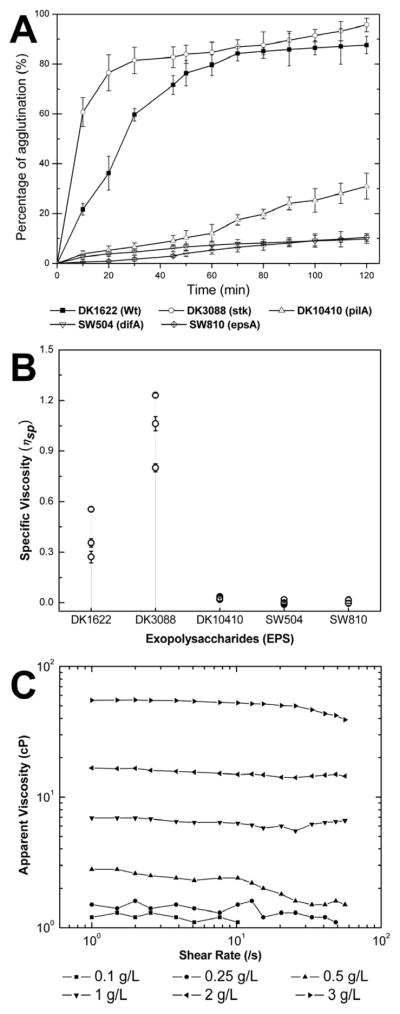
Agglutination of M. xanthus cells and rheological properties of EPS. Panel A: agglutination of mutants in broth. Cells were grown overnight in CYE medium and the OD600nm was adjusted to about 1.0. The assay was conducted as described in Materials and Methods. The data represent triplicate experiments, and mean ± SD is plotted. Panel B: specific viscosity (ηsp) of EPS suspensions isolated from 5×1010 cells of different strains. For each strain, three parallel EPS samples were prepared and each sample was tested for three times, and mean ± SD were plotted. Panel C: influence of shear rate and concentration on the rheological curves of WT-EPS suspensions.
Next, the rheological properties of EPS from different M. xanthus strains were further investigated. Because of their large molecular mass, EPS normally yield highly viscous aqueous solutions or suspensions with complicated molecular interactions, which are important chemical and physical features for its function in cell cohesion and matrix formation (Sutherland, 2001). EPS isolated from equal cell numbers of different strains were suspended in buffer to determine their specific viscosity (ηsp, equation [1]). As shown in Fig. 2B, DK3088-EPS showed the highest ηsp, while suspensions of EPS from DK10410 (ΔpilA), SW504 (ΔdifA) and SW810 (ΔepsA) exhibited very low viscosities (ηsp < 0.05). Although DK10410 (ΔpilA) cells still produce EPS and exhibit agglutination to a certain degree (Fig. 2A), the non-clumping phenotype in shaken liquid might be due to the low viscosity of its EPS. After examining the apparent viscosity of a DK1622-EPS (WT-EPS) suspension, a clear concentration-dependent viscosity against shear force was observed (Fig. 2C). At low concentrations (≤ 0.5 g/L), the viscosity was greatly decreased and the EPS suspensions exhibited a non-Newtonian behavior of shear thinning fluids, which was consistent with the observation of specific viscosity (Fig. 2B). At high concentrations (≥ 1 g/L), the highly viscous suspensions exhibited a complex behavior upon shear rates. Consistent with previous findings (Kim et al., 1999), these results confirmed the capacity of M. xanthus EPS to encase cells and form clumps in broth.
M. xanthus cell clumps formed in broth exhibit biofilm-like structures
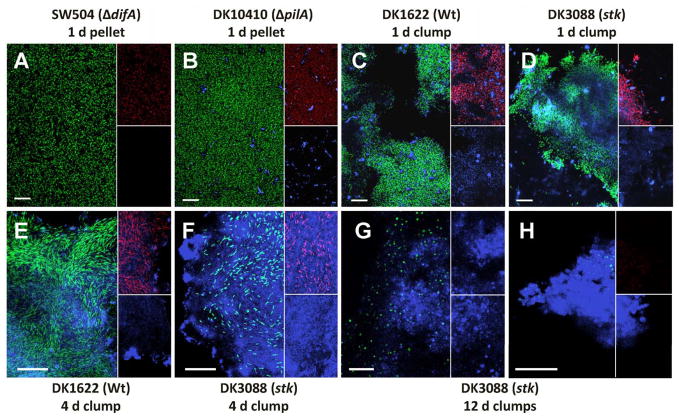
CLSM in combination with specific indicator dyes was employed to in situ visualize the distributions of cells and EPS in the clumps or pellets. Counterstaining with SYTO 9 differentiated cells from debris and the matrix (Fig. 3, green). Respiring cells were visualized with CTC (Fig. 3, red). EPS was revealed by Alexa 633-WGA labeling (Fig. 3, blue). The pellets derived from 1 d cultures of strains with reduced (DK10410) or no (SW504 and SW810) EPS production, did not contain EPS-encased clumps but the cells were metabolically active (Fig. 3A and B). No respiring cells were apparent in the respective 12 d cultures (lacking of red staining, data not shown).
Fig. 3.
Examination of cells and EPS in cell pellets or clumps derived from different M. xanthus strains. Panels A and B: pellets of 1 d cultures of SW504 (ΔdifA, and SW810-ΔepsA was similar in appearance) and DK10410 (ΔpilA) collected by centrifugation at 13,000 ×g for 5 min, respectively; panels C and D: clumps taken directly from 1 d cultures of DK1622 (Wt) and DK3088 (stk), respectively; panels E and F: clumps from 4 d cultures of DK1622 and DK3088, respectively; panels G and H: clumps from 12 d cultures of DK3088. Samples were counterstained with 5 μM SYTO 9, 5 mM CTC and 5 μg/ml Alexa 633-WGA in MOPS buffer. The panels are the overlay images of SYTO 9 (green) and Alexa 633-WGA (blue) signals, the upper small frames in the panels are the overlay images of CTC (red) and Alexa 633-WGA (blue) signals, and the lower frames are Alexa 633-WGA (blue) images, except that the upper small frame in the panel H shows the CTC (red) signal only. Bars represent 20 μm.
EPS-encased DK1622 (Wt) and DK3088 (stk) cell clumps appeared structurally similar to M. xanthus biofilms (Lux et al., 2004) with respiring cells being embedded in the EPS network (Figs. 3C–F), especially in the clumps formed in 4 d broth (Figs. 3E and F). 12 d cultures of DK1622 and DK3088 contained predominantly sphere-like cells that stained poorly with CTC (Fig. 3G) and were likely to be composed primarily of dead cells. However, these cultures also contained tight EPS matrix structures enclosing some metabolically active cells (Fig. 3H), which were observed more frequently in DK3088 (stk) cultures. The existence of these biofilm-like EPS structures might explain the longevity of DK3088 (stk) and DK1622 (Wt) under extended stationary phase culture conditions.
EPS-enclosed M. xanthus cells in clumps are more resistant to UV irradiation and SDS treatment
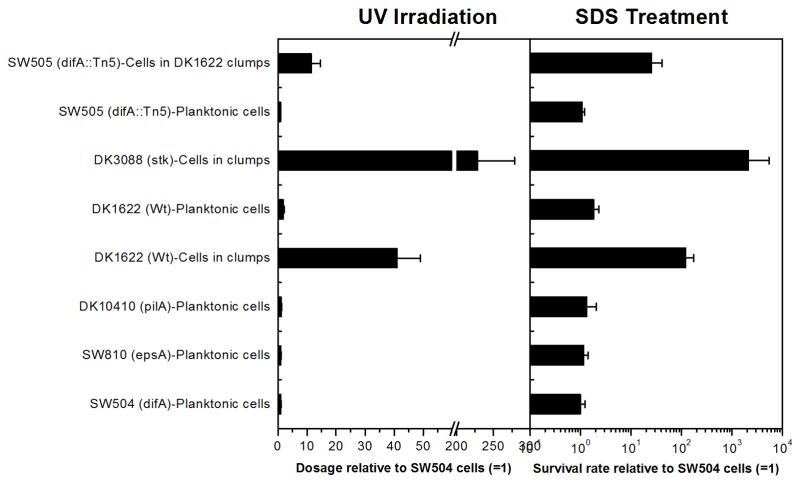
Since biofilm cells have a selective advantage for survival (Costerton et al., 1995; Flemming and Wingender, 2010), response of EPS-enclosed M. xanthus clump cells to UV irradiation was examined. Planktonic cells of SW504 (ΔdifA), SW810 (ΔepsA) and DK10410 (ΔpilA) exhibited similar resistance to UV light and required a similar dose for 99.9% of inactivation (Fig. 4, left panel). DK1622 (Wt) cells in clumps required an about 41 times higher dose for 99.9% inactivation, and DK3088 (stk) cells in clumps were the most UV-resistant cells. As expected, DK1622 planktonic cells collected from supernatant of broth were about 23 times less resistant than the clumping cells. Similar results were obtained for the sensitivity of cells to SDS (Fig. 4-right panel). The cells in DK1622 (Wt) and DK3088 (stk) clumps were more resistant to SDS-treatment than the planktonic cells. These results suggest that EPS matrix of clumps protects M. xanthus cells from environmental stresses.
Fig. 4.
Stress survival of different M. xanthus strains. Left panel: relative UV254nm doses required for 99.9% inactivation of different mutants compared to that for SW504 planktonic cells; right panel: relative survival rate after 0.01% SDS-30 min treatment of different cells compared to that of SW504 planktonic cells.
EPS produced by wild-type cells have a protective effect on mutants deficient in EPS production
To further examine the protective role of EPS, an EPS− strain, SW505, was co-cultured with DK1622 (Wt). SW505 is deficient in EPS production (Table 1) and carries a Tn5 insertion in the difA gene (Yang et al., 1998), thereby allowing distinction from the kanamycin sensitive DK1622. After 12 d shaken, SW505 cultured alone did not survive, while the mixed culture still contained viable SW505 cells, which was comparable to the ratio of survival cells in the positive control (DK1622 alone). Furthermore, SW505 cells coexisting with DK1622-clumps in the mixed culture exhibited more resistance to UV irradiation and SDS treatment than the planktonic SW505 cells; they required about 11 times more UV dosage for 99.9% inactivation and showed about 25 times higher survival rate after SDS treatment (Fig. 4).
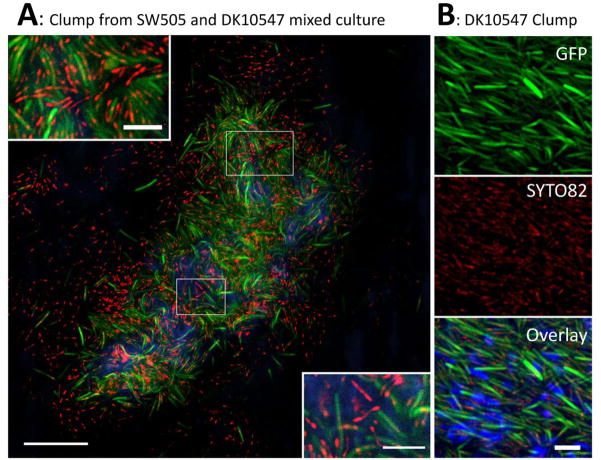
To determine whether SW505 (difA::Tn5) cells were included in DK1622 cell clumps, SW505 cells were inoculated with DK10547, a gfp-expressing derivative of DK1622 (Welch and Kaiser, 2001) with wild-type EPS production (Table 1). The SW505/DK10547 mixture from a one day culture was stained with SYTO 82 and Alexa 633-WGA (Fig. 5A). All the gfp-expressing DK10547 cells appeared green with compact red spots, since SYTO 82 bound the nucleic acids inside the cells. The aggregates were mainly composed of DK10547 cells (green with red) and EPS (blue), but also did contain SW505 cells (red only). In the clumps from DK10547 single culture, only green-red cells were observed after counterstaining (Fig. 5B). This clearly demonstrated the integration of SW505 into cell clumps with DK10547, which could be responsible for the increased survival rate and UV-resistance of the former.
Fig. 5.
Aggregates of SW505 (difA::Tn5) cells, DK10547 (gfp-expressing derivative of DK1622) cells and EPS from a mixed 1 d culture (Panel A). Samples were counterstained with SYTO 82 and Alexa 633-WGA. The image is the overlay of gfp-expressing and SYTO 82 stained DK10547 (green with yellow dots), SYTO 82 stained SW505 (red) and Alexa 633-WGA-labeled EPS (blue) signals. Bar represents 20 μm. The small panels show magnified portions of the image indicated by the white panes and the bars represent 5 μm. Panel B, clump from a DK10547 (Gfp, upper small panel) single 1 d culture. Sample was counterstained with SYTO 82 (middle small panel) and Alexa 633-WGA (overlay with green and red signals in bottom small panel). Bar represents 5 μm.
Overproduced EPS traps live cells inside the matrix and prevents re-growth
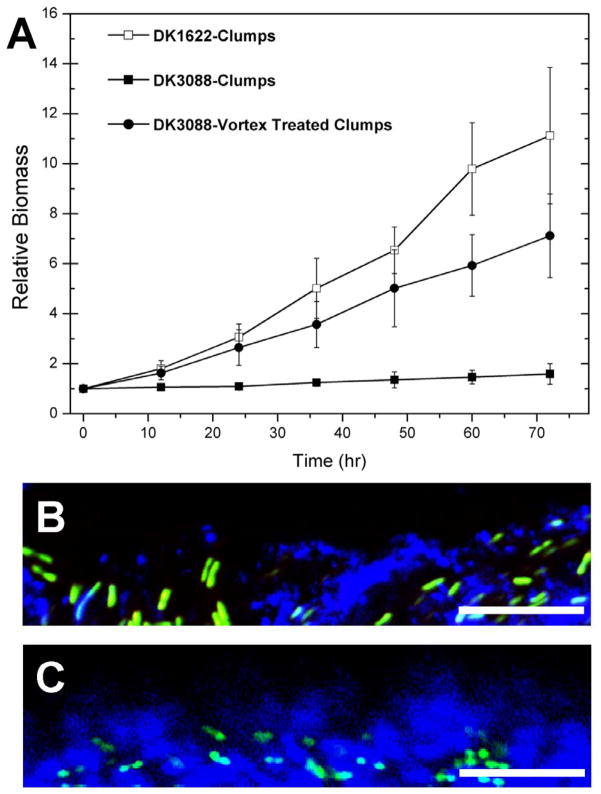
While long-term survival under nutrient limitation is an important feature in the M. xanthus life-cycle, the ability to proliferate when environmental conditions improve is another important aspect. Therefore, we examined the ability of the EPS-producing strains to re-grow upon transfer into fresh medium after extended growth under stationary phase conditions. DK1622 clumps exhibited consistent re-growth, while no re-growth was apparent for DK3088 cell clumps. However, after disassociation via vortexing, DK3088 cell clumps regained the ability of re-growth (Fig. 6A).
Fig. 6.
Re-growth and clump surface-medium interfaces of cell clumps of DK1622 (Wt) and DK3088 (stk). Panel A: re-growth curves of DK1622 and DK3088 (with or without pre-vortexing treatment) clumps form 4 d cultures. The relative biomass was calculated as dry biomass at each time point versus the dry biomass of inoculation (at 0 hr). Panels B and C show the clump surface-medium interfaces of different cell clumps examined through a 63× objective lens with CLSM and samples were counterstained with SYTO 9 (green) and Alexa 633-WGA (blue). Panels B is a clump from 4 d cultures of DK1622, and panels C is a clump from 4 d cultures of DK3088. Bars represent 10 μm.
Given the apparent effect of EPS overproduction on re-growth of DK3088 (stk), we hypothesized that the over-abundance of EPS might reduce the recovery of M. xanthus cells from clumps. We examined the clump surface-medium interface of SYTO 9 (cells) and Alexa 633-WGA (EPS) labeled cell clumps with CLSM. Cultures that were able to re-grow had loosely packed EPS matrices that allowed the cells to be released (DK1622 cell clumps, Fig. 6B). On the other hand, cell clumps with densely packed EPS matrices, such as those present in the DK3088 4 d cultures, formed a thick layer of EPS at the interface between the cells and the medium (Fig. 6C). Thus, the cells in the overproduced-EPS matrix may be trapped, and unable to escape and proliferate.
Discussion
The biochemical analysis revealed that M. xanthus ECM was mainly composed of a carbohydrate matrix (EPS) with associated proteins (Behmlander and Dworkin, 1994; Dworkin, 1993). Further experiments have shown that the EPS portion is sufficient for rescuing social interactions in strains lacking ECM (Li et al., 2003; Lu et al., 2005). Consistent with these observations, the phenotypes of the mutants with alterations in different genetic loci responsible for M. xanthus EPS production examined in this study suggested that EPS was the key component for cellular agglutination and clumping in liquid cultures. This was also confirmed by examining the rheological properties of isolated EPS samples. For some bacteria, type IV pili (TFP) are part of the important extracellular apparatus for agglutination and biofilm formation (Costerton et al., 1999). In M. xanthus, the cells lacking EPS (strain SW504, ΔdifA) were defective in the formation of clumps or submerged biofilms (Yang, 2008) even when surface pili were overproduced (Li et al., 2003). Cells lacking TFP (DK10410, ΔpilA) also failed to form clumps in liquid, which might be due to their much reduced EPS production regulated by TFP system (Black et al., 2006) and viscosity. These observations suggested that the formation of clumps in M. xanthus was most likely correlated with EPS levels rather than the presence of surface pili.
For M. xanthus on solid surfaces, EPS is able to mediate cell-to-cell aggregation and adhesion to the growth substrata forming biofilms, preventing dehydration and protecting against phagocytosis and toxins (Dworkin, 1993). It has also been proposed that EPS could protect M. xanthus cells in their habitats by preventing toxic heavy metals from contacting the cells (Merroun et al., 1998). In this study, we demonstrated that the EPS encased M. xanthus cell clumps in broth culture exhibited biofilm-like structures. In addition, physiologically like biofilm cells, the clumped cells have an increased capacity for stress resistance. For example, clumped cells exhibit prolonged cell viability in broth and increased resistance to both UV radiation and SDS treatment. The survival rate after UV irradiation of cells in the clumps was greater than that of free cells, which may be due to the physical shielding of cells by the EPS matrix or outer cells of clumps. Such a phenomenon was previously suggested in biofilms of Pseudomonas aeruginosa (Elasri and Miller, 1999). Indeed, biofilm cells are about 100 to 1000 times more resistant to UV than planktonic cells (Bak et al., 2009). The increased resistance to the anionic surfactant SDS by clumped M. xanthus cells observed in this study also supports the idea that EPS could confer increased environmental resistance to biocides (Ganeshnarayan et al., 2009; Mah and O’Toole, 2001). Our current findings are in agreement with the general observation that aggregates of bacterial cells (flocs or floccules) that are not attached to a surface share many characteristics with surface attached biofilms (Costerton et al., 1995; Hall-Stoodley et al., 2004). Considering that cells in biofilms express properties distinct from planktonic cells (Bhinu, 2005; Mah and O’Toole, 2001), M. xanthus cells may have different physiological and metabolic properties when grown in clumps relative to suspensions and this may complicate their manipulation in laboratory settings.
Just as EPS underproduction is detrimental to survival, overproduction of EPS also appears to have a counterproductive long-term influence. Excess EPS production resulted in decreased viable cell recovery during extended late stationary phase. This may be partially attributable to the increased viscosity of the EPS obtained from the EPS-overproducing strain DK3088 (stk) as compared to WT-EPS suspensions. The cells in the overproduced-EPS matrix may be trapped, and unable to escape and proliferate. Therefore, optimal EPS production by M. xanthus is important for normal physiological functions.
Myxococcus ssp. is commonly found in terrestrial habitats (Dawid, 2000; Reichenbach, 1999), while fewer strains have been isolated from aqueous samples (Li et al., 2002; Velicer and Hillesland, 2008). This observed difference in abundance between terrestrial and aquatic environments may be at least partially attributable to the fact that cells growing on terrestrial surfaces tend to produce more EPS for social behaviors, e.g. S motility (Li et al., 2003; Lu et al., 2005) and fruiting body development (Lux et al., 2004; Shimkets, 1986). Our data suggests that many of these strains may be unable to exit their gelatinous EPS encapsulation when they are washed into aqueous environments. However, some M. xanthus strains have adapted to environments with periodic dry spells while also having the capacity to live permanently in fresh water habitats (Reichenbach, 1993). Forming biofilm-like clumps of cells in liquid, instead of living as dispersed planktonic cells, provides several selective advantages for M. xanthus in nature. The cells can prolong their viability inside clumps without developing fruiting bodies or myxospores, both of which require solid surface attachment (Diodati et al., 2008). At the same time, it appears that EPS-encased M. xanthus cells in clumps are more resilient than planktonic cells in coping with environmental stresses like UV light.
Acknowledgments
We thank Drs. Mitch Singer, Lawrence Shimkets and Dale Kaiser for strains, Dr. Xuesong He for helpful discussion, and Dr. Howard Kuramitsu for editing the manuscript. This work was supported by US NIH Grant GM54666 (to W.S.) and China NSFC Grant 30870020 (to W.H.).
References
- Arnold JW, Shimkets LJ. Cell surface properties correlated with cohesion in Myxococcus xanthus. J Bacteriol. 1988;170:5771–5777. doi: 10.1128/jb.170.12.5771-5777.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling. 2009;25:289–296. doi: 10.1080/08927010802716623. [DOI] [PubMed] [Google Scholar]
- Behmlander RM, Dworkin M. Biochemical and structural analyses of the extracellular matrix fibrils of Myxococcus xanthus. J Bacteriol. 1994;176:6295–6303. doi: 10.1128/jb.176.20.6295-6303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhinu VS. Insight into biofilm-associated microbial life. J Mol Microbiol Biotechnol. 2005;10:15–21. doi: 10.1159/000090344. [DOI] [PubMed] [Google Scholar]
- Black WP, Xu Q, Yang Z. Type IV pili function upstream of the Dif chemotaxis pathway in Myxococcus xanthus EPS regulation. Mol Microbiol. 2006;61:447–456. doi: 10.1111/j.1365-2958.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- Black WP, Yang Z. Myxococcus xanthus chemotaxis homologs DifD and DifG negatively regulate fibril polysaccharide production. J Bacteriol. 2004;186:1001–1008. doi: 10.1128/JB.186.4.1001-1008.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos JM, Geisselsoder J, Zusman DR. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- Chang BY, Dworkin M. Isolated fibrils rescue cohesion and development in the Dsp mutant of Myxococcus xanthus. J Bacteriol. 1994;176:7190–7196. doi: 10.1128/jb.176.23.7190-7196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG, Johnson JD. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 1985;49:1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Dana JR, Shimkets LJ. Regulation of cohesion-dependent cell interactions in Myxococcus xanthus. J Bacteriol. 1993;175:3636–3647. doi: 10.1128/jb.175.11.3636-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid W. Biology and global distribution of myxobacteria in soils. FEMS Microbiol Rev. 2000;24:403–427. doi: 10.1111/j.1574-6976.2000.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Diodati ME, Gill RE, Plamann L, Singer M. Initiation and early developmental events. In: Whitworth DE, editor. Myxobacteria: multicellularity and differentiation. ASM Press; Washington, D.C., USA: 2008. pp. 43–76. [Google Scholar]
- Dworkin M. Cell surfaces and appendages. In: Dworkin M, Kaiser D, editors. Myxobacteria II. ASM Press; Washington, D.C., USA: 1993. pp. 63–84. [Google Scholar]
- Elasri MO, Miller RV. Study of the response of a biofilm bacterial community to UV radiation. Appl Environ Microbiol. 1999;65:2025–2031. doi: 10.1128/aem.65.5.2025-2031.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias M, Murillo FJ. Induction of germination in Myxococcus xanthus fruiting body spores. J General Micobiol. 1991;137:381–388. [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Ganeshnarayan K, Shah SM, Libera MR, Santostefano A, Kaplan JB. Poly-N-acetylglucosamine matrix polysaccharide impedes fluid convection and transport of the cationic surfactant cetylpyridinium chloride through bacterial biofilms. Appl Environ Microbiol. 2009;75:1308–1314. doi: 10.1128/AEM.01900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ramaswamy S, Downard J. Regulated exopolysaccharide production in Myxococcus xanthus. J Bacteriol. 1999;181:1496–1507. doi: 10.1128/jb.181.5.1496-1507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sun H, Ma X, Lu A, Lux R, Zusman D, Shi W. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc Natl Acad Sci USA. 2003;100:5443–5448. doi: 10.1073/pnas.0836639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YZ, Hu W, Zhang YQ, Qiu ZJ, Zhang Y, Wu BH. A simple method to isolate salt-tolerant myxobacteria from marine samples. J Microbiol Methods. 2002;50:205–209. doi: 10.1016/s0167-7012(02)00029-5. [DOI] [PubMed] [Google Scholar]
- Lu A, Cho K, Black WP, Duan XY, Lux R, Yang Z, Kaplan HB, Zusman DR, Shi W. Exopolysaccharide biosynthesis genes required for social motility in Myxococcus xanthus. Mol Microbiol. 2005;55:206–220. doi: 10.1111/j.1365-2958.2004.04369.x. [DOI] [PubMed] [Google Scholar]
- Lux R, Li Y, Lu A, Shi W. Detailed three-dimensional analysis of structural features of Myxococcus xanthus fruiting bodies using confocal laser scanning microscopy. Biofilms. 2004;1:293–303. [Google Scholar]
- Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Merroun ML, Ben Chekroun K, Arias JM, Gonzalez-Munoz MT. Lanthanum fixation by Myxococcus xanthus: cellular location and extracellular polysaccharide observation. Chemosphere. 2003;52:113–120. doi: 10.1016/S0045-6535(03)00220-0. [DOI] [PubMed] [Google Scholar]
- Merroun ML, Ben Omar N, Gonzalez-Munoz MT, Arias JM. Myxococcus xanthus biomass as biosorbent for lead. J Appl Microbiol. 1998;84:63–67. doi: 10.1046/j.1365-2672.1997.00303.x. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Killeen KP. Heat shock proteins of vegetative and fruiting Myxococcus xanthus cells. J Bacteriol. 1986;168:1100–1106. doi: 10.1128/jb.168.3.1100-1106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu TR, Manz B, Volke F, Dynes JJ, Hitchcock AP, Lawrence JR. Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol Ecol. 2010;72:1–21. doi: 10.1111/j.1574-6941.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- Reichenbach H. Biology of myxobacteria: ecology and taxonomy. In: Dworkin M, Kaiser D, editors. Myxobacteria II. ASM; Washington, D.C., USA: 1993. pp. 13–62. [Google Scholar]
- Reichenbach H. The ecology of the myxobacteria. Environ Microbiol. 1999;1:15–21. doi: 10.1046/j.1462-2920.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- Schurmann C. Growth of myxococci in suspension in liquid media. Appl Microbiol. 1967;15:971–974. doi: 10.1128/am.15.5.971-974.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets LJ. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986;166:837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets LJ. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986;166:842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo SZ, Dworkin M. Resistance of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1969;98:883–887. doi: 10.1128/jb.98.3.883-887.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology. 2001;147:3–9. doi: 10.1099/00221287-147-1-3. [DOI] [PubMed] [Google Scholar]
- Velicer GJ, Hillesland KL. Why cooperate? The ecology and evolution of myxobacteria. In: Whitworth DE, editor. Myxobacteria: multicellularity and differentiation. ASM; Washington, D.C., USA: 2008. pp. 17–40. [Google Scholar]
- Wall D, Kolenbrander PE, Kaiser D. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J Bacteriol. 1999;181:24–33. doi: 10.1128/jb.181.1.24-33.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R, Kaiser D. Cell behavior in traveling wave patterns of myxobacteria. Proc Natl Acad Sci USA. 2001;98:14907–14912. doi: 10.1073/pnas.261574598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SS, Kaiser D. Markerless deletions of pil genes in Myxococcus xanthus generated by counterselection with the Bacillus subtilis sacB gene. J Bacteriol. 1996;178:5817–5821. doi: 10.1128/jb.178.19.5817-5821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Geng Y, Shi W. A Dnak homolog in Myxococcus xanthus is involved in social motility and fruiting body formation. J Bacteriol. 1998;180:218–224. doi: 10.1128/jb.180.2.218-224.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Geng Y, Xu D, Kaplan HB, Shi W. A new set of chemotaxis homologues is essential for Myxococcus xanthus social motility. Mol Microbiol. 1998;30:1123–1130. doi: 10.1046/j.1365-2958.1998.01160.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Ma X, Tong L, Kaplan HB, Shimkets LJ, Shi W. Myxococcus xanthus dif genes are required for biogenesis of cell surface fibrils essential for social gliding motility. J Bacteriol. 2000;182:5793–5798. doi: 10.1128/jb.182.20.5793-5798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Duan X, Esmaeiliyan M, Kaplan HB. Composition, structure, and function of the Myxococcus xanthus cell envelope. In: Whitworth DE, editor. Myxobacteria: multicellularity and differentiation. ASM; Washington, D.C., USA: 2008. pp. 229–240. [Google Scholar]