Abstract
Pneumocystis organisms are airborne opportunistic pathogens that cannot be continuously grown in culture. Consequently, the follow-up of Pneumocystis stage-to-stage differentiation, the sequence of their multiplication processes as well as formal identification of the transmitted form have remained elusive. The successful high-speed cell sorting of trophic and cystic forms is paving the way for the elucidation of the complex Pneumocystis life cycle. The growth of each sorted Pneumocystis stage population was followed up independently both in nude rats and in vitro. In addition, by setting up a novel nude rat model, we attempted to delineate which cystic and/or trophic forms can be naturally aerially transmitted from host to host. The results showed that in axenic culture, cystic forms can differentiate into trophic forms, whereas trophic forms are unable to evolve into cystic forms. In contrast, nude rats inoculated with pure trophic forms are able to produce cystic forms and vice versa. Transmission experiments indicated that 12 h of contact between seeder and recipient nude rats was sufficient for cystic forms to be aerially transmitted. In conclusion, trophic- to cystic-form transition is a key step in the proliferation of Pneumocystis microfungi because the cystic forms (but not the trophic forms) can be transmitted by aerial route from host to host.
Introduction
Opportunistic fungal organisms, belonging to the Pneumocystis jirovecii species, are responsible for a severe interstitial lung disease, called Pneumocystis pneumonia (PcP), that can occur in immunocompromised patients and is fatal without effective treatment [1,2]. In addition to P. jirovecii, the fungal genus Pneumocystis encompasses a number of host-specific species that infect a wide range of mammals [3]. Studies of PcP in murine models have led to a better understanding of the pathogenesis and transmission of Pneumocystis organisms [4-7]. During PcP, Pneumocystis organisms filling the alveolar space mostly consist of trophic forms, whereas sporocytes and mature cysts are a minority [8]. Although the main transmission route has been clearly established as being aerial [9,10],, the life cycle stage responsible for disease transmission has not been formerly proven. Pneumocystis-infected mice treated with anidulafungin, an echinocandin drug inhibiting the formation of the cyst cell wall, were previously shown to be unable to transmit the infection to susceptible mice [11], thus suggesting a major role of the cysts in Pneumocystis transmission. In the present study, the life cycle of P. carinii is further dissected by independently following the growth kinetics of trophic and cystic forms both in axenic culture and in vivo after endotracheal infection of nude rats. To further determine the transmission form of PcP, a novel nude rat model of natural infection was specifically established.
Results
Growth of sorted P. carinii trophic and cystic populations in axenic culture
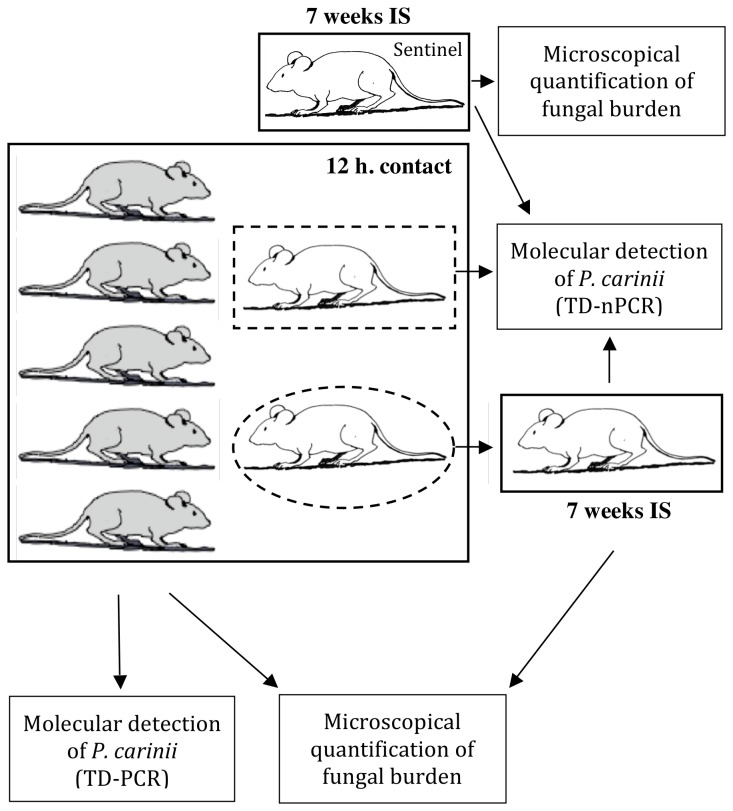
Growth of either trophic or cystic forms were followed in vitro for (i) a sorted P. carinii total population devoid of host cell debris, (ii) pure P. carinii trophic forms, or (iii) pure P. carinii cystic forms. Fungal cultures have been followed for 4 days but growth stopped after 2 days and parasite numbers decreased (Figure 1). When total sorted P. carinii organisms were cultured for 2 days, the number of trophic forms increased (P = 0.05, Figure 1A), while the increase in cystic forms was less pronounced. When cultured alone (Figure 1B), trophic forms grew significantly until day 2 (P = 0.05). Strikingly, no detectable de novo production of cystic forms occurred when the culture was set up with pure trophic forms (Figure 1B). No cysts were detected even after 4 days of culture. Conversely, pure cystic forms were able to release trophic forms during the 2 days of culture (Figure 1C). Indeed, the number of cystic forms abruptly decreased by day 2 (P = 0.05), while the number of trophic forms, starting from below the detection limit (10 P. carinii organisms per culture well), grew quickly (P = 0.037).
Figure 1. Growth of sorted P. carinii organisms in axenic culture.
Several P. carinii populations were cultured in DMEM with 10% FBS, at 37°C, in an atmosphere containing 5% CO2. Growth of (A) sorted P. carinii total population devoid of host cell debris, (B) pure P. carinii trophic forms, and (C) pure P. carinii cystic forms is followed during 4 days. Trophic forms (circles, left Y-axis) or cystic forms (triangles, right Y-axis) were microscopically quantified after RAL-555 panoptic staining [26-28]. For each population of P. carinii organisms studied, means of three replicates are represented per time point. Error bars represent standard deviations. The star (*) means P-value ≤ 0.05. The detection limit is 10 P. carinii organisms per culture well.
Differentiation of sorted P. carinii organisms in nude rats
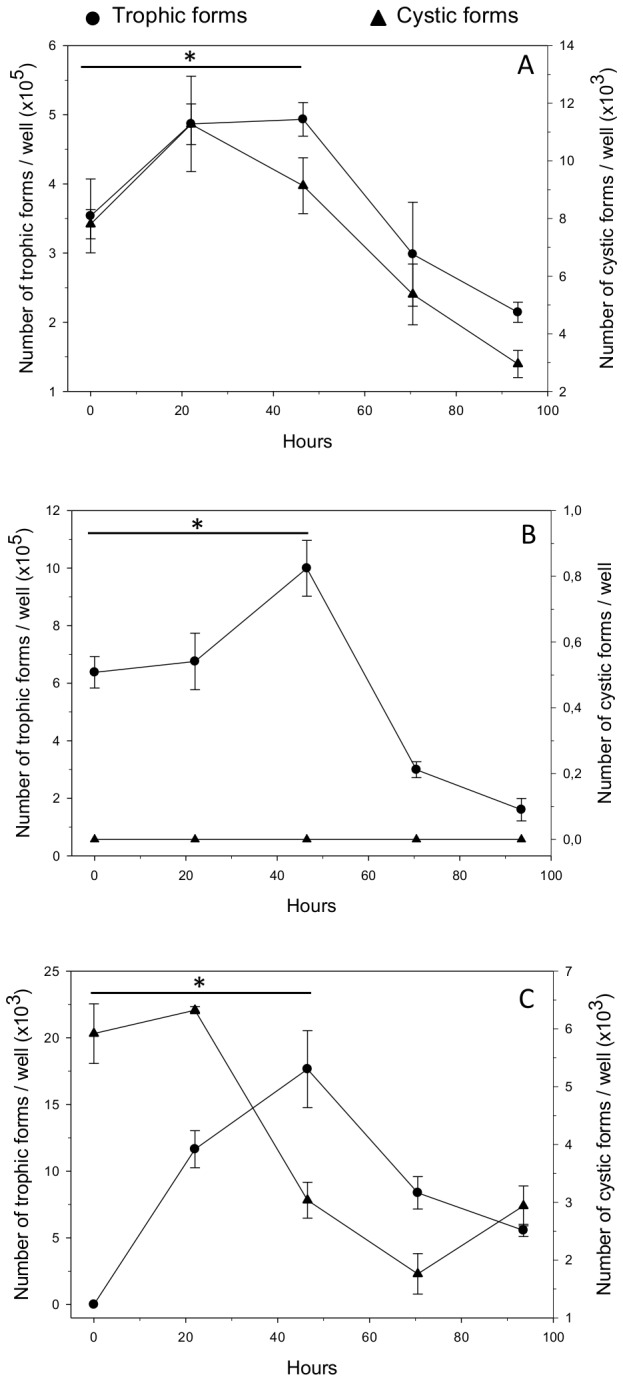
When nude rats were infected with total sorted P. carinii organisms, a significant (P = 0.05) decrease in the number of the trophic forms was obvious over the first 2.5 days following endotracheal infection (Figure 2A). At 8.5 days postinfection, this number tended to increase. Conversely, the cystic population remained stable during the course of the experiment (Figure 2A). Growth kinetics of trophic forms were similar in nude rats infected with pure trophic forms (Figure 2B). Indeed, following a decrease in the number of trophic forms (P = 0.05, Figure 2B), their development significantly resumed on day 8.5 (P = 0.034). Interestingly, cystic forms were produced as early as 12 h postinfection and their number increased significantly until the end of the experiment (P = 0.034, Figure 2B). Finally, when nude rats were infected with pure cystic forms, trophic forms were detected as early as 12 h after infection (Figure 2C). These forms actively and steadily increased thereafter (P = 0.05). The cystic-form burden significantly increased from day 2.5 until the end of the experiment (P = 0.05, Figure 2C).
Figure 2. Infection of nude rats with sorted P. carinii organisms.
Nude rats were endotracheally infected with three P. carinii sorted populations: (A) sorted P. carinii total population devoid of host cell debris, (B) pure P. carinii trophic forms, and (C) pure P. carinii cystic forms. Rats were euthanized at 0.5 (12 h), 2.5 days, and 8.5 days postinoculation and then parasites were extracted from lungs [29]. After staining with RAL-555, trophic forms (in grey) or cystic forms (in black) were microscopically quantified [26-28]. At each time point, means of either trophic- or cystic-form burdens developing in the lungs of three animals are plotted on the same Y-axis. Error bars represent standard deviations. The star (*) means P-value ≤ 0.05.
Airborne transmission of P. carinii-stage populations to nude rats
The aim of this experiment was to decipher which of the trophic and/or cystic forms are important for P. carinii aerial transmission. The choice of a short contact time (12 h) between seeder and receiver animals was dictated by the finding that trophic and cystic forms differentiate quickly once in the rat lungs (Figures 2B and C).
At the end of the contact period, all seeder rats were found to harbor a substantial burden of P. carinii organisms (Table 1). Interestingly, when molecular detection by nested-PCR (nPCR) was performed, only receiver rats that were placed in contact with seeder rats infected with pure P. carinii trophic forms were negative for the presence of P. carinii organisms (Table 1). Seeder rats infected with either sorted P. carinii total population or pure cystic forms were able to transmit P. carinii organisms to three out of four receiver rats within a contact period as short as 12 h. Nevertheless, once immunosuppressed for 7 weeks, none of these receiver animals were prone to PcP development (Table 1). Whole lung tissue extracts of sentinel noninfected nude rats were all negative for the presence of P. carinii gDNA.
Table 1. Airborne transmission of P. carinii stage populations to nude rats.
|
Detection of P. carinii organisms (at the end of contact) |
PcP development (7 weeks postcontact) | |||
|---|---|---|---|---|
|
Seeder rats
|
Receiver rats | Receiver rats | ||
| P. carinii-stage population inoculated to seeder rats | Mean P. carinii burden (1) | Molecular detection (2) | Molecular detection (3) | Microscopic and molecular detection (4) |
| Pure P. carinii total population | 6.2 × 103 ± 2.48 × 103 (n = 19) | All positive (n = 19) | 3 positive (n = 4) | All negative (n = 4) |
| Pure P. carinii trophic forms | 8.9 × 103 ± 4.9 × 103 (n = 19) | All positive (n = 19) | All negative (n = 4) | All negative (n = 4) |
| Pure P. carinii cystic forms | 3.9 × 103 ± 2.0 × 103 (n = 16) | All positive (n = 16) | 3 positive (n = 4) | All negative (n = 4) |
(1) P. carinii burden is microscopically quantified as the number of trophic forms and/or cystic forms on calibrated RAL-555 stained-smears (2). A single-round touchdown PCR (TD-PCR) targeting the multicopy gene coding for the ribosomal RNA of the large mitochondrial subunit (mtLSUrRNA) was used to detect P. carinii gDNA in lung tissue extracts of seeder rats [32]. (3) Since TD-PCR at the mtLSUrRNA locus revealed to be negative for receiver rats, nested-PCR (nPCR) at the same locus was subsequently performed on whole lung tissue extract to increase sensitivity [5]. (4) Following 7 weeks of immunosuppression, PcP development was microscopically monitored on smears of lung tissue extracts stained with RAL-555 [26-29]; as careful microscopical screening revealed to be negative, nPCR at the mtLSUrRNA was performed on all lung tissue extracts. Whole lung tissue extracts of all sentinel rats (n = 6) were also screened for the presence of P. carinii gDNA by nPCR at the mtLSUrRNA locus and all samples were negative. The number (n) of individual rats analyzed is indicated for each animal group.
Discussion
To date, no continuous culture model allows the maintenance of P. carinii organisms, thus impairing the follow-up of stage-to-stage differentiation of this still enigmatic fungus. An attempt to further elucidate its complex life cycle was to physically separate P. carinii life cycle stages with a thin cell wall (herein called trophic forms) from those with a thick cell wall (called cystic forms). High-speed cell sorting has allowed the purification of infectious P. carinii life cycle stages with high reproducibility and purity (Figure 3) [12]. In the present study, we first aimed at elucidating how trophic and cystic forms contribute to the overall P. carinii growth both in vitro and in vivo. The second aim was to evaluate whether trophic, cystic, or both forms could be aerially transmitted from seeder to receiver hosts and whether these forms could elicit PcP.
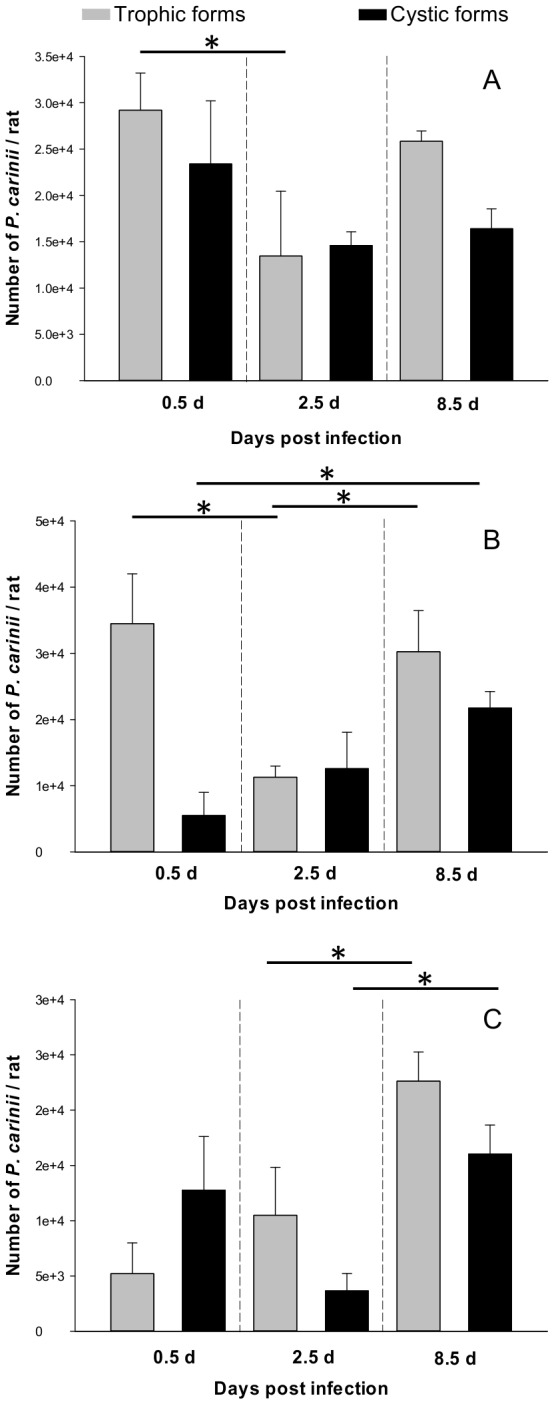
Figure 3. Purity of sorted P. carinii cystic and trophic forms.
P. carinii cyst forms were physically separated from trophic forms by cell sorting using high-speed flow cytometry [12]. To check for purity, smears of sorted P. carinii organisms were stained with RAL-555, a panoptic Giemsa-like stain. No cystic forms were noted in the sorted trophic-form fraction (A). No trophic forms were visible within the sorted cystic-form fraction (B). P. carinii cell sorting was reproducible, reaching 99.6% purity [12]. Insets represent a higher magnification. Bars = 10 μm.
First, cultivation of pure trophic forms did not lead to a detectable de novo production of cystic forms, while a significant increase in the number of trophic forms occurring at day 2 attested to their dividing capacity (Figure 1B). Conversely, when only cysts had been cultured, trophic forms were detected as early as day 2 while the number of cysts decreased (Figure 1C). The rapid occurrence of trophic forms in our model can be explained by the presence, in the starting inoculum, of mature cysts, ready to rupture and release spores that evolve toward young trophic forms. The production of trophic forms from cystic forms has already been postulated before on the basis of growth kinetics [13] or ultrastructural observations [14]. Trophic forms could subsequently initiate mitotic divisions, a quick way to boost growth, as hypothesized before [15]. Within 48 h, a decrease in the number of cystic forms concomitant to an increase in the number of trophic forms support this hypothesis (Figure 1C). Overall, these results suggest that, once cysts are formed, maturation and/or excystation can rapidly proceed in vitro and lead to the de novo production of trophic forms that are able to mitotically divide. In contrast, a blocking step seems to prevent de novo production of cystic forms in vitro when only trophic forms are incubated. This phenomenon could be related to the lack of extrinsic mating inducer signals in vitro. It also suggests that trophic-to-cystic form differentiation is an essential step in the turnover of the P. carinii life cycle, which may be one of the reasons why the P. carinii organisms do not grow continuously in culture [16,17].
The overall shape of the trophic forms’ growth kinetics in animals infected with either sorted P. carinii total population or pure trophic forms were similar (Figures 2A and B). Both populations showed a decrease in number in the first days of growth and a boost in the parasite growth towards the end of the follow-up period. Even if inflammation is reduced in nude rats treated with dexamethasone, the initial decrease could be related to the clearance of P. carinii organisms due to phagocytosis by macrophages and neutrophils. This phenomenon has already been described in the Scid mice model [18]. What is most striking, in contrast to in vitro studies, is the early detection of cystic forms in animals infected with trophic forms only (Figure 2B). Based on what is known about the P. carinii life cycle [17,19-21], two hypotheses could explain this finding: (i) thin-walled early sporocytes may be present in the starting inoculum and may be able to differentiate into thick-walled sporocytes within a short period of time and (ii) the mating between trophic forms and subsequent maturation to early and intermediate sporocytes may occur quite rapidly after inoculation. Although cystic forms appeared early in trophic-form-infected animals, their number increased rather slowly but steadily (Figure 2B). This could be due to (i) low mating frequency between trophic forms and/or (ii) a long period of time necessary for sporocytes to evolve towards mature cysts. Another reason why cystic forms cannot accumulate is related to their rupture once they are fully mature. The subsequent spore release can contribute to the late increase in the number of trophic forms, which probably also augments as a result of the mitotic divisions of these forms. When nude rats are solely infected with P. carinii cystic forms, the growth kinetics are drastically different: (i) the presence of trophic forms was rapidly detectable, at 12 h postinfection, and their number constantly increased up to day 8.5 and (ii) the cystic forms multiplied from day 2.5 until the end of the experiment (Figure 2C). In these animals, both cystic and trophic forms reached an approximately comparable organism burden as in animals infected with the total sorted P. carinii population or trophic forms only. The smaller cyst inoculum may elicit less clearance by the rat innate immune system and is sufficient to initiate active growth [22]. The rapid initial occurrence of trophic forms was probably consecutive to the spore release from mature cysts, whereas the significant increase observed at the end of the follow-up period could be a consequence of mitotic divisions.
Our novel natural aerial transmission model indicates that animals infected with P. carinii trophic forms were unable to transmit the fungal organisms to any of the receiver nude rats after 12 h of co-housing (Table 1). In contrast, animals infected with P. carinii total population or cystic forms-only, were able to seed the fungal organisms to co-housed receiver animals. Our data further strengthen the notion that cystic forms are the transmitted stage and agree with [11], who, using echinocandins, indirectly showed that these forms were important in the transmission process. Although P. carinii DNA was detected in the lungs of positive recipient nude rats, none of them developed PcP at the end of the immunosuppression period. Several hypotheses may account for this unexpected finding: (i) the contact period may have been too short to allow enough organisms to be seeded, (ii) the infectivity of sorted P. carinii is lower than nonsorted organisms, although they were shown to be able to elicit PcP [12], and (iii) the innate immune response occurring in seeder rats may be responsible for a drastic reduction in the number of inoculated organisms, thus diminishing the number of exhaled organisms.
To conclude, the growth of Pneumocystis cystic and trophic form populations has been followed up independently, both in vivo and in vitro, for the first time. The trophic-to-cystic form transition appears as a key step in the proliferation and the life cycle of Pneumocystis microfungi. Only the cystic forms can be transmitted by aerial route from host to host [11,the current study]. Thus, producing cystic forms by mating inside its mammalian host appears to be the only way for Pneumocystis to perpetuate its life cycle from host to host, as no environmental form has been evidenced so far. This is a unique trait amongst aerially transmitted ascomycetes.
Materials and Methods
Ethics statement
All animal experiments were conducted following the guidelines of the Pasteur Institute of Lille animal study board, which conforms to the Amsterdam Protocol on animal protection and welfare, and Directive 86/609/EEC on the Protection of Animals Used for Experimental and Other Scientific Purposes, updated in the Council of Europe’s Appendix A (http://conventions.coe.int/Treaty/EN/Treaties/PDF/123-Arev.pdf). The animal work also complied with the French law (nu 87-848 dated 19-10-1987) and the European Community’s 1976 Amendment of Cruelty to Animals Act. The animal house (accreditation number: A59107, agreement number: B 59-350009) was placed under the direct control of the director of the Pasteur Institute of Lille, who is the “designated responsible person” under French law. The experiment protocol used in the present study has been approved by the Ethics Committee for Experiments on Animals of the Nord-Pas-de-Calais region (approval number ECEA 022011) and was carried out by qualified personnel.
Source of Pneumocystis carinii
Athymic Pneumocystis-free Lou nu/nu rats (Pasteur Institute of Lille, France) were used as the source of Pneumocystis carinii organisms for all experiments [12]. Briefly, nude rats were administered dexamethasone (Merck Sharp & Dohme Chibret) starting from 2 weeks prior to nonsurgical endotracheal infection with cryopreserved P. carinii organisms [12,23]. All animal groups were housed in independent compartments in HEPA-filtered air isolators (Flufrance, Wissous, France) and were provided with sterile irradiated food (Scientific Animal Food & Engineering (SAFE), Augy, France) and sterile water ad libitum. Eight weeks postinfection, all animals harbored a high P. carinii burden and were euthanized. Their lungs were dissected to isolate organisms that were cryopreserved while remaining highly infectious [12,24,25].
Cell sorting of P. carinii cystic and trophic forms
To study growth kinetics and transmission of P. carinii trophic and cystic forms, high-speed cell sorting of P. carinii lifecycle-stage populations was performed [12]. Two parasite-stage fractions can be sorted simultaneously: (1) the trophic-form fraction corresponds to parasite stages bearing a thin cell wall (trophic forms, early sporocytes) and (2) the cystic-form fraction includes parasite stages possessing a thick cell wall (intermediate-to-late sporocytes, and mature cysts). The purity of cystic versus trophic forms was checked by microscopy prior to any subsequent experiments and was higher than 99.6% (Figure 3) [12]. Sorted P. carinii organisms were also shown to remain infectious for the nude rat [12].
Growth of sorted P. carinii organisms in axenic culture
The growth of (i) sorted P. carinii total population devoid of host cell debris, (ii) pure P. carinii trophic-form fraction, or (iii) pure P. carinii cystic-form fraction were followed in vitro. Axenic short-term cultures of P. carinii were carried out in 24-well plates (Costar) as described in [26] with modifications as mentioned hereafter. Either 5 × 105 P. carinii organisms (total population or trophic-form fraction) or 1.5 × 104 P. carinii of the cystic-form fraction were suspended in 2 mL of DMEM supplemented with antibiotics (100 U/mL penicillin; 100 µg/mL streptomycin; Sigma-Aldrich) and 10% heat-inactivated fetal bovine serum (FBS, GIBCO BRL, Life Technologies Inc.). P. carinii organisms were incubated for 4 days in 5% CO2 at 37°C [26]. Every day, organisms from each well were re-suspended in 50 µL of Dulbecco phosphate buffer solution (DPBS, Sigma-Aldrich). The organisms were quantified by microscopic numeration as previously described [26-28]. All experiments were conducted in triplicate.
Infection of nude rats with sorted P. carinii organisms
In order to monitor P. carinii growth in vivo, dexamethasone-treated nude rats were endotracheally infected with sorted P. carinii organisms [12,23]. Three groups of nine animals were infected with three different P. carinii populations as follows: (i) 1.3 × 106 cells per animal of sorted P. carinii total population (trophic and cystic forms), devoid of host cell debris, (ii) 1.3 × 106 pure trophic forms per animal, and (iii) 3 × 105 pure cystic forms per animal. Three animals per group were euthanized at 12 h, 2.5 days, and 8.5 days postinfection. In order to accurately evaluate parasite burdens, infected lungs were homogenized using a stomacher (Stomacher 80 Biomaster Lab Blender, Seward) as previously described [29]. The resulting pellets were then suspended in DPBS and all P. carinii forms were microscopically quantified as described previously [26-28].
Nude rat model of airborne P. carinii transmission
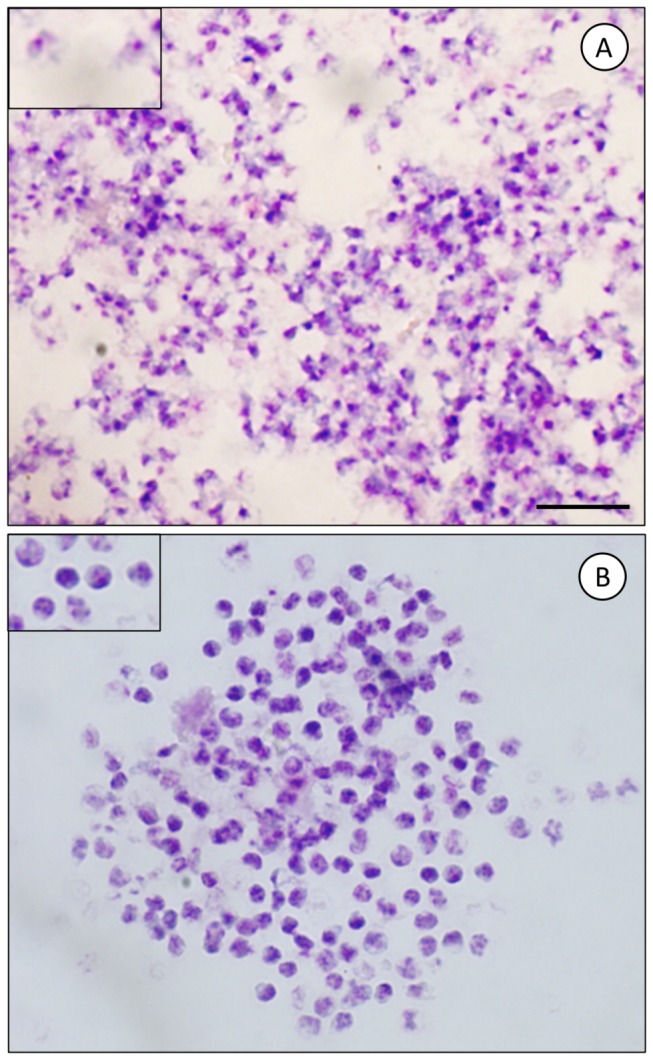
By setting up a novel nude rat model of airborne transmission of P. carinii organisms (Figure 4), we attempted to approach the natural conditions of transmission in order to identify the P. carinii airborne-transmitted form. Three groups of 16–19 immunosuppressed nude rats were endotracheally infected with (i) 6.45 × 106 P. carinii per rat from the total sorted population, (ii) 12 × 106 pure trophic forms per rat, or (iii) 9.35 × 104 pure cystic forms per rat [12,23]. These animals, called seeder rats, were allowed to recover from inoculation for 15 min and, then, were housed with eight dexamethasone-treated P. carinii-free nude rats, called receiver rats, for 12 h (Figure 4). The mean seeder-to-receiver rat ratio was 2.4 per cage. At the end of contact, all seeder and four receiver rats were euthanized. The four remaining receiver rats were kept separately under dexamethasone treatment for 7 weeks and then euthanized. Two sentinel noninfected nude rats were used as internal negative controls for each of the three groups of animals. After 7 weeks of immunosuppression, they were also euthanized. Each group of animals was kept in a separate compartment of an HEPA-filtered isolator to avoid any risk of contamination. The lungs of all rats were aseptically removed before parasite extraction [29]. Lung tissue suspensions were then screened for the presence of P. carinii genomic DNA (gDNA), either by single-round touchdown PCR (TD-PCR) in seeder rats or nested-PCR (nPCR) in receiver and sentinel rats. Lung tissue suspensions from receiver and sentinel rats were also microscopically monitored to detect PcP development 7 weeks postcontact [26-28].
Figure 4. Nude rat model of aerial P. carinii transmission.
To study the airborne transmission of P. carinii, three groups of animals were infected with either P. carinii total population or trophic or cystic forms. To ease comprehension, only a portion (1/4) of the animals belonging to a single group is represented on the figure. First, seeder rats (in grey) were endotracheally infected with P. carinii organisms (total sorted P. carinii population, pure trophic forms, or cystic forms) [23]. Second, after 15 min of recovery, seeder rats were placed in close contact with receiver animals (in white) for 12 h at a mean ratio (seeders to receivers) of 2.4 per cage. These dexamethasone-treated animals were co-housed in capped cages within an individual compartment (thick black square line) of an HEPA-filtered air isolator. Third, all the seeder and half of the receiver rats were euthanized at the end of the contact period. P. carinii organisms were extracted from the seeder rat lungs [29]: the fungal burden was microscopically quantified [26-28] and molecular detection of the P. carinii mtLSUrRNA gene was performed using a single-round touchdown PCR (TD-PCR, [32]). Whole lung tissue suspensions of half of the receiver rats (dotted square line) were screened by nested-PCR (nPCR) at the same locus to detect P. carinii gDNA [5]. Fourth, the other half of the receiver rats (dotted oval line) were kept under immunosuppression (IS) in HEPA-filtered air conditions for 7 weeks to microscopically monitor for eventual PcP development. nPCR was also performed on whole lung tissue suspensions to detect P. carinii gDNA. Whole lung tissue suspensions of sentinel rats were also screened by nPCR and by microscopy after 7 weeks of immunosuppression.
Molecular detection of P. carinii genomic DNA
For seeder rats, a single gDNA extraction was conducted starting from 25 mg of P. carinii-infected lung tissue using the tissue protocol of the QIAmp DNA MiniKit (QIAGEN). gDNA was eluted twice in Tris-EDTA (TE) buffer as recommended. For receiver and sentinel rats, since lower quantities of P. carinii gDNA were expected, gDNA was extracted from P. carinii-infected whole lungs following the tissue protocol of the NucleoBond® AXG 500 kit (Macherey Nagel). Ten gDNA eluates were collected, precipitated, and centrifuged at 20,000 g. The pellets were washed with 70% ethanol and then dissolved (55°C for 2 h.) in 500 µL of TE buffer (pH 8.0). Three series were needed to extract gDNA from a whole lung. Negative buffer controls were prepared concurrently to monitor for possible cross-contamination during gDNA extractions. Quantity and quality of gDNA were measured using the ND-1000 spectrophotometer (NanoDrop technologies).
P. carinii gDNA was detected by TD-PCR using pAZ102-E and pAZ102-H primers [30], amplifying a portion of the multicopy gene encoding the mitochondrial large-subunit ribosomal RNA (mtLSUrRNA). Nested-PCR with internal primers pAZ102-X and pAZ102-Y [31] was used when the number of P. carinii organisms in the samples was expected to be low.
DNA amplification was carried out from 100 ng of a gDNA sample in a 50-µL reaction mixture containing final concentrations of 75 mM Tris-HCl (pH 8.8), 20 mM (NH4)2SO4, 0.01% (vol./vol.) Tween 20, 3 mM MgCl2, 400 µM (each) deoxynucleoside triphosphate, 1 µM (each) oligonucleotide primer (Eurogentec), and 0.02 U.µL−1 of GoldstarTM DNA polymerase (Eurogentec). In the second round of DNA amplification, 2 µL of the first-round PCR product were used in a final volume of 50 µL; final concentrations of reagents in the PCR mixture were the same as above. DNA amplification was carried out on a PTC 200 thermocycler (MJ Research) and cycling conditions were as follows: (i) a TD-PCR [32] was performed as a first round amplification: 10 cycles comprising a denaturation step (94°C for 1 min 30 s), an annealing step (65°C to 55°C for 1 min 30 s, 1°C decrease per cycle), and an extension step (72°C for 2 min) were followed by 30 cycles comprising a denaturation step (94°C for 1 min 30 s), an annealing step (55°C for 1 min 30 s), and an extension step (72°C for 2 min), and (ii) the second round of amplification (nPCR) was performed as described by 5. Negative controls with no added gDNA as well as positive control with known P. oryctolagi gDNA were included in each amplification run. gDNA extraction, preparation of mix reagents, and pipeting of 1st-round PCR products were all performed in separate rooms to prevent possible contamination. Purified PCR products were sent for direct sequencing (GenoScreen, Lille, France). A single TD-PCR and 30 nPCR reactions per rat were performed to detect P. carinii gDNA in seeder and receiver rat lungs, respectively. For sentinel rats, the screening strategy was the same as for receiver rats (Figure 4).
Statistical analysis
Pneumocystis loads were expressed as the mean ± SD for each group. All comparisons were analyzed using the Mann-Whitney U-test. Statistical analyses were performed using the SPSS software package (version 16.0, SPSS Inc., Chicago, IL, USA). A P-value ≤ 0.05 was considered to be significant.
Acknowledgments
We thank Dr. E. Deruy (Lille-Nord-de-France BICeL-Bio-imaging Center, IFR142, Lille, France) for his help with the cell sorting set-up and JP. Decavel, head of Institute Pasteur animal facilities. We would also like to thank Dr. L. Northrup, ‘“English solutions”’, for her help editing the manuscript.
Funding Statement
This work was supported by ANR-ERA-NET ‘Pneumocystis’ PathoGenoMics (ANR-06-PATHO-009-01). We also thank the Lille-Nord-de-France University, the Pasteur Institute of Lille and the microscopy and flow cytometry platform of the BioImaging Center of Lille Nord de France (BiCeL, IFR142) for their support. The funders (ANR-ERA-NET, Lille Nord de France University and Pasteur Institute of Lille) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Calderón EJ, Gutiérrez-Rivero S, Durand-Joly I, Dei-Cas E (2010) Pneumocystis infection in humans: diagnosis and treatment. Expert Rev Anti Infect Ther 8: 683-701. doi: 10.1586/eri.10.42. PubMed: 20521896. [DOI] [PubMed] [Google Scholar]
- 2. Catherinot E, Lanternier F, Bougnoux ME, Lecuit M, Couderc LJ et al. (2010) Pneumocystis jirovecii Pneumonia. Infect Dis Clin North Am 24: 107-138. doi: 10.1016/j.idc.2009.10.010. PubMed: 20171548. [DOI] [PubMed] [Google Scholar]
- 3. Aliouat-Denis CM, Chabé M, Demanche C, Aliouat EM, Viscogliosi E et al. (2008) Pneumocystis species, co-evolution and pathogenic power. Infect Genet Evol 8: 708-726. doi: 10.1016/j.meegid.2008.05.001. PubMed: 18565802. [DOI] [PubMed] [Google Scholar]
- 4. An CL, Gigliotti F, Harmsen AG (2003) Exposure of immunocompetent adult mice to Pneumocystis carinii f. sp. muris by cohousing: growth of P. carinii f. sp. muris and host immune response. Infect Immun 71: 2065-2070. doi: 10.1128/IAI.71.4.2065-2070.2003. PubMed: 12654827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chabé M, Dei-Cas E, Creusy C, Fleurisse L, Respaldiza N et al. (2004) Immunocompetent hosts as a reservoir of Pneumocystis organisms: histological and RT-PCR data demonstrate active replication. Eur J Clin Microbiol Infect Dis 23: 89-97. doi: 10.1007/s10096-003-1092-2. PubMed: 14712369. [DOI] [PubMed] [Google Scholar]
- 6. Dumoulin A, Mazars E, Seguy N, Gargallo-Viola D, Vargas S et al. (2000) Transmission of Pneumocystis carinii disease from immunocompetent contacts of infected hosts to susceptible hosts. Eur J Clin Microbiol Infect Dis 19: 671-678. doi: 10.1007/s100960000354. PubMed: 11057500. [DOI] [PubMed] [Google Scholar]
- 7. Gigliotti F, Harmsen AG, Wright TW (2003) Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect Immun 71: 3852-3856. doi: 10.1128/IAI.71.7.3852-3856.2003. PubMed: 12819069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dei-Cas E, Aliouat EM, Cailliez JC (2004) Pneumocystis Cellular Structure. In: Walzer PD, Cushion MT. Pneumocystis carinii Pneumonia. New York: Marcel Dekker Inc. pp. 61-94. [Google Scholar]
- 9. Hughes WT (1982) Natural mode of acquisition for de novo infection with Pneumocystis carinii . J Infect Dis 145: 842-848. doi: 10.1093/infdis/145.6.842. PubMed: 6979590. [DOI] [PubMed] [Google Scholar]
- 10. Walzer PD, Schnelle V, Armstrong D, Rosen PP (1977) Nude mouse: a new experimental model for Pneumocystis carinii infection. Science 197: 177-179. doi: 10.1126/science.301657. PubMed: 301657. [DOI] [PubMed] [Google Scholar]
- 11. Cushion MT, Linke MJ, Ashbaugh A, Sesterhenn T, Collins MS et al. (2010) Echinocandin treatment of Pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLOS ONE 5: e8524. doi: 10.1371/journal.pone.0008524. PubMed: 20126455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinez A, Aliouat EM, Pottier M, Gantois N, Pinçon C et al. (2009) High-speed cell sorting of infectious trophic and cystic forms of Pneumocystis carinii . J Eukaryot Microbiol 56: 446-453. doi: 10.1111/j.1550-7408.2009.00423.x. PubMed: 19737197. [DOI] [PubMed] [Google Scholar]
- 13. Aliouat EM, Dujardin L, Martinez A, Duriez T, Ricard I et al. (1999) Pneumocystis carinii growth kinetics in culture systems and in host: involvement of each life cycle parasite stage. J Eukaryot Microbiol 46S: 116-117. [PubMed] [Google Scholar]
- 14. Itatani CA (1994) Ultrastructural demonstration of a pore in the cyst wall of Pneumocystis carinii . J Parasitol 80: 644-648. doi: 10.2307/3283204. PubMed: 8064534. [DOI] [PubMed] [Google Scholar]
- 15. Cushion MT, Stringer JR (2010) Stealth and opportunism: alternative lifestyles of species in the fungal genus Pneumocystis . Annu Rev Microbiol 64: 431-452. doi: 10.1146/annurev.micro.112408.134335. PubMed: 20528694. [DOI] [PubMed] [Google Scholar]
- 16. Schmatz DM, Powles MA, McFadden DC, Pittarelli L, Balkovec J et al. (1992) Anti-Pneumocystis activity of water-soluble lipopeptide L-693,989 in rats. Antimicrob Agents Chemother 36: 1964-1970. doi: 10.1128/AAC.36.9.1964. PubMed: 1416888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aliouat-Denis CM, Martinez A, Aliouat EM, Pottier M, Gantois N et al. (2009) The Pneumocystis life cycle. Mem Inst Oswaldo Cruz 104: 419-426. doi: 10.1590/S0074-02762009000300004. PubMed: 19547866. [DOI] [PubMed] [Google Scholar]
- 18. Aliouat EM, Escamilla R, Cariven C, Vieu C, Mullet C et al. (1998) Surfactant changes during experimental pmeunocystosis are related to the Pneumocystis development. Eur Respir J 11: 542-547. PubMed: 9596099. [PubMed] [Google Scholar]
- 19. Cushion MT (2010) Are members of the fungal genus pneumocystis (a) commensals; (b) opportunists; (c) pathogens; or (d) all of the above? PLOS Pathog 6: e1001009 PubMed: 20885786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas CF, Limper AH (2007) Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol 5: 298-308. doi: 10.1038/nrmicro1621. PubMed: 17363968. [DOI] [PubMed] [Google Scholar]
- 21. Yoshida Y (1989) Ultrastructural studies of Pneumocystis carinii . J Protozool 36: 53-60. doi: 10.1111/j.1550-7408.1989.tb05830.x. PubMed: 2651656. [DOI] [PubMed] [Google Scholar]
- 22. Ambrose HE, Keely SP, Aliouat EM, Dei-Cas E, Wakefield AE et al. (2004) Expression and complexity of the PRT1 multigene family of Pneumocystis carinii . Microbiology 150: 293-300. doi: 10.1099/mic.0.26539-0. PubMed: 14766907. [DOI] [PubMed] [Google Scholar]
- 23. Garry S, Nesslany F, Aliouat E, Haguenoer JM, Marzin D (2003) Hematite (Fe2O3) enhances benzo[a]pyrene genotoxicity in endotracheally treated rat, as determined by Comet Assay. Mutat Res 538: 19-29 [Google Scholar]
- 24. European Concerted Action on Pneumocystis carinii (1996) Invitro systems in Pneumocystis research. Parasitol Today 12: 245-249 doi: 10.1016/0169-4758(96)80812-X. PubMed: 15275206. [DOI]
- 25. Durand-Joly I, Aliouat EM, Recourt C, Guyot K, François N et al. (2002) Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J Clin Microbiol 40: 1862-1865. doi: 10.1128/JCM.40.5.1862-1865.2002. PubMed: 11980979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aviles P, Aliouat EM, Martinez A, Dei-Cas E, Herreros E et al. (2000) In vitro pharmacodynamic parameters of sordarin derivatives in comparison with those of marketed compounds against Pneumocystis carinii isolated from rats. Antimicrob Agents Chemother 44: 1284-1290. doi: 10.1128/AAC.44.5.1284-1290.2000. PubMed: 10770763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aliouat EM, Mazars E, Dei-Cas E, Cesbron JY, Camus D (1993) Intranasal inoculation of mouse, rat or rabbit-derived Pneumocystis in SCID mice. J Protozool Res 3: 94-98. [Google Scholar]
- 28. Dei-Cas E, Brun-Pascaud M, Bille-Hansen V, Allaert A, Aliouat EM (1998) Animal models of pneumocystosis. FEMS Immunol Med Microbiol 22: 163-168. doi: 10.1111/j.1574-695X.1998.tb01201.x. PubMed: 9792075. [DOI] [PubMed] [Google Scholar]
- 29. Soulez B, Dei-Cas E, Palluault F, Camus D (1991) Morphological evaluation of Pneumocystis carinii after extraction from infected lung. J Parasitol 77: 449-453. doi: 10.2307/3283134. PubMed: 2040957. [DOI] [PubMed] [Google Scholar]
- 30. Wakefield AE, Pixley FJ, Banerji S, Sinclair K, Miller RF et al. (1990) Detection of Pneumocystis carinii with DNA amplification. Lancet 336: 451-453. doi: 10.1016/0140-6736(90)92008-6. PubMed: 1974987. [DOI] [PubMed] [Google Scholar]
- 31. Wakefield AE (1996) DNA sequences identical to Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. hominis in samples of air spora. J Clin Microbiol 34: 1754-1759. PubMed: 8784583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Durand-Joly I, Chabé M, Soula F, Delhaes L, Camus D et al. (2005) Molecular diagnosis of Pneumocystis pneumonia. FEMS Immunol Med Microbiol 45: 405-410. doi: 10.1016/j.femsim.2005.06.006. PubMed: 16061360. [DOI] [PubMed] [Google Scholar]