Abstract
Objective
To examine whether early inflammation is related to cortisol levels at 18 months corrected age (CA) in children born very preterm.
Study Design
Infants born ≤ 32 weeks gestational age were recruited in the NICU, and placental histopathology, MRI, and chart review were obtained. At 18 months CA developmental assessment and collection of 3 salivary cortisol samples were carried out. Generalized least squares was used to analyze data from 85 infants providing 222 cortisol samples.
Results
Infants exposed to chorioamnionitis with funisitis had a significantly different pattern of cortisol across the samples compared to infants with chorioamnionitis alone or no prenatal inflammation (F[4,139] = 7.3996, P <.0001). Postnatal infections, necrotizing enterocolitis and chronic lung disease were not significantly associated with the cortisol pattern at 18 months CA.
Conclusion
In children born very preterm, prenatal inflammatory stress may contribute to altered programming of the HPA axis.
Keywords: preterm, chorioamnionitis, funisitis, premature infants, hypothalamic-pituitary-adrenal axis, infection, cortisol, stress
Introduction
Infections are common in very preterm infants in the fetal period as well as postnatally. Chorioamnionitis is often involved in initiation of preterm labor, occurring in 60% of preterm deliveries.(1) Postnatal sepsis occurs in up to 24% of very low birth weight preterm infants while in the neonatal intensive care unit (NICU).(2) Both fetal and postnatal infections are characterized by the presence of marked inflammation and the release of inflammatory mediators; elevated pro-inflammatory cytokine levels are reported in preterm neonates with evidence of chorioamnionitis(3), funisitis(4), and sepsis.(5) In an earlier cohort, we found elevated basal salivary cortisol levels at 8 and 18 months corrected age (CA),(6,7) in preterm infants born at extremely low gestational age (24-28 weeks gestation) compared to healthy full term controls, suggesting altered programming of the HPA axis.
Important and complex bi-directional interactions exist between the immune system and the endocrine system at the hypothalamic and pituitary levels and peripherally. In brief, cytokines (IL-1, IL-2, IL-6 and TNF, for example) elicit an acute hypothalamic-pituitary-adrenal (HPA) response, mainly by stimulating corticotropin releasing hormone (CRH) secretion, while glucocorticoid hormones modulate tissue inflammatory responses.(8) Accordingly, elevated amniotic fluid cortisol levels(9) and increased cord blood cortisol levels(10) are found in fetuses and preterm newborns exposed to intrauterine infection compared to those non-exposed.
Increased basal and stimulated cortisol levels after exposure to chorioamnionitis were shown to persist at least throughout the first 3 weeks of life.(11,12) Similarly, neonates with sepsis typically display higher cortisol levels than healthy controls.(13) Early exposure to inflammatory signals leading to activation of the HPA axis may result in reprogramming of this stress axis, causing long-term functional changes. Possible mechanisms for altered programming in these circumstances include exposure to excess endogenous glucocorticoids(14) or direct action of cytokines on the brain during critical windows of brain development; however, higher cortisol levels were not driven by either antenatal(15) or postnatal(7) glucocorticoid exposure in our previous cohort . Several animal studies have shown a long term effect of early infection on the HPA axis. For example, maternal endotoxemia (with the bacterial endotoxin lipopolysaccharide (LPS) that mimics Gram negative infection) during gestation altered offspring cortisol responses to stress and/or to immune challenge up to adulthood in sheep.(16) Neonatal exposure to LPS altered adult HPA basal function and response to various forms of stress, for example in rats.(17,18) There are no studies to our knowledge that have examined long term effects of infections on resting or stress cortisol responses in preterm infants. The aim of the present study was to examine whether early inflammation contributes to altered cortisol levels at 18 months CA in children born very preterm.
Materials and Methods
Design
Secondary analysis from a longitudinal cohort recruited for a study of neonatal pain-related stress, brain development and neurodevelopment in infants born very preterm. (19)
Participants
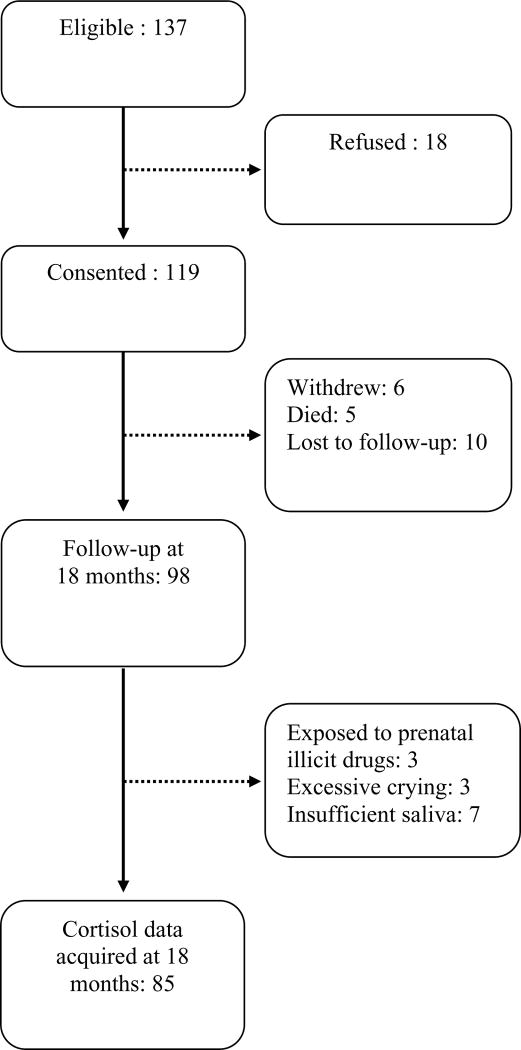
Preterm infants born ≤32 weeks gestational age (GA) were recruited from April 2006 to November 2010 from the NICU at Children's & Women's Health Centre of British Columbia. Infants with major congenital anomalies or evidence of congenital TORCH infection were not eligible; infants known to be exposed to maternal illicit drug use during pregnancy were excluded. A flow chart of participants is shown in Figure 1.
Figure 1. Study Participants Flow Chart.
Procedures
Written informed consent was obtained in the NICU, following approval by the Clinical Research Ethics Board of the University of British Columbia and the Research Review Committee of the Children's and Women's Health Centre of British Columbia. During the NICU stay, placental pathology was obtained, MRI performed, and chart review conducted on all enrolled newborns. The infants were seen at 18 months CA in the Neonatal Follow up Clinic, and underwent developmental testing and collection of three saliva samples for cortisol assay. All infants were healthy on the test day by parent report.
Measures
Placental Histopathology
Macroscopic and microscopic analysis of the placenta was performed on a minimum of 4 sections for histopathological examination (reflected membranes, distal and proximal umbilical cord, fetal and maternal surfaces), as well as a full thickness section. Tissue sections were fixed in formalin, cut to 3–5μm thickness and stained with hematoxylin and eosin. An experienced placental pathologist (D.E.M.) assessed for placental inflammation and noted the presence of chorioamnionitis alone, chorioamnionitis with funisitis or neither.
Magnetic Resonance Imaging
All newborns were scanned using an MR-compatible isolette (Lammers Medical Technology, Luebeck, Germany) and specialized neonatal head coil (Advanced Imaging Research, Cleveland, OH), in a Siemens (Berlin, Germany) 1.5T Avanto using VB 13A software. An experienced neuroradiologist (K.J.P.) reviewed the images blinded to medical history and placental pathology. As described previously, white matter injury (WMI) was defined as foci of abnormal white matter T1 hyperintensity in the absence of marked T2 hypointensity, or by low-intensity T1 foci (cysts). The severity of WMI was scored as minimal, moderate, or severe, using a system with high reliability that is predictive of adverse neurodevelopmental outcome.(20) The presence of intraventricular hemorrhage, ventriculomegaly, and cerebellar hemorrhage was also noted.
Cortisol
At 18 months CA, 3 saliva samples were collected using our established procedures (7): pretest, when the child was awake and settled following arrival at the center, post-1 after developmental assessment (approximately one hour after the pretest sample, mean 70 ± 42 minutes), and post-2 at the end of the session (approximately 30 minutes after the post-1 sample, mean 27 ±7 minutes). Testing was done in the morning (first saliva sample mean time 10:00 am ± 1 hour). A salivette was placed in the mouth for about 1 minute, without prior stimulants. Saliva was extracted and stored frozen at -20°C until assayed, using the Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics LLC, Philadelphia, PA). Intra-assay and inter-assay coefficients of variation were 2.92% and 3.41%, respectively.
Medical Chart Review
Medical and nursing chart review was carried out from birth to term-equivalent or discharge home, whichever came first, by a research nurse, and included: gestational age, birth weight, sex, antenatal and postnatal steroid exposure, SNAP II score on day 1, number of days of mechanical ventilation (conventional and oscillation) and other respiratory support, surgeries, number of skin-breaking procedures, morphine, midazolam and fentanyl exposure, postnatal steroid exposure (dexamethasone and hydrocortisone), postnatal infections, grade of intraventricular hemorrhage (IVH), Bell's stage of NEC, and presence of chronic lung disease (CLD), defined as receiving supplemental O2 at 36 weeks corrected age. Postnatal infections were defined by the following criteria(21): a clinical infection as the presence of clinical suspicion, a negative blood culture and antibiotic therapy for ≥ 5 days, sepsis was defined as a culture positive infection and antibiotic therapy for ≥ 5 days, and meningitis was defined as a positive cerebrospinal fluid (CSF) culture. In cases with more than one infection, the most severe was used for analysis. Exposure to morphine, midazolam, fentanyl, dexamethasone and hydrocortisone was calculated as the average dose (mg/kg) per day adjusted for daily weight multiplied by the number of days on the drug, combining I.V. and P.O. administration after appropriate conversion.
Statistical Analysis
Generalized least squares analysis was performed to allow for correlation over time while accommodating cases with incomplete cortisol samples, using the nlme package of the R environment for statistical computing. Cortisol values were log-transformed. We examined whether the cortisol pattern across phases was associated with early infection by testing the interaction of pre- and post-natal infection and phase on log cortisol levels, adjusting for clinical confounders. This analysis was also conducted for necrotizing enterocolitis (NEC) and CLD.
Results
Of 98 preterm infants followed up at 18 months, three were excluded due to prenatal exposure to maternal illicit drugs (cocaine), three due to excessive crying during the follow-up visit, and seven due to insufficient saliva for all 3 samples. The final study sample comprised 85 infants providing 222 valid cortisol samples with no feeding within 20 minutes of sample collection: 65 infants had the complete set of 3 saliva samples, 7 had 2 samples, and 13 had 1 sample. Infant characteristics are presented in Table 1. Of the 85 infants, placental histopathology was available for 78 infants: 24 had chorioamnionitis and 11 had chorioamnionitis with funisitis. Further, among the 85 infants: 37 infants had a postnatal infection (11 clinical infection, 17 sepsis, 8 concurrent sepsis and NEC stage I-III, 1 meningitis).
Table 1. Infant characteristics N=85.
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Gestational age (wks) | 28.1 ± 2.2 |
| Birth weight (gr) | 1079 ± 343 |
| Apgar 1 min | 5.2 ± 2.5 |
| Apgar 5 min | 7.3 ± 2 |
| SNAP II score day 1 | 16.8 ± 15.1 |
| Chorioamnionitis 1 | 24/78 (31%) |
| Funisitis 1 | 11/78 (14%) |
| Postnatal infections | 37/85 (44%) |
| Chronic lung disease (CLD) | 24/85 (28%) |
| NEC (stage II/III) | 5/85 (6%) |
| White matter injury 2 | 22/80 (28%) |
| IVH grade III/IV 2 | 4/80 (5%) |
| Pre-test Cortisol at 18 months CA (μg/dl) | .242 ± .478 |
| Post-1 Cortisol at 18 months CA (μg/dl) | .162 ± .296 |
| Post-2 Cortisol at 18 months CA (μg/dl) | .188 ± .373 |
n=78 placental histopathology available
n=80 with MRI
Multiple infections were found in 18 infants. NEC was suspected in 8 (Bell's stage I) and definite in 5 infants (1 with Bell's stage II, 4 with Bell's stage III requiring surgical intervention). CLD was found in 24 infants. MRI was performed in 80 infants: 32 infants had IVH (9 grade I, 19 grade II, 4 grade IV), and 22 had white matter injury (WMI; 9 minimal, 10 moderate, and 3 severe). Postnatal steroids were given to 22 infants: 5 received dexamethasone, 11 received hydrocortisone, and 6 received both.
Preliminary Analyses
There were no significant associations between WMI severity or IVH grades and cortisol values, and no interaction with the pattern of cortisol over phases (pretest, post-1, post-2).
Prenatal Infections in Relation to Cortisol
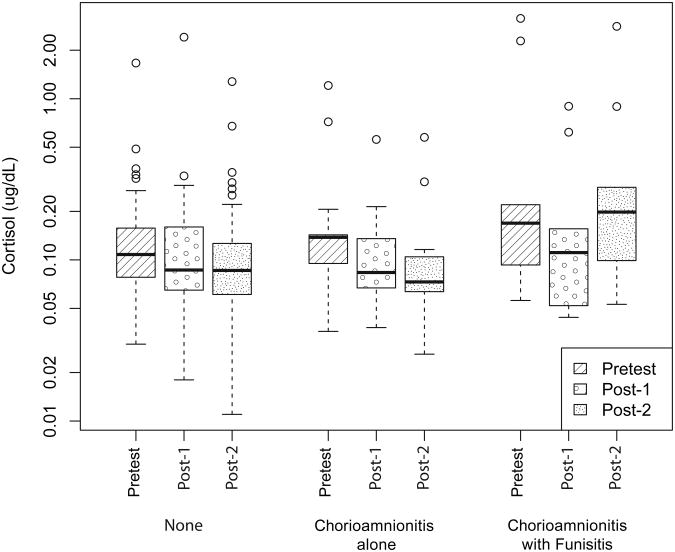
We compared infants with no prenatal infection (n=54), chorioamnionitis alone (n=13), and chorioamnionitis with funisitis (n=11). Infant characteristics by prenatal infection group are presented in Table 2. There was a significant interaction between prenatal infection status and cortisol pattern over assessment phases (pretest, post-1, post-2). This reflected the fact that the chorioamnionitis with funisitis group showed a cortisol increase from post-1 to post-2 whereas there was no change in cortisol levels from post-1 to post-2 in the other two groups (after adjustment: F[4,139] = 7.3996, P <.0001). The cortisol pattern is shown in Figure 2. The estimated difference (unadjusted) between the increase from post-1 to post-2 in log cortisol in the chorioamnionitis with funisitis group and the average change (in log cortisol) from post-1 to post-2 in the other two groups (combined) was 0.569 (95% C.I .278 - 0.859 P = .0001). Back-transforming from the log scale yielded an estimate of 1.77 (95% CI: 1.32 to 2.36) which corresponds to the ratio of the proportional increases for chorioamnionitis with funisitis compared to the other two groups. We adjusted for the following clinical confounders as covariates: gestational age at birth, antenatal and postnatal steroid exposure, WMI and IVH, cumulative number of skin-breaking procedures, morphine, midazolam and fentanyl exposure, days of mechanical ventilation and of any respiratory support, and time of day at cortisol sampling. After adjusting for these covariates, the log scale estimate was 0.810 (95% CI:0.506 to 1.114. P<.0001), which back-transforms to 2.25 (95% CI:.1.658 to 3.047).
Table 2. Infant characteristics by prenatal infection group (n=78).
| Characteristic | No infection (n=54) | Chorioamnionitis alone (n=13) | Chorioamnionitis with Funisitis (n=11) |
|---|---|---|---|
| Gestational age1 (wks) | 28.6±2.3 | 27.4±2.0 | 27.6±2.1 |
| Birth weight1 (g) | 1123±345 | 1047±388 | 1084±320 |
| Prenatal Steroids | 47/54 (87%) | 13/13 (100%) | 10/11 (91%) |
| SNAP II score day | 17±15 | 15±15 | 18±17 |
| Postnatal infections | 22/54 (41%) | 6/13 (46%) | 7/11 (64%) |
| Mechanical ventilation1 (days) | 16.3±21.4 | 26.8±35.3 | 15±20.2 |
| Postnatal steroids | 12/54 (22%) | 4/13 (31%) | 3/11 (27%) |
| NEC (stage II/III) | 4/54 (7%) | 0/13 (0%) | 1/11 (9%) |
| White matter injury 2 | 16/54 (30%) | 1/13 (8%) | 5/11 (45%) |
| IVH grade III/IV 2 | 3/54 (6%) | 1/13 (8%) | 0/11 (0%) |
| Skin breaking procedures1 | 119±81 | 125±73 | 131±75 |
| Small-for-gestational-age (SGA) | 10 (19%) | 1 (8%) | 0 (0%) |
Mean±SD
n=80 with MRI
Figure 2. Cortisol Pattern by Prenatal Infection Group.
Cortisol across assessment phase (pretest, post-1 and post-2) by prenatal infection group: no infection (n=54), chorioamnionitis alone (n=13) and chorioamnionitis with funisitis (n=11). The lower and upper quartiles are represented by the top and bottom limits of each box, and the minimum and maximum data values by the “whiskers”. The median is represented by the bold line. Outliers (represented as points outside “whiskers”) were identified if they surpassed the upper or lower quartile by more than 1.5 times the inter-quartile range (IQR).
Postnatal Infections in Relation to Cortisol
Due to the small number of patients with meningitis (1 infant) and concurrent sepsis and NEC (8 infants), they were combined with the 17 infants with sepsis forming a group of 26 infants with culture positive infections. This group did not differ significantly before or after adjusting for covariates.
NEC and CLD in Relation to Cortisol
Cortisol values and patterns were comparable between infants with and without the presence of NEC at any stage, and with and without CLD. There was no statistically significant difference before or after adjusting for multiple covariates.
Discussion
To our knowledge, this is the first study to examine whether early infection is associated with long-term alterations to HPA axis function in preterm infants born ≤ 32 weeks gestation, after hospital discharge. We found that funisitis, a severe form of chorioamnionitis with fetal inflammatory response in the blood vessels of the umbilical cord, was associated with an altered pattern of salivary cortisol levels at 18 months CA. This finding persisted after adjusting for multiple potential clinical confounders, and may indicate altered programming of the HPA axis by exposure to inflammatory stress and likely, associated cytokines and HPA hormones.
Early life plasticity of physiological systems is well established, with the HPA axis being one of the most sensitive and well studied systems. Several prenatal and postnatal environmental factors have been shown in animal models to program the HPA axis and affect function into adulthood, including, for example, prenatal (22) and postnatal stress (23). In humans, prenatal maternal stress (24), low birth weight (25), and postnatal procedural pain-related stress in preterm infants (6,7), have been associated with altered HPA function later. Given the close inter-relationship between the endocrine and immune systems, and the bidirectional signaling between the two systems from fetal life onward (26), there is growing interest in the long term effects of early inflammation on the HPA axis. Indeed, early inflammatory stress has recently been examined in experimental animal studies and found to influence programming of HPA function.(8). Ours is the first study to address the possible role of prenatal or postnatal infections in programming HPA function in humans, focusing on a population of patients especially prone to infections – very low gestational age preterm infants.
Importantly, in our study, only the infants with funisitis and not those with chorioamnionitis without funisitis showed a significantly altered cortisol pattern at 18 months CA. Unlike the broad definition of chorioamnionitis typically used to describe the presence of intrauterine infection and inflammation, which includes cases of maternal placental membrane involvement alone, funisitis specifically indicates a fetal inflammatory response. Other studies have shown poorer neurological outcome following funisitis compared to inflammation limited to maternal membranes. For example, funisitis was found to be more predictive of periventricular leucomalacia(27) and neurologic impairment(28) compared to chorioamnionitis without funisitis. Furthermore, funisitis was found to elicit a stronger cytokine response than chorioamnionitis alone(29). Therefore our present finding of altered HPA responses at 18 months only in infants with funisitis, likely reflects the spectrum of severity of the intrauterine infection, with funisitis being the severe end of the spectrum. Surprisingly, postnatal infection was not related to cortisol levels in our study, which is not consistent with the animal data reporting a programming effect of neonatal LPS exposure on HPA axis function. However, we did not examine other components of endocrine-immune balance such as the HPA response to illness, which have been demonstrated to be altered with neonatal inflammatory stress.(16). CLD and NEC are common diseases of prematurity in which an inflammatory cascade is central to the disease process. Due to the highly inflammatory nature of these conditions, we explored their relationship with HPA function. However, in our study CLD and NEC did not affect cortisol values at 18 months CA. Interpretation of these observations should be cautious. Although the incidence of NEC in our sample was comparable to previous reports, our study may be underpowered to detect a difference. CLD was more prevalent in this cohort, however we used the classic definition of CLD that does not differentiate severity. Potential differences may therefore be masked.
Strengths of this study were the careful consideration of multiple potential confounders, such as gestational age at birth, respiratory support, and prenatal and postnatal synthetic corticosteroid exposure. Several recent studies have suggested an association between elevated inflammatory markers(30) as well as positive culture sepsis(31) with white matter injury. Given this, and despite the lack of association of chorioamnionitis with WMI in this cohort, we nonetheless examined the relationship between WMI or IVH and cortisol but found none. This makes them unlikely to be confounders, although we still adjusted for these factors in statistical analyses. This also implies that chorioamnionitis may be associated with adverse outcomes through mechanisms other than WMI. In addition, we used strict criteria for the definitions of infections, including histological criteria for chorioamnionitis rather than clinical suspicion, and standardized categorization for postnatal infections.
One limitation to our study is that we could not evaluate all aspects of HPA axis function. This was due to the fact that there are ethical constraints that limit the extent to which the HPA axis can be assessed for research purposes in young children. Subjecting infants to a physical stressor or performing a pharmacological challenge is not possible when there is no medical indication or benefit. Moreover, it is more difficult to elicit a cortisol response after the first few months of age, even using routine immunization injections.(32,33) In light of this, the observation of an altered cortisol pattern following cognitive assessment in the present study may suggest a programming effect of exposure to funisitis on HPA function. Another important limitation is the low sample size. This was a secondary analysis from an existing database of a longitudinal cohort that was not powered for the current question. Our results therefore should be interpreted with caution. Larger studies are needed to confirm the role of early infection on HPA axis programming.
In summary, we found that fetal inflammatory response to prenatal infection may be associated with an altered cortisol pattern following behavioral testing at 18 months CA, long after NICU discharge. This may indicate reprogramming of the HPA axis due to early inflammatory stress. Why we did not find associations with other inflammatory conditions of prematurity including postnatal infections, NEC and CLD remains to be determined. It is possible that the timing of inflammation is a critical factor in HPA programming. Our study adds to the growing body of literature on the importance of prenatal infection, a potentially preventable and treatable condition, for later morbidity. Future human research examining the effect of early inflammation on later immune response may contribute to a fuller understanding of long term morbidities following prenatal infection.
Acknowledgments
We thank the families who participated, and Ivan Cepeda and Gisela Gosse for project management.
Funding: Canadian Institutes for Health Research (MOP86489 to REG, MOP79262 to SPM), and the Eunice Kennedy Shriver Institute of Child Health and Human Development (NICHD/NIH) grant RO1 HD039783 to REG. REG is supported by a Senior Scientist award from the Child & Family Research Institute. SPM is supported by a Tier 2 Canada Research Chair in Neonatal Neuroscience and Scholar award from the Michael Smith Foundation for Health Research.
Footnotes
Financial Disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have indicated there is no conflict of interest. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
References
- 1.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;13319(15):972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27(4):293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 3.Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A, et al. Relationship Between Neonatal Blood Protein Concentrations and Placenta Histologic Characteristics in Extremely Low GA Newborns. Pediatr Res. 2011;69(1):68–73. doi: 10.1203/PDR.0b013e3181fed334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirbelauer J, Seidenspinner S, Thomas W, Kunzmann S, Speer CP. Funisitis is associated with increased interleukin-10 gene expression in cord blood mononuclear cells in preterm infants </=32 weeks of gestation. Eur J Obstet Gynecol Reprod Biol. 2011;155(1):31–35. doi: 10.1016/j.ejogrb.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Harris MC, D'Angio CT, Gallagher PR, Kaufman D, Evans J, Kilpatrick L. Cytokine elaboration in critically ill infants with bacterial sepsis, necrotizing entercolitis, or sepsis syndrome: correlation with clinical parameters of inflammation and mortality. J Pediatr. 2005;147(4):462–468. doi: 10.1016/j.jpeds.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114(1):e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150(2):151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karrow NA. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine-immune axis during fetal and neonatal development: lessons learned from the model inflammagen, lipopolysaccharide. Brain Behav Immun. 2006;20(2):144–158. doi: 10.1016/j.bbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol. 2000;182(6):1404–1413. doi: 10.1067/mob.2000.106180. [DOI] [PubMed] [Google Scholar]
- 10.Miralles R, Hodge R, Kotecha S. Fetal cortisol response to intrauterine microbial colonisation identified by the polymerase chain reaction and fetal inflammation. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F51–4. doi: 10.1136/adc.2006.110130. [DOI] [PubMed] [Google Scholar]
- 11.Watterberg KL, Scott SM, Naeye RL. Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics. 1997;99(2):E6. doi: 10.1542/peds.99.2.e6. [DOI] [PubMed] [Google Scholar]
- 12.Ng PC, Wong SP, Chan IH, Lam HS, Lee CH, Lam CW. A prospective longitudinal study to estimate the “adjusted cortisol percentile” in preterm infants. Pediatr Res. 2011;69(6):511–516. doi: 10.1203/PDR.0b013e31821764b1. [DOI] [PubMed] [Google Scholar]
- 13.Soliman AT, Taman KH, Rizk MM, Nasr IS, Alrimawy H, Hamido MS. Circulating adrenocorticotropic hormone (ACTH) and cortisol concentrations in normal, appropriate-for-gestational-age newborns versus those with sepsis and respiratory distress: Cortisol response to low-dose and standard-dose ACTH tests. Metabolism. 2004;53(2):209–214. doi: 10.1016/j.metabol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29(2):209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Gover A, Brummelte S, Synnes AR, Miller SP, Brant R, Weinberg J, et al. Single course of antenatal steroids did not alter cortisol in preterm infants up to 18 months. Acta Paediatr. 2012;101(6):604–608. doi: 10.1111/j.1651-2227.2012.02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher RE, Karrow NA, Quinton M, Finegan EJ, Miller SP, Atkinson JL, et al. Endotoxin exposure during late pregnancy alters ovine offspring febrile and hypothalamic-pituitary-adrenal axis responsiveness later in life. Stress. 2010;13(4):334–342. doi: 10.3109/10253891003663762. [DOI] [PubMed] [Google Scholar]
- 17.Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, et al. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97(10):5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granger DA, Hood KE, Ikeda SC, Reed CL, Block ML. Neonatal endotoxin exposure alters the development of social behavior and the hypothalamic-pituitary-adrenal axis in selectively bred mice. Brain Behav Immun. 1996;10(3):249–259. doi: 10.1006/brbi.1996.0022. [DOI] [PubMed] [Google Scholar]
- 19.Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes A, Miller SP. Procedural pain and brain development in premature newborns. Annals of Neurology. 2012 Mar;71(3):385–96. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 22.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13(2):113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 23.Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology (Berl) 2011;214(1):33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glover V, O'Connor TG, O'Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010;35(1):17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Ward AM, Syddall HE, Wood PJ, Chrousos GP, Phillips DI. Fetal programming of the hypothalamic-pituitary-adrenal (HPA) axis: low birth weight and central HPA regulation. J Clin Endocrinol Metab. 2004;89(3):1227–1233. doi: 10.1210/jc.2003-030978. [DOI] [PubMed] [Google Scholar]
- 26.Chesnokova V, Melmed S. Minireview: Neuro-immuno-endocrine modulation of the hypothalamic-pituitary-adrenal (HPA) axis by gp130 signaling molecules. Endocrinology. 2002;143(5):1571–1574. doi: 10.1210/endo.143.5.8861. [DOI] [PubMed] [Google Scholar]
- 27.Wharton KN, Pinar H, Stonestreet BS, Tucker R, McLean KR, Wallach M, et al. Severe umbilical cord inflammation-a predictor of periventricular leukomalacia in very low birth weight infants. Early Hum Dev. 2004;77(1-2):77–87. doi: 10.1016/j.earlhumdev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Rovira N, Alarcon A, Iriondo M, Ibanez M, Poo P, Cusi V, et al. Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants. Early Hum Dev. 2011;87(4):253–257. doi: 10.1016/j.earlhumdev.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Dollner H, Vatten L, Halgunset J, Rahimipoor S, Austgulen R. Histologic chorioamnionitis and umbilical serum levels of pro-inflammatory cytokines and cytokine inhibitors. BJOG. 2002;109(5):534–539. [PubMed] [Google Scholar]
- 30.Leviton A, Kuban K, O'Shea TM, Paneth N, Fichorova R, Allred EN, et al. The Relationship between Early Concentrations of 25 Blood Proteins and Cerebral White Matter Injury in Preterm Newborns: The ELGAN Study. J Pediatr. 2011;158(6):897–903.e5. doi: 10.1016/j.jpeds.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 31.Chau V, Poskitt KJ, McFadden DE, Bowen-Roberts T, Synnes A, Brant R, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66(2):155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 32.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1-2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 33.Glover V, Miles R, Matta S, Modi N, Stevenson J. Glucocorticoid exposure in preterm babies predicts saliva cortisol response to immunization at 4 months. Pediatr Res. 2005;58(6):1233–1237. doi: 10.1203/01.pdr.0000185132.38209.73. [DOI] [PubMed] [Google Scholar]