Abstract
Introduction:
The temporal relationship between incident tuberculosis (TB) and virological outcomes during antiretroviral therapy (ART) is poorly defined. This was studied in a cohort in Cape Town, South Africa.
Methods:
Data regarding TB diagnoses, ART regimens, and 4-monthly updated viral load (VL) and CD4 count measurements were extracted from a prospectively maintained database. Rates of virological breakthrough (VL > 1000 copies/mL) and failure (VL > 1000 copies/mL on serial measurements) following initial VL suppression were calculated. Poisson models were used to calculate incidence rate ratios (IRRs) and identify risk factors for these virological outcomes.
Results:
Incident TB was diagnosed in 391 (28.5%) of 1370 patients during a median of 5.2 years follow-up. Five hundred seventy-eight episodes of virological breakthrough and 231 episodes of virological failure occurred, giving rates of 10.0 episodes per 100 person-years and 4.0 episodes per 100 person-years, respectively. In multivariate analyses adjusted for baseline and time-updated risk factors, TB was an independent risk factor for adverse virological outcomes. These associations were strongly time dependent; the 6-month period following diagnosis of incident TB was associated with a substantially increased risk of virological breakthrough (IRR: 2.3, 95% confidence interval: 1.7 to 3.2) and failure (IRR: 2.6, 95% confidence interval: 1.6 to 4.3) compared with time without a TB diagnosis. Person-time preceding TB diagnosis or more than 6 months after a TB diagnosis was not associated with poor virological outcomes.
Conclusions:
Incident TB during ART was strongly associated with poor virological outcomes during the 6-month period following TB diagnosis. Although underlying mechanisms remain to be defined, patients with incident TB may benefit from virological monitoring and treatment adherence support.
Key Words: tuberculosis, HIV, viral load, virological failure, antiretroviral, Africa
INTRODUCTION
HIV-associated tuberculosis (TB) remains a substantial challenge to global health, especially in sub-Saharan Africa where the majority of new cases and deaths occur.1 This burden of disease is of particular importance to antiretroviral treatment (ART) programs in the region as HIV-associated TB is concentrated in patients accessing these services.2–4 Significant progress has been made in strategies and policies regarding screening, diagnosis, management, and prevention of HIV-associated TB in ART programs.4–8 Nevertheless, the burden of incident TB in patients receiving long-term ART remains high, even in those with good immunological recovery, and this may potentially have adverse effects on ART outcomes.9–11
Establishing and maintaining virological suppression during ART is key to achieving favorable long-term clinical outcomes and to reducing HIV transmission risk. Although incident TB in patients receiving ART has been independently associated with mortality, some evidence also suggests a link between incident TB and viremia or virological failure.11–14 However, much of this evidence is from cross-sectional studies, the relationship is incompletely defined, and, in particular, the temporal relationship is unknown. It is mechanistically plausible that virological failure, and subsequent immunological failure, could result in increased risk of incident TB. Alternatively, the risk of virological failure might be increased during concurrent ART and TB treatment because of impaired treatment adherence or pharmacokinetic drug interactions. The aim of this study conducted in a community-based ART program with high burden of TB in Cape Town, South Africa, was to characterize the association between incident TB and poor virological outcomes and to specifically characterize the temporal relationship.
METHODS
Study Setting
This study was part of ongoing research at the ART service in Gugulethu township, Cape Town, which has previously been well described.3,15–17 Patients enrolled consecutively into the ART program from September 2002 were eligible for the study. Blood CD4 cell counts and plasma viral load (VL) levels were measured routinely pretreatment, every 4 months during ART, and when clinically indicated. VL assays were measured using the Versant HIV-1 RNA 3.0 assay performed on a 340 bDNA analyzer (Bayer Diagnostics, Tarrytown, NY) with a lower limit of detection of 50 RNA copies per milliliter.
First-line ART comprised the nonnucleoside reverse transcriptase inhibitor efavirenz (EFV) or nevirapine (NVP) with a nucleoside reverse transcriptase inhibitor backbone (stavudine and lamivudine). The second-line regimen was protease inhibitor (PI) based (lopinavir/ritonavir) with nucleoside reverse transcriptase inhibitor backbone (zidovudine and didanosine). For those receiving NVP, a standard induction dose was used for all patients as per standard practice at the time. The PI dose was doubled during rifampicin-based TB treatment. A secure supply of medication was maintained by the local health authority with no stockouts during the study period, and all treatment was supplied free of charge. Patients were allocated to community-based therapeutic counselors for ongoing support. Patients did not receive isoniazid preventive therapy as was practice at that time.
Virological Definitions
Virological suppression was defined as at least 1 plasma VL measurement < 50 copies per milliliter. Virological breakthrough was defined as a single plasma VL > 1000 copies per milliliter having previously achieved virological suppression. Two or more consecutive VL measurements > 1000 copies per milliliter at least 4 weeks apart were defined as virological failure. Patients with a VL > 1000 copies per milliliter were reviewed and received a targeted adherence intervention including counselor home visits, as previously described.17 Although a previous study reported similar proportions of patients with VL measurements >400 and >1000 copies per milliliter during ART,17 the threshold of 1000 copies per milliliter was chosen as it is less likely to reflect random variation and more likely to reflect significant viral breakthrough.
TB Screening, Diagnosis, and Definitions
TB screening, diagnosis, and treatment have been previously described in this cohort.10 TB screening was conducted at baseline using a multisymptom screening questionnaire similar to the subsequently developed WHO-recommended screen6 to identify patients who required further investigation. All cases of TB fulfilled WHO diagnostic criteria for resource-constrained settings with high HIV prevalence.18 Any culture-negative TB diagnoses were defined by radiological abnormalities consistent with active TB, strong histological, or clinical evidence of active TB and a clinician’s decision to treat with a full course of anti-TB chemotherapy. All microbiological specimens were analyzed in accredited laboratories, and all positive cultures were speciated by polymerase chain reaction. TB treatment was rifampicin based and administered via a local network of community clinics providing a directly observed treatment short-course strategy.
“History of TB” was defined as a diagnosis of TB with completion of anti-TB treatment before enrollment for ART. “Prevalent TB” was defined as patients taking anti-TB treatment at the time of starting ART. “Incident TB” was defined as a new clinical episode of TB diagnosed after initiation of ART irrespective of date of symptom onset. Patients with multiple diagnoses of incident TB had completed a full course of anti-TB treatment with resolution of symptoms between episodes.
Data Collection and Ethics
Data were collected from patients enrolled consecutively into the ART programme between September 2002 and May 2006 until censoring of observations on January 1, 2011. Patients not initiating ART, nonnaive to ART at enrollment, aged younger than 16 years, or not achieving virological suppression were excluded. Information on TB events was obtained from prospectively maintained structured clinical records. Laboratory results were transferred on a monthly basis to an electronic database and information on ART regimen was attained from electronic pharmacy records. Outcomes were assigned to all patients as follows: alive and receiving ART, death from any cause, transfer to another ART service, or lost to follow-up (LTFU). LTFU was defined as patients on ART who were over 12 weeks late for a scheduled clinic appointment, failed to return to the ART program, and could not be located by active community-based follow-up.
All patients enrolling in this cohort study provided written, informed consent for data collection for research purposes, and this was approved by the Research Ethics Committee of the University of Cape Town.
Data Analysis
Data were analyzed using Stata version 11.0 (College Station, TX). Person-time was accrued from the date of starting ART until death, transfer, LTFU, or data censorship. Chi-squared tests were used for comparing proportions, t tests for comparing means, and Wilcoxon rank-sum tests for comparing medians. All statistical tests were 2 sided at α value of 0.05.
Person-time was divided into intervals predefined by the laboratory monitoring done at least 4 monthly. Each interval was categorized into strata according to the CD4 cell count and viral load at the start of the interval. If CD4 cell count or viral load measurements were missing for 1 or more intervals, a mean of the values for the preceding and subsequent intervals were used (1.6% intervals). Person-time accrued with plasma VL > 1000 copies per milliliter was excluded from the analysis. After virological failure, if participants subsequently achieved suppression they began accruing person-time again from repeat virological suppression until a further outcome event or data censorship. Person-time was also stratified by time related to incident TB diagnoses.
Poisson regression models were used to calculate incidence rate ratios (IRRs) with 95% confidence intervals (CIs) for time-updated and baseline characteristics. Random effects modeling was used to account for clustering of person-time for individuals with more than 1 episode of virological breakthrough or failure. Multivariate models were constructed using Poisson regression and based on forward selection; a priori risk factors (age and ART regimen) and variables identified as being associated with virological breakthrough or failure during the crude analysis were included. Likelihood ratio tests were used to investigate interactions between variables, statistical hypotheses, and linear trends.
RESULTS
Patient Enrollment and Follow-Up
During the study period, 2000 patients enrolled in the ART program. A total of 630 patients were excluded from this study because they were aged younger than 16 years (n = 161), were nonnaive to ART (n = 85), did not start ART for a variety of reasons (n = 210), or did not achieve virological suppression (n = 174) (Fig. 1). The remaining 1370 patients were eligible for inclusion in the study.
FIGURE 1.
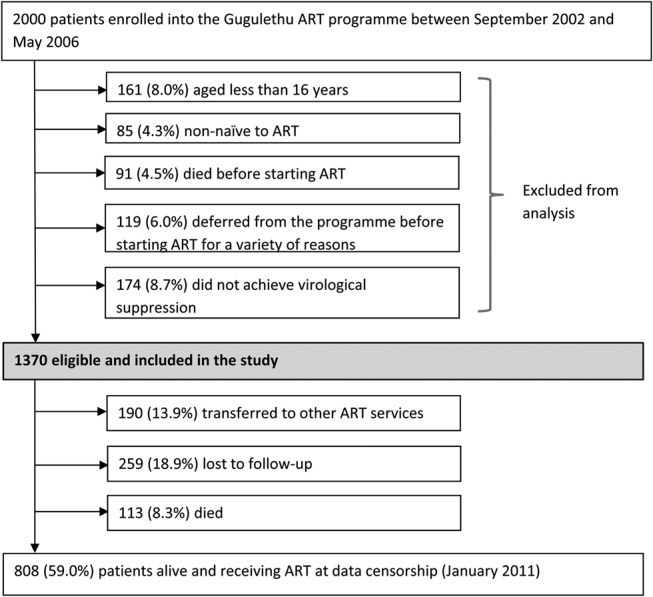
Patient enrollment and outcomes during follow-up.
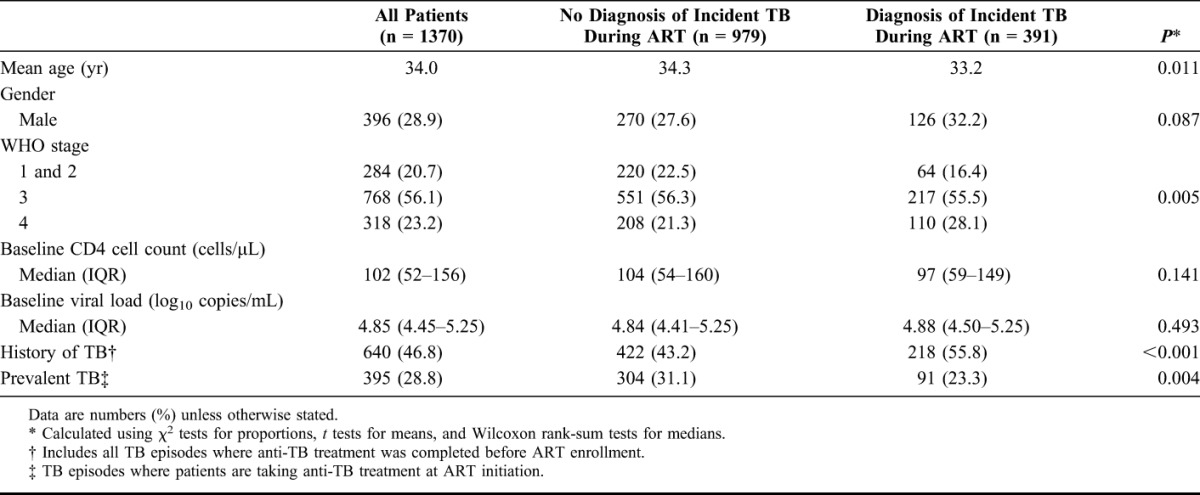
The mean age of the cohort was 34 years, and the majority of the patients were women (71.1%). Overall, patients had advanced immunodeficiency at enrollment with a median baseline CD4 cell count of 102 cells per microliter [interquartile range (IQR): 52–156 cells/µL]. Patients in whom incident TB was diagnosed during ART were younger, less likely to have WHO stage 1 or 2 disease, and more likely to have WHO stage 4 disease (Table 1). They were also more likely to have a history of TB and prevalent TB compared with those who did not.
TABLE 1.
Patient Characteristics at Initiation of Antiretroviral Therapy
During a median follow-up of 5.2 years (IQR: 3.2–5.9 years) of ART, 71.9% of person-time was on an EFV-based ART regimen, 18.7% on an NVP-based regimen, and 9.2% on a PI-based regimen (second line). One hundred thirteen (8.2%) patients died and 259 (18.9%) were lost to follow-up. A total of 449 episodes of incident TB were diagnosed in 391 (28.5%) patients, with 338 patients having 1 diagnosis and 53 patients having 2 diagnoses. Diagnoses of incident TB were microbiologically confirmed in 73.5% cases.
Virological Breakthrough and Failure
During a total of 5790 person-years (PY) of follow-up, 578 episodes of virological breakthrough were detected. One episode was recorded in 339 (24.7%) patients, 102 (7.5%) patients had multiple episodes, and 929 (67.8%) patients maintained virological suppression throughout. Of the virological breakthrough episodes, 231 (40.0%) subsequently met the criteria for virological failure. In total, 200 (14.6%) patients experienced virological failure. Overall, rates of virological breakthrough and virological failure were 10.0 episodes/100 PY and 4.0 episodes/100 PY, respectively.
Incident TB and Virological Outcomes
Higher rates of virological breakthrough occurred in the period following a diagnosis of incident TB compared with person-time without a diagnosis of incident TB [14.3 episodes/100 PY (95% CI: 12.2 to 16.7) versus 9.0 episodes/100 PY (95% CI: 8.2 to 9.9), respectively]. The corresponding rates for virological failure showed a similar association [5.5 episodes/100 PY (95% CI: 4.3 to 7.1) versus 3.6 episodes/100 PY (95% CI: 3.1 to 4.2), respectively). Of patients with diagnoses of incident TB, 35% subsequently had episodes of virological breakthrough, of which one third developed within 6 months (median: 83 days, IQR: 50–170 days). Virological failure developed in 12% patients following diagnoses of incident TB, with over half of these episodes developing within 6 months of TB diagnosis (median: 102 days, IQR: 40–164 days).
Risk Factors for Poor Virological Outcomes and Temporal Relationship With Incident TB
Rates of virological breakthrough and failure were calculated for person-time accrued within a range of updated CD4 cell strata (Fig. 2). The highest rates were associated with person-time with counts of 0–100 cells per microliter (46.8 episodes/100 PY; 95% CI: 37.2 to 58.9; 156 PY), and the lowest rates were associated with person-time with counts of >500 cells per microliter (3.2 episodes/100 PY; 95% CI: 2.4 to 4.1; 1802 PY). The relationship between rates of virological failure and updated CD4 cell counts showed a similar pattern (Fig. 2).
FIGURE 2.
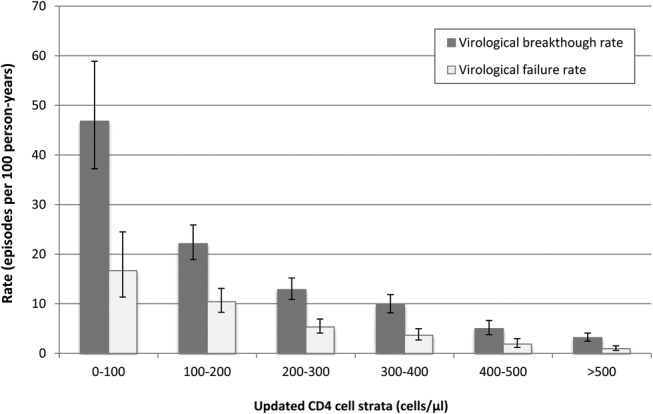
Unadjusted association between virological breakthrough rates (95% CI), virological failure rates (95% CI), and time-updated CD4 cell counts.
In unadjusted analyses, time-updated CD4 cell counts were the strongest risk factor for virological breakthrough (Table 2). Other risk factors for increased rates of virological breakthrough included younger age, increased duration of ART, NVP- or PI-based ART, and diagnoses of incident TB. Age, duration of ART, updated CD4 cell counts, ART regimen, and incident TB were included in the multivariate model (Table 2). There were no interactions between variables. Time-updated CD4 cell counts remained the strongest risk factor for virological breakthrough, with the lowest CD4 cell count stratum having over 30 times the risk of virological breakthrough than the highest stratum (IRR: 33.7, 95% CI: 22.2 to 51.2, P < 0.001). Duration of ART and age also remained strongly associated with virological breakthrough (P < 0.001 for both). After adjustment for other risk factors, NVP-based ART was independently associated with increased risk of virological breakthrough (IRR: 1.35, 95% CI: 1.1 to 1.7) compared with EFV-based ART, but no independent association was observed with PI-based ART (Table 2).
TABLE 2.
Unadjusted and Multivariate Analyses of Risk Factors for Virological Breakthrough and Virological Failure During ART
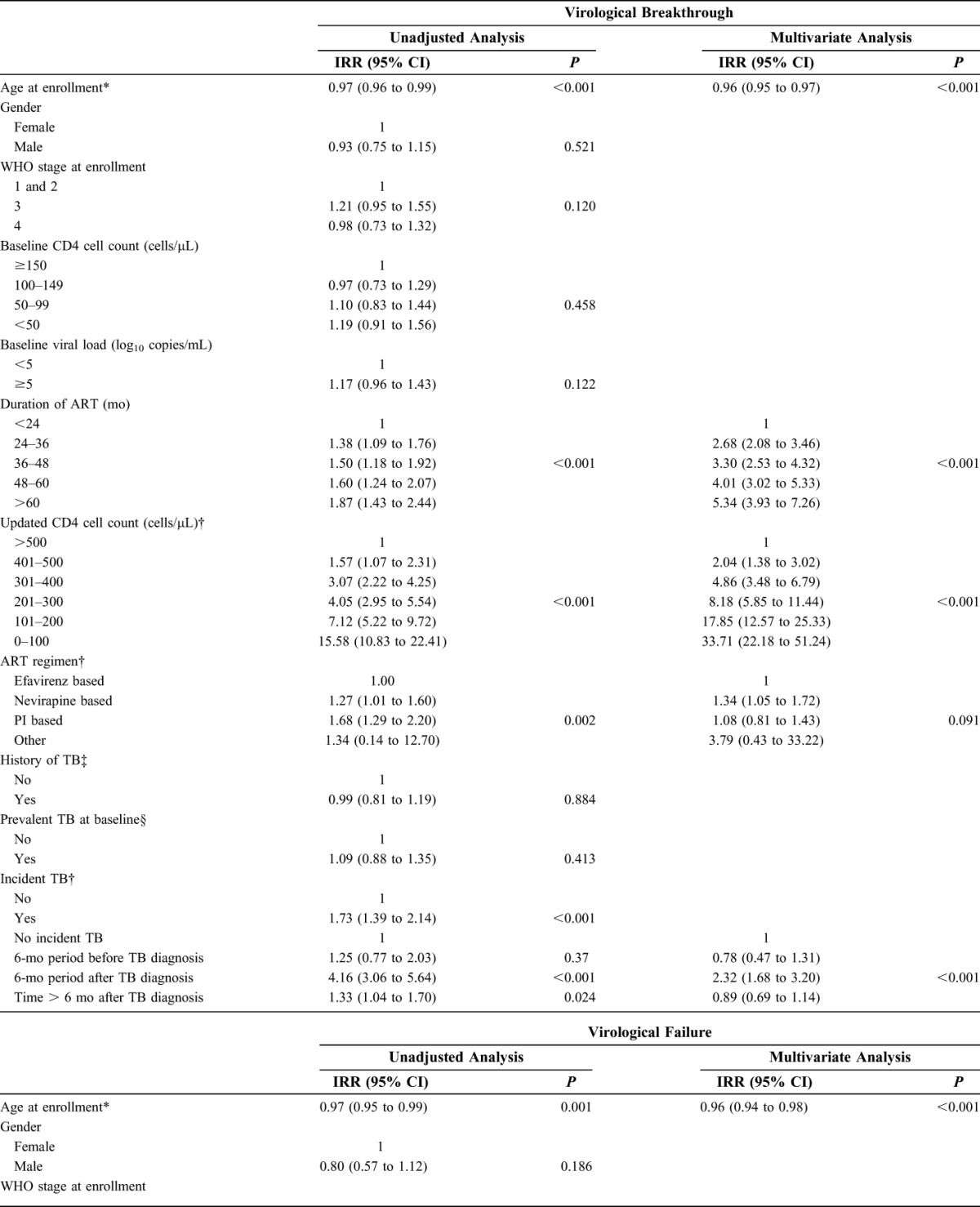
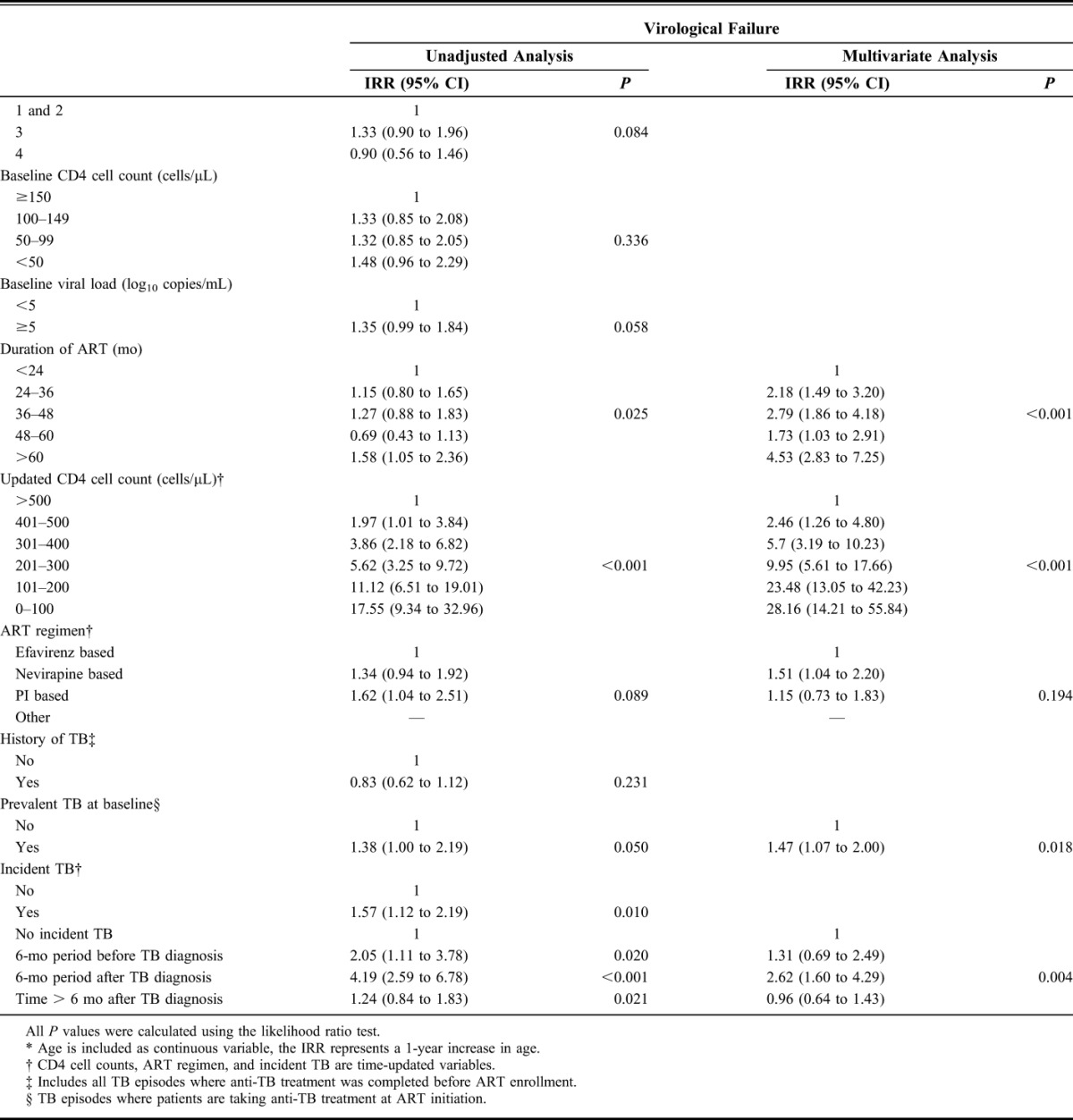
In multivariate analysis, the risk of virological breakthrough in the 6-month period following a diagnosis of TB was associated with over twice the risk of virological breakthrough compared with person-time without a TB diagnosis (IRR: 2.3, 95% CI: 1.7 to 3.2, P < 0.001). The 6-month period before TB diagnosis or time more than 6 months after TB diagnosis were not associated with increased risk of virological breakthrough after adjustment for other risk factors (IRR: 0.8, 95% CI: 0.5 to 1.3, and IRR: 0.9 95% CI: 0.7 to 1.1, respectively) (Fig. 3).
FIGURE 3.
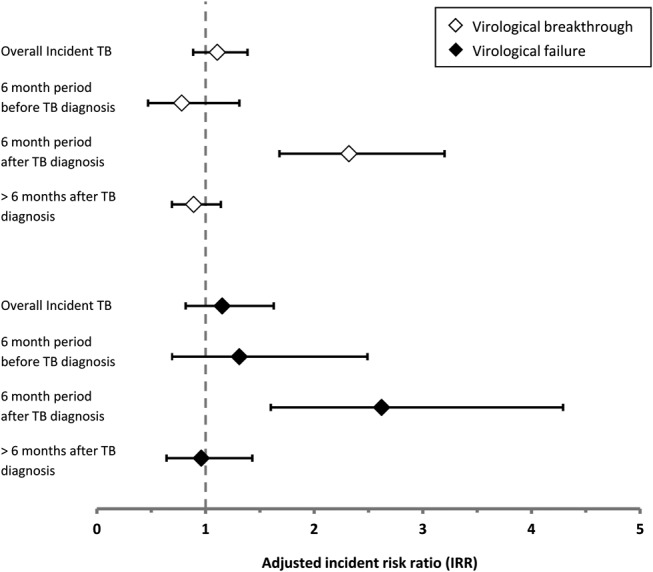
Virological breakthrough ( ) and virological failure (
) and virological failure ( ) IRR associated with person-time accrued following a diagnosis of incident TB, 6-month period before TB, 6-month period after TB diagnosis, and time > 6 months after TB diagnosis, compared with person-time with no incident TB diagnosis.
) IRR associated with person-time accrued following a diagnosis of incident TB, 6-month period before TB, 6-month period after TB diagnosis, and time > 6 months after TB diagnosis, compared with person-time with no incident TB diagnosis.
In unadjusted and multivariate analyses (Table 2), risk factors for virological failure were similar to those for virological breakthrough. Diagnoses of incident TB remained an independent risk factor for virological failure, even after adjustment for other risk factors (P = 0.004). Risk of virological failure was increased over 2.6 times during the 6-month period following a diagnosis of incident TB compared with person-time without a TB diagnosis (IRR: 2.6, 95% CI: 1.6 to 4.3). The 6-month period preceding incident TB diagnosis or time more than 6 months after TB diagnosis were not associated with an increased risk virological failure after adjustment for other risk factors (IRR: 1.3, 95% CI: 0.7 to 2.5, and IRR: 1.0 95% CI: 0.6 to 1.4, respectively) (Fig. 3). ART regimen was not a risk factor for virological failure overall, but NVP-based ART regimens were associated with an increased risk of virological failure (IRR: 1.5, 95% CI: 1.0 to 2.2).
A sensitivity analysis similarly found increased adjusted risks of virological breakthrough and failure (IRR: 2.3, 95% CI: 1.6 to 3.3, P < 0.001, and IRR 3.1, 95% CI: 1.9 to 5.2, P = 0.002, respectively) in the 6 months after microbiologically confirmed diagnosis of incident TB but not in the periods either preceding or more than 6 months after TB diagnosis.
Temporal Relationship With Prevalent TB at Baseline
An exploratory analysis was also performed of the association between prevalent TB and virological failure. As previously, only person-time following initial virological suppression was included and to provide adequate person-time for analysis, a 12-month period was considered. In adjusted analysis, the risks of virological breakthrough or failure following prevalent TB were greater during the first 12 months of ART compared with >12 months after starting ART (IRR: 1.7, 95% CI: 1.0 to 2.8, P = 0.053 and IRR: 1.4, 95% CI: 0.9 to 2.0, P = 0.072, respectively).
DISCUSSION
Virological breakthrough and virological failure were common events during ART in this South African cohort, and the key finding of this study was that the rates of both were substantially higher in the 6-month period after a diagnosis of incident TB (but not in the periods either preceding or more than 6 months after TB diagnosis). Over one third of patients who developed incident TB during ART subsequently developed virological breakthrough and 12% developed virological failure. Incident TB was an independent risk factor for both virological outcomes, having adjusted for a wide range of baseline and time-updated variables. These data highlight an important issue that has not previously been clearly defined and indicate the need, where possible, for virological monitoring following diagnosis of incident TB, adherence support, and other interventions that may be required to address the underlying mechanisms.
Few studies have reported data on virological breakthrough and failure rates during ART in sub-Saharan Africa.19 The high cumulative proportion of patients experiencing virological breakthrough and failure in this study may reflect the long duration of follow-up, and rates are consistent with data from other studies elsewhere in the world.20–23 Virological breakthrough differs from virological failure in that the majority of patients experiencing breakthrough will virologically suppress following adherence interventions.17 The significance of virological breakthrough has yet to be clearly defined, and a better understanding and correlation with clinical outcomes is needed. There is conflicting evidence as to whether low-level viremia (commonly defined as 50–400 copies/mL) during ART is associated with virological failure.24–28 Blips of increasing magnitude (eg, >500 copies/mL), however, have been associated with virological failure and resistance.29 Virological breakthrough in this study was conservatively defined as >1000 copies per milliliter and was thus unlikely to be because of stochastic variation and represented patients at increased risk of virological failure, resistance, and poor clinical outcomes.
The data from this study did not support the hypothesis that virological failure in this cohort results in the development of incident TB independently of immune function. Thus, poor virological control does not explain the high TB incidence rates that persist during ART.10 In marked contrast, this study specifically demonstrated that risk of virological failure was increased in the 6-month period after a diagnosis of TB. There are several possible mechanisms for this strong temporal association. TB and anti-TB treatment may reduce adherence to ART because of high pill burden, poor tolerability, adverse effects, or individuals being too unwell to take ART. Impaired adherence may be exacerbated by poorly integrated TB and HIV services, requiring patients to obtain ART and TB medication from separate clinical services.30,31 In addition, pharmacokinetic interactions between rifampicin-based TB treatment and ART may also be a contributory mechanism.32–34 Systemic release of proinflammatory cytokines in patients with active TB may serve to provide stimulus to HIV-1 replication.35,36 Prevalent TB was also found to have a weak association with virological failure during the first year of ART and may be attributable to similar underlying mechanisms, although these findings may also be because of chance.
In this study, PI-based ART regimens were associated with increased risk of virological breakthrough and failure before but not after adjustment for other risk factors. This is most likely because of PI-based regimens being used as second-line treatment, most commonly following virological failure. However, there was some evidence of an independent association between NVP-based ART regimens and poor virological outcomes. This could be a direct association with NVP; existing studies have shown NVP-based regimens to have inferior virological outcomes compared with EFV- or PI-based regimens, especially in the context of rifampicin-based TB treatment, although a recent randomized trial found no difference.37–41 Alternatively, the relationship could be confounded by pregnancy (for which data were not available and adjustment was not made) as NVP-based regimens were mostly prescribed for women who were pregnant or at risk of pregnancy. Pregnancy has been independently associated with worse virological outcomes.41
The findings of this study have several implications for ART programs. First, further research is needed to explore the mechanisms underlying the association between incident TB and poor virological outcomes and to identify appropriate interventions. However, there is also an immediate need to recognize that patients with TB during ART are at increased risk of poor virological outcomes and should, therefore, be prioritized for virological monitoring where possible and increased adherence support. The adherence support system used in this cohort has previously been shown to be associated with successful virological suppression in approximately two thirds of patients with virological breakthrough as also reported in this analysis.17 During the period of this study, HIV care and TB treatment were provided by separate nonintegrated services, which compromises efficient delivery of care as described elsewhere.30,31 Delivery of care for patients with HIV-associated TB must be strengthened, colocated, and integrated. Finally, adjunctive interventions are needed to prevent incident TB during ART, including the use of isoniazid preventive therapy and effective infection control measures in health care facilities.5,42,43
Strengths of this study include the large sample size, long median duration of follow-up, completeness of the dataset, high frequency of CD4 and VL testing, availability of time-updated ART regimen data, high proportion of microbiologically confirmed TB diagnoses, and the use of multiple-event analysis techniques. Although the observational design of the study has inherent limitations, including the inability to establish a direct causal relationship between TB and virological breakthrough and failure, the routine programmatic conditions may improve the generalizability of the findings. The lack of data on treatment adherence and side-effects are a limitation as we were unable to explore these as potential mechanisms. Although this study cannot exclude VL > 1000 copies per milliliter being a risk factor for incident TB, the strong temporal relationship and lack of association between time preceding TB diagnosis and VL breakthrough do not support this explanation. Although other opportunistic infections not accounted for in this study may also be implicated in virological failure, most opportunistic infections are strongly related to CD4 cell count, which was adjusted for. There may be other social and biological risk factors for poor virological outcomes that were not possible to quantify or adjust for in this study. Although the LTFU rate (18.9% over a median follow-up of 5.2 years) was considerably lower than that of other ART programs in sub-Saharan Africa,44 some may have had unascertained TB. However, patients LTFU from this cohort in fact do not have poor prognostic characteristics45 and do not have higher TB incidence rates before LTFU.10
In conclusion, this study shows an association between TB occurring during ART and virological breakthrough and virological failure, and this association is strongly time dependent. Patients developing TB during ART may benefit from targeted VL monitoring, intensified treatment support, and adherence interventions that may be best delivered through integrated TB-HIV services. Further research into the underlying mechanisms and potential solutions is needed.
ACKNOWLEDGMENTS
The authors are grateful to the staff and patients of the Hannan Crusaid ART Clinic in Gugulethu, Cape Town, South Africa.
Footnotes
S.D.L. is funded by the Wellcome Trust, London, United Kingdom, Grant 074641. R.W. was funded in part by the International Epidemiologic Database to Evaluate Aids with a grant from the National Institute of Allergy and Infectious Diseases (NIAID: 5U01AI069924-02); Cost-Effectiveness of Preventing AIDS Complications (CEPAC) funded by the National Institutes of Health (NIH, 5 R01AI058736-02); USAID Right to Care (CA 674 A 00 08 0000,700), and the South African Centre for Epidemiological Modelling and Analysis (SACEMA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2012. Geneva, Switzerland: World Health Organization; 2012. Available at: http://www.who.int/tb/publications/global_report/en/index.html. Accessed January 13, 2013 [Google Scholar]
- 2.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719 [DOI] [PubMed] [Google Scholar]
- 3.Lawn SD, Myer L, Bekker LG, et al. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612 [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Harries AD, Meintjes G, et al. Reducing deaths from tuberculosis in antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2012;26:2121–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO Three “I”s Meeting: Intensified Case Finding, Isoniazid Preventive Therapy and TB Infection Control for People Living With HIV. Geneva, Switzerland: World Health Organization; 2008 [Google Scholar]
- 6.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375:1906–1919 [DOI] [PubMed] [Google Scholar]
- 8.Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis. 2011;204(suppl 4):S1159–S1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr. 2011;56:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Wood R, Kaplan R, et al. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7:e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komati S, Shaw PA, Stubbs N, et al. Tuberculosis risk factors and mortality for HIV-infected persons receiving antiretroviral therapy in South Africa. AIDS. 2010;24:1849–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Khatib Z, Ekstrom AM, Ledwaba J, et al. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: a cross-sectional study in Soweto, South Africa. AIDS. 2010;24:1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahoua L, Guenther G, Pinoges L, et al. Risk factors for virological failure and subtherapeutic antiretroviral drug concentrations in HIV-positive adults treated in rural northwestern Uganda. BMC Infect Dis. 2009;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Wood R, Kaplan R, et al. Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PLoS One. 2013;8:e55824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekker L, Myer L, Orrell C, et al. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–320 [PubMed] [Google Scholar]
- 16.Lawn SD, Myer L, Orrell C, et al. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148 [DOI] [PubMed] [Google Scholar]
- 17.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12:83–88 [PubMed] [Google Scholar]
- 18.World Health Organisation. Improving the Diagnosis and Treatment of Smear-Negative Pulmonary and Extrapulmonary Tuberculosis Among Adults and Adolescents. Recommendations for HIV-Prevalent and Resource-Constrained Settings. Geneva, Switzerland: WHO; 2007 [Google Scholar]
- 19.Barth RE, van der Loeff MFS, Schuurman R, et al. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–166 [DOI] [PubMed] [Google Scholar]
- 20.Geretti A, Smith C, Haberl A. Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther. 2008;13:927–936 Available at: http://www.intmedpress.com/serveFile.cfm?sUID=5489f510-8e0f-49eb-a436-f83ce450b3e8. Accessed November 10, 2012 [PubMed] [Google Scholar]
- 21.Smith CJ, Phillips AN, Dauer B, et al. Factors associated with viral rebound among highly treatment-experienced HIV-positive patients who have achieved viral suppression. HIV Med. 2009;10:19–27 [DOI] [PubMed] [Google Scholar]
- 22.Li JZ, Brumme ZL, Brumme CJ, et al. Factors associated with viral rebound in HIV-1-infected individuals enrolled in a therapeutic HIV-1 gag vaccine trial. J Infect Dis. 2011;203:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khienprasit N, Chaiwarith R, Sirisanthana T, et al. Incidence and risk factors of antiretroviral treatment failure in treatment-naïve HIV-infected patients at Chiang Mai University Hospital, Thailand. AIDS Res Ther. 2011;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829 [DOI] [PubMed] [Google Scholar]
- 25.Henrich TJ, Wood BR, Kuritzkes DR. Increased risk of virologic rebound in patients on antiviral therapy with a detectable HIV load <48 copies/mL. PLoS One. 2012;7:e50065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore AL, Youle M, Lipman M, et al. Raised viral load in patients with viral suppression on highly active antiretroviral therapy: transient increase or treatment failure? AIDS. 2002;16:615–618 [DOI] [PubMed] [Google Scholar]
- 27.Easterbrook PJ, Ives N, Waters A, et al. The natural history and clinical significance of intermittent viraemia in patients with initial viral suppression to <400 copies/ml. AIDS. 2002;16:1521–1527 [DOI] [PubMed] [Google Scholar]
- 28.Raboud JM, Rae S, Woods R, et al. Consecutive rebounds in plasma viral load are associated with virological failure at 52 weeks among HIV-infected patients. AIDS. 2002;16:1627–1632 [DOI] [PubMed] [Google Scholar]
- 29.Grennan JT, Loutfy MR, Su D, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205:1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawn SD, Campbell L, Kaplan R, et al. Delays in starting antiretroviral therapy in patients with HIV-associated tuberculosis accessing non-integrated clinical services in a South African township. BMC Infect Dis. 2011;11:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawn SD, Campbell L, Kaplan R. Time to initiation of antiretroviral therapy among patients with HIV-associated tuberculosis in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2011;57:15–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Cortés LF, Ruiz-Valderas R, Viciana P, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–690 [DOI] [PubMed] [Google Scholar]
- 33.Manosuthi W, Kiertiburanakul S, Sungkanuparph S, et al. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS. 2006;20:131–132 [DOI] [PubMed] [Google Scholar]
- 34.McIlleron H, Meintjes G, Burman WJ, et al. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(suppl 1):S63–S75 [DOI] [PubMed] [Google Scholar]
- 35.Kedzierska K, Crowe SM, Turville S, et al. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39–56 [DOI] [PubMed] [Google Scholar]
- 36.Pawlowski A, Jansson M, Sköld M, et al. Tuberculosis and HIV co-infection. PLoS Pathog. 2012;8:e1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–539 [DOI] [PubMed] [Google Scholar]
- 38.Lockman S, Hughes M, Sawe F, et al. Nevirapine- versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial. PLoS Med. 2012;9:e1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amoroso A, Etienne-Mesubi M. Treatment outcomes of recommended first-line antiretroviral regimens in resource-limited clinics. J Acquir Immune Defic Syndr. 2012;60:314–320 [DOI] [PubMed] [Google Scholar]
- 40.Swaminathan S, Padmapriyadarsini C, Venkatesan P, et al. Efficacy and safety of once-daily nevirapine- or efavirenz-based antiretroviral therapy in HIV-associated tuberculosis: a randomized clinical trial. Clin Infect Dis. 2011;53:716–724 [DOI] [PubMed] [Google Scholar]
- 41.Westreich D, Cole SR, Nagar S, et al. Pregnancy and virologic response to antiretroviral therapy in South Africa. PLoS One. 2011;6:e22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598 [DOI] [PubMed] [Google Scholar]
- 43.Golub J, Saraceni V. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: systematic review. Trop Med Int Health. 2010;15(suppl 1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawn SD, Myer L, Harling G, et al. Determinants of mortality and nondeath losses from an antiretroviral service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776 [DOI] [PubMed] [Google Scholar]


