Abstract
The oculomotor role of the basal ganglia has been supported by extensive evidence, although their role in scanning eye movements is poorly understood. Nineteen Parkinsońs disease patients, which underwent implantation of deep brain stimulation electrodes, were investigated with simultaneous intraoperative microelectrode recordings and single channel electrooculography in a scanning eye movement task by viewing a series of colored pictures selected from the International Affective Picture System. Four patients additionally underwent a visually guided saccade task. Microelectrode recordings were analyzed selectively from the subthalamic nucleus, substantia nigra pars reticulata and from the globus pallidus by the WaveClus program which allowed for detection and sorting of individual neurons. The relationship between neuronal firing rate and eye movements was studied by crosscorrelation analysis. Out of 183 neurons that were detected, 130 were found in the subthalamic nucleus, 30 in the substantia nigra and 23 in the globus pallidus. Twenty percent of the neurons in each of these structures showed eye movement-related activity. Neurons related to scanning eye movements were mostly unrelated to the visually guided saccades. We conclude that a relatively large number of basal ganglia neurons are involved in eye motion control. Surprisingly, neurons related to scanning eye movements differed from neurons activated during saccades suggesting functional specialization and segregation of both systems for eye movement control.
Introduction
In everyday life we scan the environment with a series of eye movements, pointing the fovea towards objects of interest and the most salient areas of the scene. The pattern of such eye movements (EM) carried out while exploring an image, also called scanning EM, is composed of a succession of small saccades and fixations, corresponding to successive re-allocation of attention from one detail to another [1], [2]. Therefore, scanning EM can be considered as internally triggered EM, as the subject moves the gaze around a complex visual image actively searching for information relevant to current motivations and goals. The visual scanpath is generated by complex parallel strategies [3] and depends on planning, visuospatial attention, spatial working memory and emotional state [4], [5]. Scanning EM have mostly been the domain of psychiatric research which has focused on the behavioral aspects of the eye scanning path rather than to pathophysiological origin and scanning EM control [6].
The structures and mechanisms involved in scanning EM are still poorly understood. At the subcortical level, an involvement of the basal ganglia during scanning EM was suggested by early research using regional cerebral blood flow in healthy controls and schizophrenic patients [7]. The importance of the basal ganglia in EM control was further confirmed by animal studies [8], [9], [10], [11], [12], which discovered neurons co-activated during EM by single cell recordings in several regions of the basal ganglia and brainstem [9], [11], [13]. However, subcortical neuronal activity during scanning EM is still unknown and has never been studied in animals or in humans before. Several human studies supported the participation of the basal ganglia in EM control but just with results based on reflexive and voluntary saccades analyzed from oculographic recordings [14], [15], [16], [17], [18], [19] or local field potentials [20]. The only evidence of human EM-related neurons was obtained from the subthalamic nucleus during saccade tasks and smooth pursuit movements in patients with Parkinson’s disease [21].
In our study, we systematically searched for basal ganglia neurons participating in scanning EM. We took advantage of intraoperative microelectrode recordings of single neuronal activity routinely used to identify the basal ganglia based on specific electrophysiological pattern [22]. We have focused on the subthalamic nucleus (STN), substantia nigra pars reticulata (SNr) and globus pallidus (GP) – i.e. nuclei in which EM-related activity was previously reported [11], [13] and which are easily accessible during the implantation procedure for deep brain stimulation in Parkinsońs disease (PD).
Besides EM-related neurons firing selectively when a specific position, velocity or acceleration of the eyeballs is reached, we expected to find less specialized neurons with activity depending on two or more kinematic features simultaneously. This comes from the hypothesis of functional overlap based on neuronal convergence along the striato-pallido-thalamic projection and assuming compression of information when travelling from larger to smaller nuclei [23]. Findings of STN neurons showing co-activation during various eye movement tasks are in agreement with this theory [9], [21]. On the other hand, there is a segregation hypothesis which expects different neuronal populations to selectively respond to specific kinematic parameters or to fire only during a specific kind of the EM. Indeed, functional and anatomical segregation between various EM tasks has been previously observed at different levels involving the cortex, basal ganglia or cerebellum [4], [8], [24]. Therefore, in a subgroup of patients, we additionally studied the basal ganglia neurons during externally triggered EM using a visually guided saccade task. To further elucidate the function of neurons related to EM, we explored temporal relations of EM kinematic parameters with respect to their preceding and following activity, which may suggest their involvement in execution or control processes.
Methods
Ethics statement
The study was approved by the Ethics Committee of the General University Hospital in Prague, Czech Republic and was conducted according to the Declaration of Helsinki.
Patients
Nineteen PD patients were enrolled consecutively from 2008 to 2011 (15 men, 4 women; mean age: 54.5, SD 9.8, range 28–69 years; mean PD duration: 13.8, SD 6.1, range 3–30 years; Hoehn-Yahr stage 2-4; mean motor score of the Unified Parkinsońs Disease Rating scale – UPDRS III in OFF condition: mean 36.5, SD 13.6, range 10–65). All of them were suffering from motor fluctuations and/or disabling dyskinesias (demographic details in Table 1) and were indicated for treatment with deep brain stimulation due to motor fluctuations and dyskinesias. All of them met the UK Brain Bank Criteria for diagnosis of PD [25] and all gave their written informed consent for participation. Patients with dementia and/or depression had been excluded by a routine psychiatric examination and neuropsychological testing (Mini-mental state examination, Mattis dementia rating scale, Beck depression inventory). As a normal cognitive state was requested to fulfill the general indication criteria for implantation surgery, all patients understood the nature of the experiment. They had been informed that procedures related exclusively for study purposes could be skipped if desired. It had been emphasized that they were allowed to forego the experiment at any time before or during the surgery. Four days before surgery, dopamine agonists were substituted by equivalent doses of levodopa. Other anti-PD medication (amantadine, anticholinergics) was suspended earlier for the surgery preparation. Levodopa was withdrawn at least 12 hours before the surgery.
Table 1. Description of patients with Parkinson’s disease.
| patient | Age [years] | DD [years] | levodopa [mg] | UPDRS III | H-Y | DBS target | task | neurons |
| 1 | 64 | 14 | 1375 | 31 | 2.0 | STN | SEM | 12 |
| 2 | 61 | 14 | 1200 | 37 | 2.5 | STN | SEM | 7 |
| 3 | 46 | 15 | 1000 | 40 | 3.0 | STN | SEM | 15 |
| 4 | 63 | 30 | 1250 | 50 | 3.0 | STN | SEM | 3 |
| 5 | 53 | 12 | 700 | 37 | 2.5 | STN | SEM | 14 |
| 6 | 69 | 9 | 750 | 47 | 3.0 | STN | SEM | 5 |
| 7 | 49 | 12 | 1550 | 65 | 4.0 | STN | SEM | 7 |
| 8 | 59 | 12 | 600 | 30 | 2.5 | STN | SEM | 8 |
| 9 | 63 | 14 | 1350 | 21 | 2.0 | GPi | SEM | 4 |
| 10 | 53 | 10 | 750 | 42 | 4.0 | GPi | SEM | 11 |
| 11 | 53 | 11 | 1663 | 45 | 2.5 | STN | SEM | 5 |
| 12 | 57 | 26 | 2000 | 59 | 4.0 | STN | SEM | 12 |
| 13 | 28 | 3 | 720 | 10 | 2.0 | GPi | SEM | 6 |
| 14 | 64 | 17 | 1500 | 31 | 2.5 | STN | SEM | 12 |
| 15 | 53 | 12 | 1000 | 40 | 4.0 | STN | SEM | 9 |
| 16 | 44 | 10 | 1130 | 23 | 2.0 | GPi | SEM, VGS | 2 |
| 17 | 42 | 9 | 740 | 33 | 3.0 | STN | SEM, VGS | 20 |
| 18 | 55 | 19 | 1980 | 35 | 2.0 | STN | SEM, VGS | 16 |
| 19 | 60 | 14 | 1060 | 18 | 2.0 | STN | SEM, VGS | 15 |
Age – age on the day of surgery; STN – subthalamic nucleus; GPi – globus pallidus interna; DD – Parkinsońs disease duration; Levodopa – dose/day in mg including levodopa equivalent dosage of dopamine agonist; patient 4 was also treated with mianserin; patients 6, 7, 8, 9, 10 with citalopram and 16 with bupropion; UPDRS III – motor score of the Unified Parkinsońs Disease Rating Scale in OFF medication condition; H-Y – Hoehn and Yahr stage in OFF medication condition; DBS target – nucleus chosen for bilateral deep brain stimulation; SEM – scanning eye movement task; VGS – visually guided saccade task; neurons – number of neurons identified in the basal ganglia.
Surgery and intraoperative microrecording
Implantation of the deep brain stimulation system was performed separately in two steps: (i) stereotactic insertion of the permanent quadripolar electrode into the STN bilaterally and (ii) implantation of connection leads and the neurostimulator to the subclavial region. The Leksell frame and SurgiPlan software system (Elekta, Stockholm, Sweden) were employed in the stereotactic procedure. Pre-surgical planning was based on 1.5 T MRI with direct visualization of the target. The central trajectory was intentionally focused on the STN center near the anterior part of the red nucleus (15 patients) or to the posteroventrolateral portion of the GP interna (4 patients). The first surgery was performed while awake under local anesthesia. The extracellular neuronal activity was mapped by conventional microelectrode recordings (MER) using parallel insertion of five tungsten microelectrodes spaced 2 mm apart in a “Ben-gun” configuration to select sites for the macroelectrode intraoperative stimulation [26], [27]. Four out of five channels of the Leadpoint recording system (Medtronic, MN) were used for the MER, filtered with 500 Hz high pass filter and 5 kHz low pass filter, sampled at 24 kHz and stored for off-line processing. As the firing pattern of the external globus pallidus could not always be distinguished from the internal globus pallidus, we classified both areas as one structure – GP. Up to six recording positions in the STN, SNr or GP were used for the EM tasks in each patient. The number of positions depended on the time course of the surgery, patients' clinical conditions and compliance. Tasks were not performed if patients demonstrated discomfort from being in the supine position or exhibited painful symptoms relating to the off-medication state as well as increased fatigue or sleepiness during surgery. Immediately after the procedure, the position of each permanent electrode was verified by two orthogonal X-ray images co-registered with a presurgical MRI plan. No dislocation larger than 1 mm was found in any patient.
Eye movement recording
Eye movements during scanning and visually guided EM tasks were recorded using electrooculography (EOG), a technique measuring the position of the eye in terms of the electric potential induced by the eye dipole. Technical constraints during surgery (limited space around the stereotactic frame and a limited number of recording channels) did not allow for more elaborate recordings than the use of one single-channel EOG. The signal was band-pass filtered in the range of 0.1–20 Hz and recorded using the Leadpoint recording system simultaneously with MER acquisition through a pair of surface electrodes attached near the outer canthus and the lower lid of the left eye. This setup enabled the orthogonal projection of the eye position on the axis connecting the two EOG electrodes. All eye movements except those which were orthogonal to the axis could be recorded with this technique.
Tasks
The EM tasks were presented on a 17“-computer screen placed approximately 55 cm in front of the eyes of patients lying in supine position.
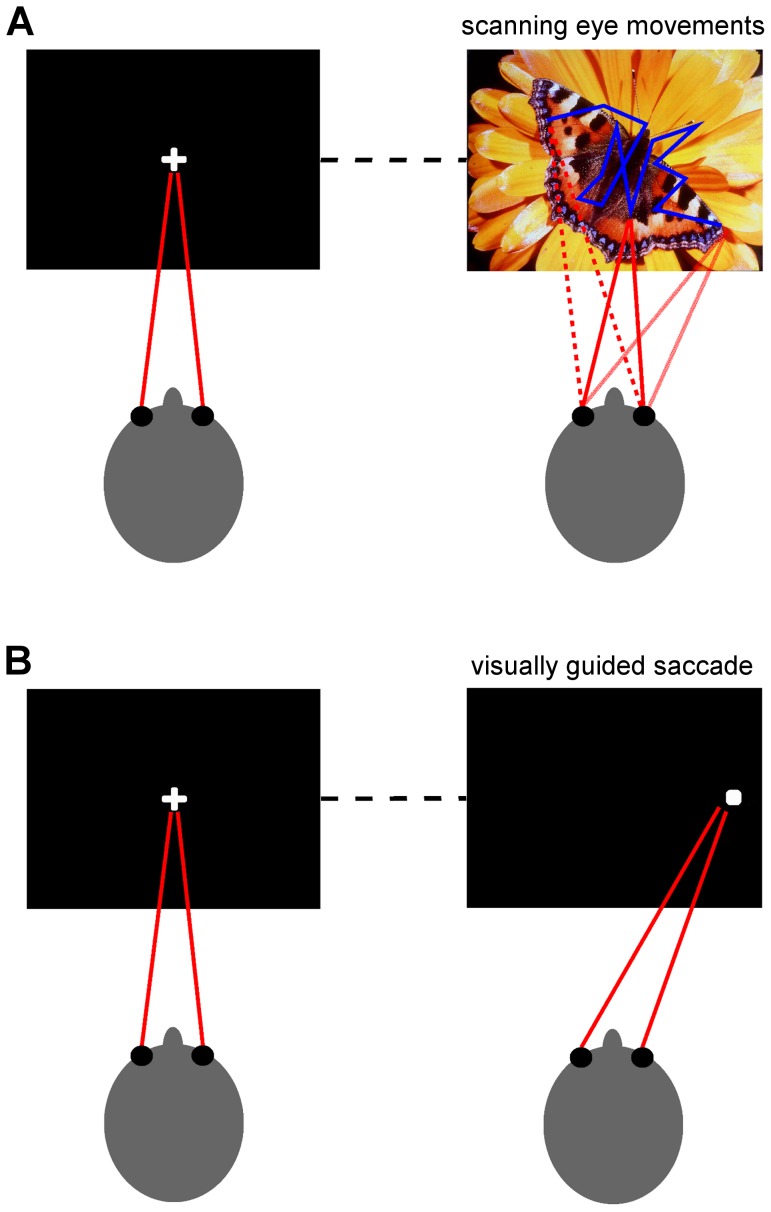
The scanning EM task. The goal of this task was to induce self-initiated free-direction scanning EM. The task consisted of a presentation of a series of photographs selected from the International Affective Picture System (IAPS, Figure 1A) [28], depicting objects, persons, animals and landscapes. To avoid showing the same picture more than once, six unique variants of the test, each containing 24 pictures, were prepared. Each picture was presented for a period of 2 s and was preceded by a black screen for various durations (3500–5500 ms) with a white cross in the center. Patients were asked to fix their eyes on the cross on the black screen and then to simply watch the pictures presented. The MER and EOG signals were acquired in 2 s epoch intervals recorded both during the picture presentation and the black screen. The task lasted approximately for 2.5 minutes.
Figure 1. Eye movement (EM) tasks employed in the study.
A - The scanning EM task. After the presentation of the black screen with a central cross, a photograph chosen from the International Affective Picture System was presented for 2 s. Patients were asked to initially fix their eyes on the cross (left picture) and then simply watch the photograph (right picture). In total, 24 pictures were consecutively used during the task. The blue line highlights a possible eye scanpath. B - The visually guided saccade task consisted of a presentation of 10 pairs of indifferent central (left picture) and lateral GO (right picture) targets positioned pseudorandomly on the left/right side of the screen. Patients were instructed to initially fixate the central cross and then track to the lateral targets as fast as possible.
The visually guided saccade task. The goal of the task was to induce externally generated horizontal saccades (Figure 1B). Initially, a black screen with a central white cross was shown for a pseudorandom period of 2, 2.25, or 2.5 seconds. Subsequently, a peripheral target, a small white square, was presented for 1 s, 17 degrees laterally from the central fixation cross, pseudorandomly to the left (5 trials) or right (5 trials). Patients were instructed to initially fixate on the central cross and then to track the lateral target as fast as possible. The MER and EOG signals of 2 s durations were recorded during all 10 trials. The task lasted for 32.5 seconds.
Data analysis
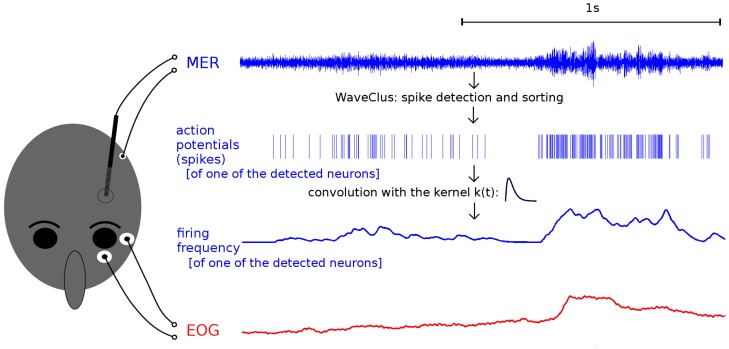
Microelectrode recordings. WaveClus [29], an unsupervised spike detection and sorting tool, which performed reasonably well on the single channel MER [30], was used to extract the series of action potentials of individual neurons from MER signals (Figure 2). Instantaneous firing rate (IFR) of each neuron was estimated by convolving the series of action potentials with the causal kernel function α2*t*exp(-α*t) defined for positive time t, where 1/α was empirically set to 20 ms.
Figure 2. Microelectrode recording (MER) and electrooculography (EOG) signal acquisition and processing.
Action potentials of individual neurons were identified using the WaveClus algorithm in the MER signal. The instantaneous firing rate (IFR) was then estimated by convolving a series of extracted action potentials generated by a single neuron with a causal kernel function. Finally, the IFR was correlated with the eye movement kinematic parameters derived from the EOG.
Each neuron was then mapped relative to the border of the STN, GPi and SNr identified by intraoperative MER. One-dimensional positions along the dorso-ventral microelectrode trajectory were determined using this technique (Figure S1).
EM recordings. EOG signals were rated manually and those contaminated with technical or major blinking artifacts, usually represented by large amplitude changes oversaturating the recording channel, were excluded from further analyses. As we presumed that neuronal activity could be related not only to the position of the eye, but also to its motion and the dynamics of the motion [21], [31] we characterized EM by: i) the eye position (POS), defined by the EOG signal itself, ii) the eye velocity (VELOC), defined as the derivative of POS, and iii) the acceleration of the eye (ACCEL), defined as the derivative of VELOC. The derivative of the signal was defined in terms of the differences between successive samples in a low-pass filtered signal computed using a sliding rectangular window with the cutoff frequency of 12.5 Hz. The maximum and typical amplitude of the EM was extracted in each recording position in each task for each patient. While the maximum amplitude was defined as the extreme value in VELOC, the typical amplitude was defined as the median peak exceeding ±1 SD of the VELOC.
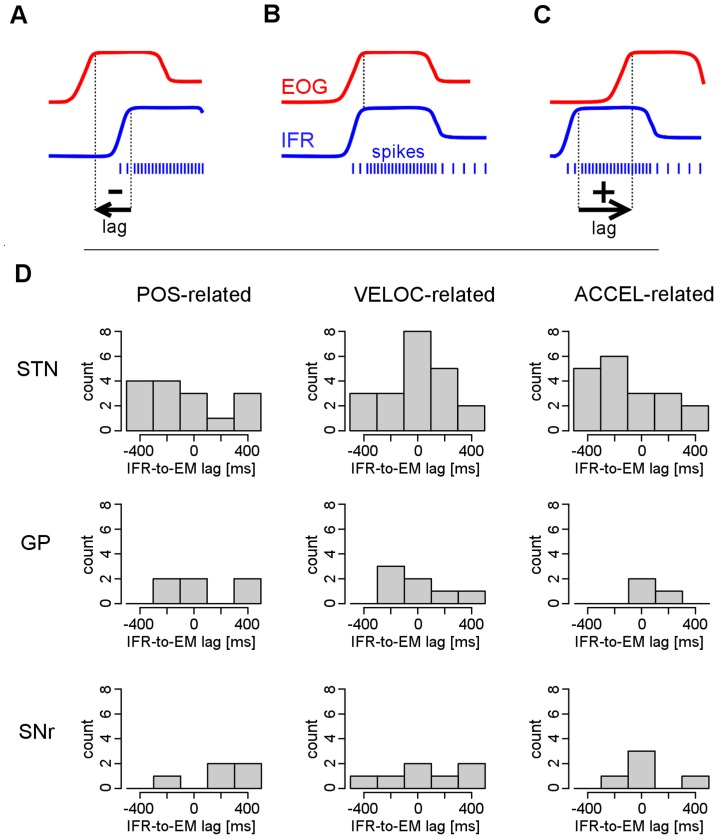
To identify neurons whose activity was associated with EM, the relationships between IFR and POS, IFR and VELOC, and IFR and ACCEL were assessed. A neuron was considered connected to EM if its IFR was related to at least one of POS, VELOC, and ACCEL at the Bonferroni-corrected significance level of p<0.05. The relationships between IFR and the EM characteristics were analyzed using cross-correlation, which could reveal not only the link between concurrent IFR and EM, but also the link of IFR to preceding and following EM (Figure 3A-C). The maximal cross-correlation lag considered was ±500 ms with steps of 2.5 ms. Biased estimates of correlation coefficients were computed to diminish uncertainty in estimates of correlation coefficients over longer lags. The cross-correlation coefficient between two signals was defined as the extreme correlation coefficient between the signals over all the lags considered. The lag in which the extreme cross-correlation was reached was called the optimal EM-to-IFR cross-correlation lag. The statistical significance of the cross-correlation coefficient between two signals was assessed with Monte-Carlo simulations [32], [33] using original and surrogate signals generated by randomly changing the phases of the spectral representation of the original signal.
Figure 3. Time lag of neuronal activity with respect to electrooculography (EOG).
A, B, C - Explanation of the cross-correlation procedure in three examples. Action potentials of three hypothetical neurons along with corresponding instantaneous firing rate (IFR) were correlated with the theoretical EOG signal. Figure A – the IFR correlates with the past EOG signal suggesting a sensory function of the neuron. Figure B – the IFR correlates with the concurrent EOG signal suggesting an executive function of the neuron. Figure C – the IFR correlates with the future EOG signal suggesting a preparatory function of the neuron. The time lag of the IFR in which the maximal (and significant) correlation with EM is reached is called the optimal IFR-to-EM cross-correlation lag. This lag is negative in A, zero in B and positive in C. Figure D - Frequency histograms of the optimal instantaneous firing rate (IFR) to eye movement cross-correlation lags in all eye movement-related neurons during the scanning eye movement task across the subthalamic nucleus (STN), globus pallidus (GP), and substantia nigra pars reticulata (SNr) considering kinematic parameters of the electrooculography (POS, VELOC, ACCEL, in columns). No significant differences in the locations of these distributions were found.
The binomial test, Pearson's correlation coefficient test, Fisher exact test, two-sample proportion test, likelihood ratio test comparing Poisson regression models of dependence and independence in a 2-by-2-by-2 contingency table and paired t-test were used for statistical analysis. Data processing and analyses were performed in MATLAB (R2007b, The MathWorks, Natick, MA) and “R” software [34].
Results
We acquired 137 pairs of MER and EOG signals from 91 recording positions: 97 MERs were assigned to the STN, 21 to the GP and 19 to the SNr according to their firing pattern. In total, 183 neurons were detected using the spike sorting procedure, out of which 130 were located in the STN, 23 in the GP and 30 in the SNr (Table 2).
Neuronal activity related to scanning eye movements
Thirty seven (20%) out of 183 neurons identified in the basal ganglia during the scanning EM task were related to at least one of the EM kinematic parameters (POS, VELOC, ACCEL) (Table 3). Their proportion was higher than the expected false positive rate in each of the analyzed nuclei (binomial test, p<0.001): 26/130 neurons (20%) in the STN, 5/23 neurons (22%) in the GP and 6/30 neurons (20%) in the SNr. Locations of the EM-related neurons are depicted in the Figure S1. In the STN, the ratio of the EM-related neurons was higher in the ventral part (0 to 1 mm from the ventral STN border) compared to the rest of the nucleus (proportion test, χ2 = 2.722, df = 1, P<0.05).
Table 3. Number of neurons related to eye movements in the scanning eye movement task.
| STN (130 neurons) | GP (23 neurons) | SNr (30 neurons) | Total (183 neurons) | |
| EM-related neurons† | 26 (20%)*** | 5 (22%)*** | 6 (20%)*** | 37 (20%)*** |
| POS-related | 15 (12%)** | 6 (26%)*** | 5 (17%)* | 26 (14%)*** |
| VELOC-related | 21 (16%)*** | 7 (30%)*** | 7 (23%)*** | 35 (19%)*** |
| ACCEL-related | 19 (15%)*** | 3 (13%) | 5 (17%)* | 27 (15%)*** |
| POS & VELOC-related | 10 (8%) | 4 (17%)* | 5 (17%)* | 19 (10%)** |
| POS & ACCEL-related | 7 (5%) | 3 (13%) | 3 (10%) | 13 (7%) |
| VELOC & ACCEL-related | 10 (8%) | 3 (13%) | 4 (13%) | 17 (9%)* |
| POS & VELOC & ACCEL-related | 7 (5%) | 3 (13%) | 3 (10%) | 13 (7%) |
EM-related neurons – the number of eye movement-related neurons associated with at least one kinematic parameter (†Bonferroni-corrected number of neurons for three kinematic parameters). Neurons functionally associated with one or more kinematic parameters (POS – eye position; VELOC – eye velocity; ACCEL – eye acceleration) are reported for each nucleus separately (STN – subthalamic nucleus; GP – globus pallidus; SNr – substantia nigra pars reticulata). Number of neurons significantly greater than expected 5% false positivity rate is denoted: *(p<0.05), **(p<0.01) ***(p<0.001).
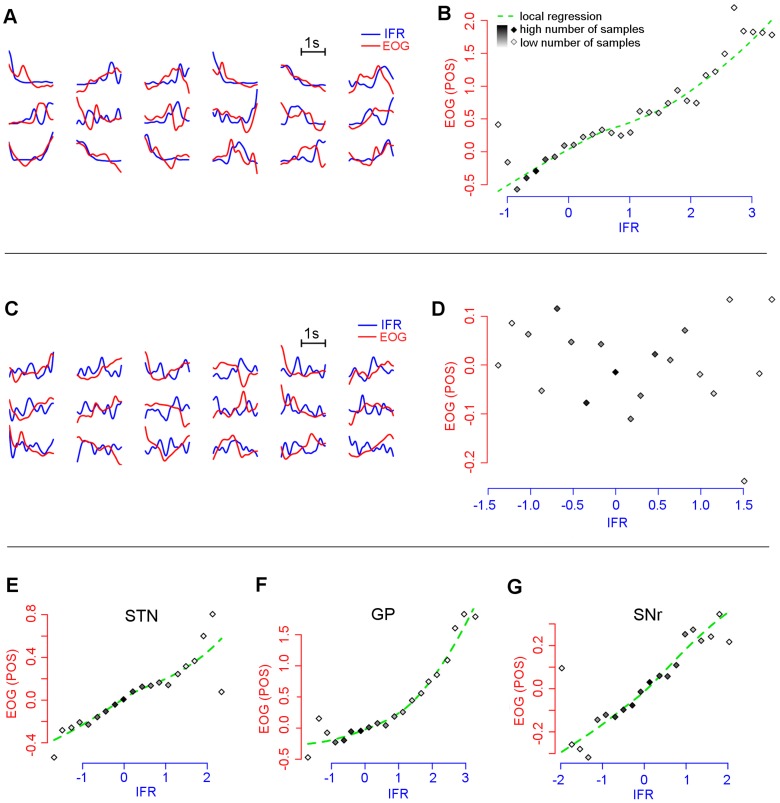
The firing rate of the neurons relating to eye position (POS) significantly correlated with fluctuations of the EOG (Pearson’s r = 0.89 (STN), 0.91 (GP), 0.86 (SNr); df = 18, p<0.001) (Figure 4). A relatively large number of neurons were related to more than one kinematic parameter (likelihood ratio test, D = 42.2 (STN), 19.8 (GP), 28.0 (SNr); df = 3, p<0.001).
Figure 4. Neuronal activity during the scanning movement task.
Example of neuron related (A, B) and unrelated (C, D) to eye movements based on correlation analysis of the instantaneous firing rate (IFR) and eye position (POS) derived from the electrooculography (EOG). All eye movement-related neuronal populations in the STN, GP and SNr are plotted in figures E, F, and G. Figures A, C show the IFR (blue) and EOG (red) pairs recorded during epochs of the task involving both the black screen and pictures presentations. Figures B, D, E, F, G show the dependency of the normalized eye position (POS) derived from the electrooculography (EOG) on the normalized, sorted and binned amplitude of the instantaneous firing rate (IFR). While the IFR from a single neuron was used on figures B and D; the IFR from all eye sensitive neurons were used on figures E, F, and G for each nucleus separately. The amplitudes of the POS signals which correlated negatively with the IFR signal were reversed. The number of signal samples in each bin is expressed by different shades of grey in the diamond glyphs.
As follows from cross-correlation analysis, the firing rate of the neurons was related either to concurrent, previous, or future EM (Figure 3). However, none of the nuclei predominantly contained any kind of the time-related neurons.
Neuronal activity related to visually guided saccades
There were 10/46 neurons (22%) whose activity was related to visually guided saccades in the STN, 1/2 of the neurons were in the GP and 2/5 were in the SNr. A description of neurons related to all EM kinematic parameters (POS, VELOC, ACCEL) is shown in Table 4.
Table 4. Eye movement-related neurons detected in the scanning eye movement task and/or visual guided saccade tasks.
| STN (46 neurons) | GP (2 neurons) | SNr (5 neurons) | |||||||
| SEM | VGS | Both | SEM | VGS | Both | SEM | VGS | Both | |
| EM-related neurons † | 10 | 10 | 2 | 0 | 1 | 0 | 1 | 2 | 0 |
| POS-related | 4 | 9 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| VELOC-related | 9 | 4 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| ACCEL-related | 8 | 11 | 3 | 0 | 1 | 0 | 2 | 0 | 0 |
| POS & VELOC-related | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| POS & ACCEL-related | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VELOC & ACCEL-related | 4 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| POS & VELOC & ACCEL-related | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
EM-related neurons – the number of eye movement-related neurons associated with at least one kinematic parameter (†Bonferroni-corrected number of neurons for three kinematic parameters) identified from patients 16-19 which performed both the scanning eye movement task (SEM) and visual guided saccade task (VGS) in the subthalamic nucleus (STN), globus pallidus (GP) and substantia nigra pars reticulata (SNr). Neurons functionally associated with one or more kinematic parameters (POS – eye position; VELOC – eye velocity; ACCEL – eye acceleration) are reported for each nucleus separately.
Eye movements in the scanning and saccadic tasks
As both the scanning EM and visually guided saccades tasks were executed by only four patients, 19 relevant recording positions were analyzed. Neurons related to scanning EM were usually not activated in the visually guided saccades task and vice versa. Out of 46 STN neurons found in these patients, ten neurons related to scanning EM, ten neurons related to visually guided saccades and only two were activated during both tasks. These neuronal populations seemed to be independent in each of the two tasks as no evidence against the null hypothesis of independence was found (Fisher exact test, p = 1.0) although the test had enough power to reject the null hypothesis had the number of co-activated neurons been higher. In the GP and SNr, an insufficient number of neurons were detected for proper assessment of independence in neuronal activity between the two tasks. However, no GP or SNr neurons were co-activated during both tasks.
Descriptive analyses of the EM amplitude revealed that the maximal amplitude of the scanning EM and visually guided saccades were nearly identical. As requested by the visually guided task, patients executed large saccades, while small EM predominated in the scanning task where large EM occurred only rarely. The amplitude of the typical EM made during the visually guided saccades task was greater than during the scanning task (t = 5.7, df = 18, p<0.001). On average, the median saccade amplitude was 2.6 times larger in the visually guided task than in the scanning EM task.
Discussion
We showed that nuclei of the basal ganglia (namely, STN, GP and SNr) contain neurons whose firing rates correlated with eye movements during the scanning EM task. The proportion of EM-related neurons was relatively high reaching 20-22% in each of those nuclei (Table 3). Despite technical limitations due to the single-channel EOG recording we found relationships between different kinematic parameters of the EM and the firing rate in many neurons (Table 3, Figure 4). These findings point to the role of the basal ganglia in the static and dynamic representation of the EM, a role of importance for the maintenance of accuracy in goal-directed movements.
Table 2. Numbers of microelectrode recordings and neurons detected.
| STN | GP | SNr | Total | |
| MER count | 97 | 21 | 19 | 137 |
| neuron count (SEM task) | 130 | 23 | 30 | 183 |
| neuron count (SEM & VGS task) | 46 | 2 | 5 | 53 |
MER count – number of microelectrode recordings obtained in each nucleus; SEM – scanning eye movement task; VGS – visually guided saccade task; neuron count – number of neurons identified in each nucleus during the SEM task (patients 1-19) and during both the SEM and VGS tasks (patients 16-19); STN – subthalamic nucleus; GP – globus pallidus; SNr – subtantia nigra pars reticulata.
Eye movement activity in basal ganglia
Our single unit records from the STN showed that the proportion of EM-related neurons was higher in its ventral part (Figure S1). A 20% share of oculomotor neurons in the ventral part of the STN has already been noted in monkeys [10] and in humans [21]. However, those were solely neurons involved in saccadic EM. As suggested by our results, the SNr and GP are probably as equally important for control of voluntary scanning EM as the STN. We consider this as one of the major outcomes of our study because in both of these nuclei, the oculomotor activity had previously been noted during EM only in animals [11], [13], [35].
The role of the STN in EM has been largely explored in deep brain stimulation treated patients with Parkinson’s disease. A high intensity STN neurostimulation resulted in contraversive eyeball deviations [36], [37], similar to STN inactivation after locally injected GABA in animals [38] or after unilateral traumatic striato-subthalamic lesion [39]. An electrode penetration to the STN has an impact on the EM parameters as well. It causes a transitory microlesion [40] prolonging the latency of reflexive saccades [41] which are already prolonged due to Parkinson’s disease [42]. Unlike microlesion, deep brain stimulation has an opposite effect on the STN as the latency of visually initiated reflexive saccades become shorter and normalized [41], [43], [44] while their gain is growing [45]. In addition, the STN deep brain stimulation improves some of the parameters of voluntary saccades [18], [46] and suppresses interruptive saccades during fixation [47].
The significance of the STN in EM control is also well documented by other studies. The STN participates in the initiation of voluntary EM and in the inhibition of automatic EM [9], [46], probably reflecting the influence of a hyperdirect pathway connecting the SMA and the motor cortex [48], [49], [50] including the supplementary eye field [51] with the STN, bypassing slower projections through the basal ganglia [52], [53]. By the hyperdirect pathway, the motor plan can be rapidly implemented at the STN level and interfere with automatic EM [9], [11]. The STN influence is then propagated by the following two main outputs [54], [55]. The first is an excitatory glutamatergic projection to the SNr [56], whose activity is reduced or increased during saccades or smooth pursuit movements [12], [13], [35]. The SNr subsequently sends ipsi- as well as contralateral projections to the superior colliculus [57] which is an important nucleus involved in the control of automatic reflexive saccades [8]. The second glutamatergic output from the STN projects to the internal part of the GP [56] through which the oculomotor pattern can be further modified. The GP is more than just a skeletomotor structure as confirmed by several findings of EM-related neurons in its external and internal role during visually guided saccades [11] and anti-saccades in animals [58]. Moreover, bilateral pallidotomy affects the fixation [17] and reduces the velocity of self-initiated saccades [15]. On the other hand, deep brain stimulation of the GP interna modifies other parameters of automatic as well as voluntary saccades (Fawcett et al., 2005). Hence the fact that during the scanning EM task we found EM-related neurons in the STN, SNr and GP was not surprising.
Segregation and convergence in eye movement control
Scanning EM are an important tool in the exploration of complex visual stimuli [6], [59]. Their trajectory is made up of a sequence of variably large saccades and fixations with the visual field maintained for tens to hundreds of milliseconds. As a result, a certain detail is steadily projected on the fovea. This is followed by a saccade, a rapid voluntary movement, by means of which the fovea moves on to a new point of interest while information from the other parts of the retina is being concurrently assessed in search of another point of fixation. This distributed parallel processing has been recently confirmed by the sequential scanning task [60]. As expected, in four patients where both tasks were used, the median amplitude of scanning EM was smaller than that of the saccades in the visually guided task. At the same time, the amplitudes of largest EM executed in both tasks were similar. This is in agreement with previous studies, indicating that the amplitudes of scanning EM follow a heavily skewed distribution towards low values, with relatively rare movements of larger amplitude [61].
From what structures and in which way the scanning movements are controlled is still poorly understood. Since they are under voluntary control, they can be seen as a model with internally generated movements – unlike reflexive saccades which are initiated by external stimuli. Internally and externally triggered movements are generally subject to different control and executive mechanisms [62], [63]. Hence, we assumed that both oculomotor systems are functionally segregated even at basal ganglia level. This hypothesis proved to be correct because in a subgroup of patients engaged in tasks which involved scanning as well as visually guided saccades, we observed that different EM-related neurons were involved in each of the tasks (Table 4). The principle of functional segregation in the control of voluntary and automatic EM had already been previously implied in connection with the interpretation of deep brain stimulation effects [16] or cerebellar lesions [24]. Animal studies have identified spatially segregated functional territories for the control of saccadic EM in the basal ganglia [64], [65]. In primates, the majority of visuo-oculomotor neurons were found in the ventral part of the STN, one third of them being active during reflexive externally triggered saccades and another third being active predominantly during internally triggered (memory guided) saccades [10]. Our results go even further in terms of this specialization hierarchy. Apart from the segregation of populations of EM neurons for scanning movements and visually guided saccades, we identified a higher degree of segregation in all three nuclei neurons. In fact, some neurons responded exclusively to a specific kinematic parameter of the EM associated with an increasing or decreasing firing rate depending on whether or not the eye had reached a particular position, velocity or acceleration of movement (Figure 3).
Some of our results conform to the opposite principle arising from the convergence of cortico-striato-pallido-thalamic projection, i.e. from input nuclei which are larger, to output nuclei which are smaller [23], [66] implying that initially complex information undergoes compression and simplification on its way to the output [67], [68]. Indeed, a small percentage of the STN neurons showed the same neuronal activity in both types of tasks (Table 4). The convergence theory is supported by our observation of 5–8% of STN neurons, whose activity correlated with several kinematic parameters simultaneously (Table 3, 4) suggesting the presence of universal oculomotor neurons. This is in agreement with previous findings of STN neurons which become activated by switching from automatic to voluntary controlled EM [9], with the STN neurons activated from saccades and also during passive movements of the limb [21], with the SNr neurons activated during both pursuit and saccadic EM [13], or with anatomical connections documenting overlap between saccadic and pursuit oculomotor system at the brainstem level [69]. The functional convergence is further supported by the STN deep brain stimulation joint effect on the oculomotor and motor system of the neck and trunk in Parkinson’s disease, marked by simultaneously improved orienting eye-head movements [45] or by improved oculomotor performance associated with body turning [70].
Time relation between EOG and neuronal activity
In our study, the eye-movement neurons in the STN, SNr or GP were not firing solely in a particular phase of the scanning EM task. In all three nuclei, these neurons became active 200–400 ms before EM, in its course and also 200–400 ms after its onset (Figure 3D). While STN neuronal activity expressed in saccade-related potentials already began 0.8–1.8 s before the saccade, suggesting the involvement of nonspecific readiness non-motor mechanisms [20], single unit neuronal STN and SNr activity culminated within 250 ms after the saccade onset [21] suggesting monitoring or sensory function. Our results are more in agreement with observations of the STN showing modified neuronal activity before, during and after the saccade [10]. This means that scanning EM-related neurons of the STN could be involved in all the preparatory, executive and monitoring phases of EM. This cannot be concluded for GP and SNr due to a relatively low amount of data.
Limitations
As there were several limitations we should interpret our results with caution. The main problem arised from the impossibility of using infra-red oculography or two-channel EOG during surgery. While their use would definitely have improved the accuracy of the kinematic parameters during EM, they would also have interfered with the established implantation procedure. The use of single-channel EOG, which failed to capture the full extent of free-direction EM and yielded no more than EM projection into a one-dimensional space, is clearly a limitation which to some extent compromised the sensitivity of our study. Another limitation is connected with the assessment of neuronal activity during the oculomotor tasks based on just correlation analysis. Neuronal firing does not have to relate to EM activity alone but it may also reflect visual perception, planning, visuo-spatial attention or other cognitive processing which coincide with oculomotor activity. In addition, our results could be affected by the fact that our data was obtained from patients with Parkinson’s disease in whom abnormal saccadic EM were repeatedly reported [42], [44], [46], [71], [72], [73], [74]. Whether any abnormalities exist in Parkinson’s disease during scanning EM also is not clearly known since, with the exception of one study which showed a deficit in trans-saccadic working memory [75], no-one has systematically focused on scanpath or other parameters of complex exploratory EM in these patients.
Conclusions
As our results showed, the STN, SNr and GP contain neuronal populations related to scanning EM. Their representation reached about 20% in each of the three nuclei. Basal ganglia are thus not limited to previously described saccade control and perhaps play a more general role in EM circuitry. Oculomotor systems responsible for the execution and monitoring of scanning EM and visually guided saccades are mostly segregated as suggested by neurons involved exclusively in one of two EM tasks or by neurons selectively co-activated in association with a specific kinematic parameter. However, some functional overlap of the two oculomotor systems does exist, albeit confined to small groups of neurons conforming to the complementary convergence principle. Further studies combining clinical and electrophysiological approaches are needed to clarify the role of the basal ganglia in automatic and voluntary oculomotor behavior. We should emphasize, that the large representation of basal ganglia neurons showing activity during all phases of the EM is also an argument for taking them into account when designing new tasks using single unit microrecording. Many visual, ocular or motor experiments are potentially oculomotor in their nature which may compromise results if the EM-related neuronal activity was not considered.
Supporting Information
Positions of the eye movement-related neurons along dorso-ventral microelectrode trajectory within the basal ganglia. A – length of the subthalamic nucleus (STN), B – length of the globus pallidus (GP) and C – length of the substantia nigra pars reticulata (SNr) explored intraoperatively by the five microelectrodes in both the left and right hemispheres and projected to one-dimensional space aligned to the ventral border of the STN and GPi and to the dorsal border of the SNr. Position of each neuron along the dorso-ventral axis is shown in each subject. The proportion of eye movement-related neurons (EM) was significantly higher in the ventral part of the STN.
(TIFF)
Acknowledgments
We are grateful to all the patients who participated in the study. We thank Markéta Fialová for administrative support, Martin Voleman for technical support, Henri Bonnet and Valerie Reeves for reviewing the manuscript.
Funding Statement
This work was supported by the Czech Science Foundation: grant project 309/09/1145, by the Czech Ministry of Health: IGA MZ ÄŒR NT12282-5/2011, IGA MZ ÄŒR NT12288-5/2011, by the Czech Ministry of Education: research project MÅ M 0021620849, MÅ M 6840770012 Transdisciplinary Research in the Area of Biomedical Engineering II, by the Charles University in Prague: research project PRVOUK-P26/LF1/4. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Araujo C, Kowler E, Pavel M (2001) Eye movements during visual search: the costs of choosing the optimal path. Vision Res 41: 3613–3625. [DOI] [PubMed] [Google Scholar]
- 2. Burman DD, Segraves MA (1994) Primate frontal eye field activity during natural scanning eye movements. J Neurophysiol 71: 1266–1271. [DOI] [PubMed] [Google Scholar]
- 3. Wolfe JM, Vo ML, Evans KK, Greene MR (2011) Visual search in scenes involves selective and nonselective pathways. Trends Cogn Sci 15: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, et al. (2003) Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage 18: 231–246. [DOI] [PubMed] [Google Scholar]
- 5. Zihl J, Hebel N (1997) Patterns of oculomotor scanning in patients with unilateral posterior parietal or frontal lobe damage. Neuropsychologia 35: 893–906. [DOI] [PubMed] [Google Scholar]
- 6. Toh WL, Rossell SL, Castle DJ (2011) Current visual scanpath research: a review of investigations into the psychotic, anxiety, and mood disorders. Compr Psychiatry 52: 567–579. [DOI] [PubMed] [Google Scholar]
- 7. Tsunoda M, Kurachi M, Yuasa S, Kadono Y, Matsui M, et al. (1992) Scanning eye movements in schizophrenic patients. Relationship to clinical symptoms and regional cerebral blood flow using 123I-IMP SPECT. Schizophr Res 7: 159–168. [DOI] [PubMed] [Google Scholar]
- 8. Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978. [DOI] [PubMed] [Google Scholar]
- 9. Isoda M, Hikosaka O (2008) Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci 28: 7209–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsumura M, Kojima J, Gardiner TW, Hikosaka O (1992) Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol 67: 1615–1632. [DOI] [PubMed] [Google Scholar]
- 11. Shin S, Sommer MA (2010) Activity of neurons in monkey globus pallidus during oculomotor behavior compared with that in substantia nigra pars reticulata. J Neurophysiol 103: 1874–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sato M, Hikosaka O (2002) Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci 22: 2363–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basso MA, Pokorny JJ, Liu P (2005) Activity of substantia nigra pars reticulata neurons during smooth pursuit eye movements in monkeys. Eur J Neurosci 22: 448–464. [DOI] [PubMed] [Google Scholar]
- 14. Averbuch-Heller L, Stahl JS, Hlavin ML, Leigh RJ (1999) Square-wave jerks induced by pallidotomy in parkinsonian patients. Neurology 52: 185–188. [DOI] [PubMed] [Google Scholar]
- 15. Blekher T, Siemers E, Abel LA, Yee RD (2000) Eye movements in Parkinson's disease: before and after pallidotomy. Invest Ophthalmol Vis Sci 41: 2177–2183. [PubMed] [Google Scholar]
- 16. Fawcett AP, Gonzalez EG, Moro E, Steinbach MJ, Lozano AM, et al. (2010) Subthalamic Nucleus Deep Brain Stimulation Improves Saccades in Parkinson's Disease. Neuromodulation 13: 17–25. [DOI] [PubMed] [Google Scholar]
- 17. O'Sullivan JD, Maruff P, Tyler P, Peppard RF, McNeill P, et al. (2003) Unilateral pallidotomy for Parkinson's disease disrupts ocular fixation. J Clin Neurosci 10: 181–185. [DOI] [PubMed] [Google Scholar]
- 18. Rivaud-Pechoux S, Vermersch AI, Gaymard B, Ploner CJ, Bejjani BP, et al. (2000) Improvement of memory guided saccades in parkinsonian patients by high frequency subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry 68: 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Temel Y, Visser-Vandewalle V, Carpenter RH (2008) Saccadic latency during electrical stimulation of the human subthalamic nucleus. Curr Biol 18: R412–414. [DOI] [PubMed] [Google Scholar]
- 20. Fawcett AP, Cunic D, Hamani C, Hodaie M, Lozano AM, et al. (2007) Saccade-related potentials recorded from human subthalamic nucleus. Clin Neurophysiol 118: 155–163. [DOI] [PubMed] [Google Scholar]
- 21. Fawcett AP, Dostrovsky JO, Lozano AM, Hutchison WD (2005) Eye movement-related responses of neurons in human subthalamic nucleus. Exp Brain Res 162: 357–365. [DOI] [PubMed] [Google Scholar]
- 22. Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, et al. (1998) Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol 44: 622–628. [DOI] [PubMed] [Google Scholar]
- 23. Selemon LD, Goldman-Rakic PS (1990) Topographic intermingling of striatonigral and striatopallidal neurons in the rhesus monkey. J Comp Neurol 297: 359–376. [DOI] [PubMed] [Google Scholar]
- 24. Alahyane N, Fonteille V, Urquizar C, Salemme R, Nighoghossian N, et al. (2008) Separate neural substrates in the human cerebellum for sensory-motor adaptation of reactive and of scanning voluntary saccades. Cerebellum 7: 595–601. [DOI] [PubMed] [Google Scholar]
- 25. Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pollak P, Krack P, Fraix V, Mendes A, Moro E, et al. (2002) Intraoperative micro- and macrostimulation of the subthalamic nucleus in Parkinson's disease. Mov Disord 17 Suppl 3S155–161. [DOI] [PubMed] [Google Scholar]
- 27. Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL (2006) Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson's disease and tremor. Mov Disord 21 Suppl 14S259–283. [DOI] [PubMed] [Google Scholar]
- 28.Lang PJ, Bradley MM, Cuthbert BN (2005) International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: Florida TRA-Uo.
- 29. Quiroga RQ, Nadasdy Z, Ben-Shaul Y (2004) Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput 16: 1661–1687. [DOI] [PubMed] [Google Scholar]
- 30. Wild J, Prekopcsak Z, Sieger T, Novak D, Jech R (2012) Performance comparison of extracellular spike sorting algorithms for single-channel recordings. J Neurosci Methods 203: 369–376. [DOI] [PubMed] [Google Scholar]
- 31. Kitama T, Omata T, Mizukoshi A, Ueno T, Sato Y (1999) Motor dynamics encoding in cat cerebellar flocculus middle zone during optokinetic eye movements. J Neurophysiol 82: 2235–2248. [DOI] [PubMed] [Google Scholar]
- 32. Simpson DM, Infantosi AF, Rosas DA (2001) Estimation and significance testing of cross-correlation between cerebral blood flow velocity and background electro-encephalograph activity in signals with missing samples. Med Biol Eng Comput 39: 428–433. [DOI] [PubMed] [Google Scholar]
- 33.Manly BEJ (1997) Randomization, bootstrap and Monte Carlo methods in biology. London: Chapman & Hall.
- 34.R-Core-Team (2012) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- 35. Hikosaka O, Wurtz RH (1983) Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol 49: 1230–1253. [DOI] [PubMed] [Google Scholar]
- 36. Sauleau P, Pollak P, Krack P, Pelisson D, Vighetto A, et al. (2007) Contraversive eye deviation during stimulation of the subthalamic region. Mov Disord 22: 1810–1813. [DOI] [PubMed] [Google Scholar]
- 37. Shields DC, Gorgulho A, Behnke E, Malkasian D, DeSalles AA (2007) Contralateral conjugate eye deviation during deep brain stimulation of the subthalamic nucleus. J Neurosurg 107: 37–42. [DOI] [PubMed] [Google Scholar]
- 38. Baron MS, Wichmann T, Ma D, DeLong MR (2002) Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J Neurosci 22: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bartanusz V, Daniel RT, Villemure JG (2005) Conjugate eye deviation due to traumatic striatal-subthalamic lesion. J Clin Neurosci 12: 92–94. [DOI] [PubMed] [Google Scholar]
- 40. Jech R, Mueller K, Urgosik D, Sieger T, Holiga S, et al. (2012) The subthalamic microlesion story in Parkinson's disease: electrode insertion-related motor improvement with relative cortico-subcortical hypoactivation in fMRI. PLoS One 7: e49056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Antoniades CA, Buttery P, FitzGerald JJ, Barker RA, Carpenter RH, et al. (2012) Deep brain stimulation: eye movements reveal anomalous effects of electrode placement and stimulation. PLoS One 7: e32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pinkhardt EH, Jurgens R, Lule D, Heimrath J, Ludolph AC, et al. (2012) Eye movement impairments in Parkinson's disease: possible role of extradopaminergic mechanisms. BMC Neurol 12: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Temel Y, Visser-Vandewalle V, Carpenter RH (2009) Saccadometry: a novel clinical tool for quantification of the motor effects of subthalamic nucleus stimulation in Parkinson's disease. Exp Neurol 216: 481–489. [DOI] [PubMed] [Google Scholar]
- 44. Antoniades CA, Carpenter RH, Temel Y (2012) Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: similar improvements in saccadic and manual responses. Neuroreport 23: 179–183. [DOI] [PubMed] [Google Scholar]
- 45. Sauleau P, Pollak P, Krack P, Courjon JH, Vighetto A, et al. (2008) Subthalamic stimulation improves orienting gaze movements in Parkinson's disease. Clin Neurophysiol 119: 1857–1863. [DOI] [PubMed] [Google Scholar]
- 46. Yugeta A, Terao Y, Fukuda H, Hikosaka O, Yokochi F, et al. (2010) Effects of STN stimulation on the initiation and inhibition of saccade in Parkinson disease. Neurology 74: 743–748. [DOI] [PubMed] [Google Scholar]
- 47. Wark HA, Garell PC, Walker AL, Basso MA (2008) A case report on fixation instability in Parkinson's disease with bilateral deep brain stimulation implants. J Neurol Neurosurg Psychiatry 79: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nambu A, Takada M, Inase M, Tokuno H (1996) Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci 16: 2671–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci Res 43: 111–117. [DOI] [PubMed] [Google Scholar]
- 50. Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, et al. (2011) Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage 55: 1728–1738. [DOI] [PubMed] [Google Scholar]
- 51. Huerta MF, Kaas JH (1990) Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293: 299–330. [DOI] [PubMed] [Google Scholar]
- 52. Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- 53. Bauswein E, Fromm C, Preuss A (1989) Corticostriatal cells in comparison with pyramidal tract neurons: contrasting properties in the behaving monkey. Brain Res 493: 198–203. [DOI] [PubMed] [Google Scholar]
- 54. Deniau JM, Hammond C, Chevalier G, Feger J (1978) Evidence for branched subthalamic nucleus projections to substantia nigra, entopeduncular nucleus and globus pallidus. Neurosci Lett 9: 117–121. [DOI] [PubMed] [Google Scholar]
- 55. Kita H, Kitai ST (1987) Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol 260: 435–452. [DOI] [PubMed] [Google Scholar]
- 56. Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, et al. (2000) Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur J Neurosci 12: 4141–4146. [DOI] [PubMed] [Google Scholar]
- 57. Beckstead RM, Edwards SB, Frankfurter A (1981) A comparison of the intranigral distribution of nigrotectal neurons labeled with horseradish peroxidase in the monkey, cat, and rat. J Neurosci 1: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoshida A, Tanaka M (2009) Enhanced modulation of neuronal activity during antisaccades in the primate globus pallidus. Cereb Cortex 19: 206–217. [DOI] [PubMed] [Google Scholar]
- 59. Noton D, Stark L (1971) Eye movements and visual perception. Sci Am 224: 35–43. [PubMed] [Google Scholar]
- 60.Trukenbrod HA, Engbert R (2012) Eye movements in a sequential scanning task: evidence for distributed processing. J Vis 12. [DOI] [PubMed]
- 61. von Wartburg R, Wurtz P, Pflugshaupt T, Nyffeler T, Luthi M, et al. (2007) Size matters: saccades during scene perception. Perception 36: 355–365. [DOI] [PubMed] [Google Scholar]
- 62. Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, et al. (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain 118 ( Pt 4): 913–933. [DOI] [PubMed] [Google Scholar]
- 63. Wiese H, Stude P, Nebel K, de Greiff A, Forsting M, et al. (2004) Movement preparation in self-initiated versus externally triggered movements: an event-related fMRI-study. Neurosci Lett 371: 220–225. [DOI] [PubMed] [Google Scholar]
- 64. Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, et al. (2010) Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci 11: 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cui DM, Yan YJ, Lynch JC (2003) Pursuit subregion of the frontal eye field projects to the caudate nucleus in monkeys. J Neurophysiol 89: 2678–2684. [DOI] [PubMed] [Google Scholar]
- 66. Haber SN, Calzavara R (2009) The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Filion M, Tremblay L, Matsumura M, Richard H (1994) [Dynamic focusing of informational convergence in basal ganglia]. Rev Neurol (Paris) 150: 627–633. [PubMed] [Google Scholar]
- 68. Ramanathan S, Hanley JJ, Deniau JM, Bolam JP (2002) Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci 22: 8158–8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yan YJ, Cui DM, Lynch JC (2001) Overlap of saccadic and pursuit eye movement systems in the brain stem reticular formation. J Neurophysiol 86: 3056–3060. [DOI] [PubMed] [Google Scholar]
- 70. Lohnes CA, Earhart GM (2012) Effect of subthalamic deep brain stimulation on turning kinematics and related saccadic eye movements in Parkinson disease. Exp Neurol 236: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Corin MS, Elizan TS, Bender MB (1972) Oculomotor function in patients with Parkinson's disease. J Neurol Sci 15: 251–265. [DOI] [PubMed] [Google Scholar]
- 72. DeJong JD, Jones GM (1971) Akinesia, hypokinesia, and bradykinesia in the oculomotor system of patients with Parkinson's disease. Exp Neurol 32: 58–68. [DOI] [PubMed] [Google Scholar]
- 73. Shibasaki H, Tsuji S, Kuroiwa Y (1979) Oculomotor abnormalities in Parkinson's disease. Arch Neurol 36: 360–364. [DOI] [PubMed] [Google Scholar]
- 74. Ventre J, Zee DS, Papageorgiou H, Reich S (1992) Abnormalities of predictive saccades in hemi-Parkinson's disease. Brain 115 ( Pt 4): 1147–1165. [DOI] [PubMed] [Google Scholar]
- 75. Hodgson TL, Tiesman B, Owen AM, Kennard C (2002) Abnormal gaze strategies during problem solving in Parkinson's disease. Neuropsychologia 40: 411–422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Positions of the eye movement-related neurons along dorso-ventral microelectrode trajectory within the basal ganglia. A – length of the subthalamic nucleus (STN), B – length of the globus pallidus (GP) and C – length of the substantia nigra pars reticulata (SNr) explored intraoperatively by the five microelectrodes in both the left and right hemispheres and projected to one-dimensional space aligned to the ventral border of the STN and GPi and to the dorsal border of the SNr. Position of each neuron along the dorso-ventral axis is shown in each subject. The proportion of eye movement-related neurons (EM) was significantly higher in the ventral part of the STN.
(TIFF)