Abstract
Prenatal alcohol exposure can lead to behavioral and cognitive impairments across multiple domains. Many of the brain regions impacted by prenatal alcohol exposure are also linked with olfactory processing, and odor identification deficits have been documented in certain neurological disorders associated with these brain regions. As odor identification following prenatal alcohol exposure is not well studied, we compared odor identification in children with prenatal exposure to alcohol (AE) to typically developing controls (CON) (N = 16/group). It was hypothesized that children in the AE group would perform more poorly than children in the CON group on the San Diego Odor Identification Test, an identification test of 8 common household odorants. Children exposed to alcohol during prenatal development were significantly impaired in olfactory identification (M = 5.95, SE = 0.37) compared to typically developing controls (M = 7.24, SE = 0.37). These findings confirmed the hypothesis that prenatal exposure to alcohol is associated with odor identification deficits, and suggest that further research is warranted to identify the mechanisms underlying these deficits, the integrity of brain areas that are involved, and to determine whether olfactory performance might contribute to better identification of children at risk for behavioral and cognitive deficits.
Keywords: Prenatal Alcohol Exposure, Fetal Alcohol Spectrum Disorders, Fetal Alcohol Syndrome, Odor Identification, Smell Impairment
Introduction
Recent research suggests that prevalence rates of Fetal Alcohol Syndrome (FAS) in the U.S. are at least 2 to 7 for every 1,000 in typical mixed race and socioeconomic populations, with this number growing as high as 2-5% of school-aged children when all diagnoses of Fetal Alcohol Spectrum Disorders (FASD) are considered (May et al., 2009). As one of the most prevalent preventable causes of birth defects and cognitive impairments in North America, the effects of prenatal exposure to alcohol have received a significant amount of attention in recent research (Mattson et al., 2011; Mattson & Riley, 2011; Sowell et al., 2008; Murthy et al., 2009).
While a diagnosis of FAS is based primarily on physical features, FASD incorporates a broader spectrum of alcohol-affected individuals; alcohol-exposed children with or without the physical markers show similar cognitive and behavioral deficits (Sampson et al., 1997; Mattson et al., 1998). Characteristic cognitive deficits include impairments in attention, IQ, executive functioning, verbal skills, arithmetic skills, visual-spatial functioning, and verbal and non-verbal learning (Chiodo et al., 2009; Kaemingk et al., 2003; Mattson et al., 2006; McGee et al., 2009; Streissguth et al., 1990; Streissguth et al., 1994; Vaurio et al., 2008). Behavioral problems associated with FASD include poor social and communication skills, adaptive abilities, hyperactivity, attention deficits, depression, and various conduct disorders (Crocker, et al., 2009; Streissguth et al., 1996). Overall, these children are more likely to be classified as disruptive, impulsive or delinquent and to suffer academic failure (Fast et al., 1999; Howell et al., 2006; Mattson et al., 2001).
In general, known neural effects of prenatal alcohol exposure in humans include overall reductions in brain volume, particularly within the frontal, temporal, and parietal cortices. Imaging studies have also shown the basal ganglia to be particularly reduced in size as well as both the hippocampus and the cerebellum, even when controlling for overall brain size (Mattson et al., 1996; Lebel et al., 2011). Overall increases in gray matter and decreases in white matter throughout the brain are also commonly seen, along with abnormalities of the corpus callosum. Specifically, prenatal exposure to alcohol results in the corpus callosum lying more anterior and inferior in posterior regions with relatively normal localization of anterior regions. Importantly, studies have linked the amount of corpus callosum displacement with deficits in verbal learning, as well as on other tasks requiring communication between the left and right hemispheres (Korkman et al., 1998; Sowell et al., 2001). Within the frontal region, the cortex has been shown to be thicker for those exposed to alcohol than for controls, while extent of brain surface was smaller in the anterior and orbitofrontal areas (Sowell et al., 2001; Lebel et al., 2011).
Animal studies have also found neurological and behavioral changes resulting from prenatal exposure to alcohol, particularly in the domain of olfactory functioning. In rodents, exposure to ethanol in utero resulted in a “tuning” of the olfactory epithelium to ethanol, leading infantile rats to develop a specific behavioral response to ethanol and to also become less responsive to other odorants (Youngentob et al., 2007). This “tuned” behavioral response to ethanol is characterized by an unconditioned sniffing and neurophysiological response to ethanol odor in infantile and adolescent rats, and is linked to the increased ethanol avidity during adolescence (Barron & Riley, 1992; Middleton et al., 2009).
Odor identification is a task that requires not only detection of an odor, but the ability to name it, implying memory for previous experience with the odor. Quantitative measurement of the ability to identify odors may be beneficial in helping to identify individuals with histories of prenatal alcohol exposure. Importantly, many of the brain regions required for successful odor identification, such as the orbitofrontal cortex, anterior and medial temporal lobe areas, and limbic system, are all known to be affected by prenatal exposure to alcohol, even when the full criteria for FAS are not met (Jones-Gotman et al., 1997; Larsson et al., 1999; Mattson et al., 2001; Mattson, et al., 2010). Moreover, functional imaging studies have reported that those with an FASD show abnormal patterns of neural activation during tasks of verbal working memory, verbal learning, and sustained attention, which are also important to successful odor identification (Lebel et al., 2010). Tests of odor identification have exhibited significant ability to identify other neurological disorders also known to affect orbitofrontal cortex, anterior and medial temporal lobe areas, and the limbic system, such as Alzheimer's disease (Murphy et al., 1998; Murphy, 2002; Murphy et al., 2003; Calhoun-Haney & Murphy, 2005; Wilson et al., 2011).
However, to the best of our knowledge, there are no published studies that investigated the relationship between prenatal exposure to alcohol and odor identification abilities. Thus, the aim of the current study was to investigate the effects of prenatal exposure to alcohol on odor identification performance. It was hypothesized that those with confirmed histories of prenatal exposure to alcohol would perform significantly worse than normal controls on the San Diego Odor Identification Test.
Materials and Methods
Subjects
Subjects were 16 children and adolescents with heavy prenatal alcohol exposure (AE) and a control group of 16 typically developing peers with no prenatal exposure to alcohol (CON). Both the AE and CON groups ranged from 6 to 16 years in age. The two groups were matched on age, sex, and race/ethnicity (Table 1). All subjects were recruited as part of a larger ongoing study of the behavioral teratogenicity of alcohol. Alcohol-exposed children were recruited via professional referral or caregiver self-referral, while children in the control group were recruited from the community through advertising at child-related agencies and venues.
Table 1. Participant Demographics and Performance.
| Gender, M/F |
| Age, Mean (SE) |
| Race |
| Caucasian |
| African American |
| White/Asian |
| Ethnicity |
| Hispanic |
| Non-Hispanic |
| SDOIT, Mean (SE) |
Note. SDOIT = San Diego Odor Identification Test.
To be included in the current study, children were required to be at least 5 years old or have entered kindergarten prior to administration of the odor identification test. Inclusion in the AE group required a history of heavy prenatal alcohol exposure, which was defined as at least 4 drinks per occasion at least once per week or 14 drinks per week during pregnancy. Prenatal alcohol exposure was determined through multisource collateral report, including review of available medical, social service, and adoption agency records or maternal report, when available. Subjects in the AE group were also evaluated by a dysmorphologist with expertise in teratogenesis (Kenneth Lyons Jones, MD). None of the children included in this study met the criteria for FAS diagnosis.
Subjects were excluded if they had a history of significant head injury or loss of consciousness greater than 30 minutes, evidence of other known causes of mental deficiency, were non-fluent in English, were adopted from abroad after age 5 or less than 2 years before assessment, or had current allergies or upper respiratory infection, or had a psychiatric or physical disability that would prevent study completion.
Procedure
All procedures were conducted in accordance with the ethical standards of the San Diego State University Review Board and with the Helsinki Declaration of 1975, as revised in 1983. Written parental consent and subject assent was obtained for each subject prior to testing, and all test administrators were blind to group status of the subject. Testing was completed at the Center for Behavioral Teratology (CBT), San Diego State University.
Odor Identification Test
The San Diego Odor Identification Test (SDOIT; Murphy et al., 1994; Murphy et al., 2002) is an 8-item test consisting of common odors typically found in the home (e.g., chocolate, peanut butter). The task is ideal for children because of the simple nature of the instructions, and it requires no reading ability. Previous research demonstrates high (0.86) test-retest reliability in children (Murphy et al., 1994), and it has been used successfully with other samples of children, e.g., those with Down Syndrome (Sliger et al., 2004). Odorants were presented to the subject in opaque plastic jars to prevent visual clues that might influence odor identification responses. Prior to administration, subjects were presented with a picture-based cue sheet depicting 20 pictures: 8 of the pictures were of the target odor items and twelve were distracters. The cue sheet was used as a non-lexical identification response aid, and to prevent any response bias related to naming problems. All subjects were asked to identify all the pictures on the cue sheet prior to testing, and all were able to do so. They were then presented each of the eight target odors in random order. The subject was asked to close his/her eyes, the odor jar was held beneath the subject's nose for 5 seconds and the subject was instructed to smell. The subject was allowed to respond verbally or by pointing to the picture they perceived as identifying the odor. An interstimulus interval of 45 seconds was used to avoid adaptation. Performance was evaluated using raw scores by summing the number of correct target odors identified, with a possible score ranging from 0 to 8.
Statistical Analyses
We initially examined the data to ensure compliance with the assumptions of analysis of covariance and to evaluate descriptive characteristics of the variables. Group differences on odor identification performance were analyzed with Analysis of variance (ANOVA). Alpha level was set at p < 0.05. Analysis was conducted using SPSS version 18.0.
Results
Table 1 lists demographics and group means on the SDOIT. Groups were well matched on age, sex, and race/ethnicity.
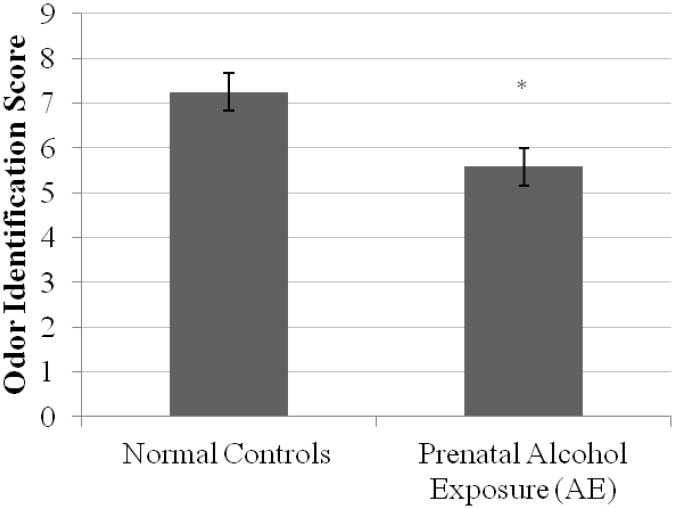
As shown in Figure 1, results of the ANOVA revealed that subjects in the AE group performed significantly poorer than those in the CON group on the odor identification task, F(1, 30) = 4.81, p < 0.05, Cohen's d = 0.77. The mean number of odors identified for the AE group was 5.95 (S.E.M. = 0.37) odors, while for the CON group it was 7.24 (S.E.M. = 0.37) odors.
Figure 1.
Mean +/- S.E.M. odor identification scores by group.
Discussion
The aim of the current study was to investigate odor identification performance in children and adolescents with prenatal alcohol exposure. The findings confirmed the hypothesis that children and adolescents who were exposed to alcohol prenatally would perform worse on the San Diego Odor Identification test than typically developing peers who were not exposed to alcohol during prenatal development.
These results are consistent with the finding that many children who do not manifest the physical features necessary to meet diagnostic criteria for FAS nevertheless sustain underlying central nervous system deficits that result in cognitive and behavioral abnormalities (Maier et al., 1999; Sampson et al., 1997; Mattson et al., 1998).
To our knowledge this is the first study to report the results of direct testing of odor identification in children exposed to alcohol during prenatal development. Caregivers of children with FASD have suggested the potential for general sensory processing deficits (Franklin et al., 2008), including tactile, taste, and smell sensitivity, problems with auditory filtering, visual and auditory sensitivity, and either an under-responsiveness to or excessive seeking of sensation. Studies that relied on caregiver reports and questionnaires have noted the limitations that such measures may be biased depending on which caregiver completes the report (Franklin et al., 2008) and may not reflect the child's internal experience. The use of a quantitative psychophysical measure of odor identification in children with FASD addresses these limitations by testing the child directly on a standard measure of olfactory processing. Furthermore, the nature of the response measure reduces the confounding effects of verbal limitations, and allows for the inclusion of younger subjects.
Effects on fetal development may be dependent upon the timing and/or duration of alcohol exposure during pregnancy. For example, Maier et al. (1999) found decreased volume in the olfactory bulb of rats exposed to alcohol during a time point equivalent to the third trimester in humans, but not during the first or second trimesters. However, overall volume of the olfactory bulb was reduced in those exposed across all trimesters compared to those exposed during the first or second trimester only. Similarly, regional brain volume decreases correlating with level of prenatal alcohol exposure have been found in multiple brain regions in humans (Roussotte et al., 2012; Lebel et al., 2011). It is speculated that olfactory deficits seen in the present study could be due to cellular or volume changes in brain structures involved in olfactory processing or decreased connectivity between sensory processing regions. Investigation of the effects of timing and duration of exposure are important areas for future study.
There are some limitations to this study. It is important to note that further studies are needed to determine the relative contributions of olfactory system integrity and impairment in cognitive domains such as attention or memory to the performance deficits seen in this study. Utilizing a battery of measures that includes an olfactory threshold test will contribute to elucidation of the nature of olfactory functional impairments. It should also be noted that reports of maternal alcohol consumption were obtained retrospectively, which could have biased responses. Despite these limitations, the current study sets a precedent and points to the need for future explorations into olfactory function and psychophysical testing in children and adolescents prenatally exposed to alcohol.
In summary, the current study supports the hypothesis that olfactory identification is impaired in children with prenatal alcohol exposure compared to non-exposed typically developing peers. The results suggest that further research is warranted to identify the mechanisms underlying these impairments, the integrity of brain areas that are involved, and to determine whether olfactory performance might contribute to better identification of children at risk for behavioral and cognitive deficits. Developing a comprehensive description of the impact of fetal alcohol exposure on olfactory function will contribute to the neurobehavioral profile of FASD, which may improve clinical identification and intervention for this group of children.
Acknowledgments
This research was supported by NIH grants R01AG004085-25 to CM and R01AA010417-14 to EPR. Ms. Szajer was supported by an NIH Diversity Supplement to R01AG004085-25.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barron S, Riley EP. The effects of prenatal alcohol exposure on behavioral and neuroanatomical components of olfaction. Neurotoxicology and Teratology. 1992;14(4):291–297. doi: 10.1016/0892-0362(92)90009-y. [DOI] [PubMed] [Google Scholar]
- Calhoun-Haney R, Murphy C. Apolipoprotein e4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain and Cognition. 2005;58:178–182. doi: 10.1016/j.bandc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Janisse J, Delaney-Black V, Sokol RJ, Hannigan JH. A metric of maternal prenatal risk drinking predicts neurobehavioral outcomes in preschool children. Alcoholism: Clinical and Experimental Research. 2009;33:634–644. doi: 10.1111/j.1530-0277.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attentiondeficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2009;33:2015–2023. doi: 10.1111/j.1530-0277.2009.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin L, Deitz J, Jerikowic T, Astley S. Children with fetal alcohol spectrum disorders: Problem behaviors and sensory processing. American Journal of Occupational Therapy. 2008;62(3):265–273. doi: 10.5014/ajot.62.3.265. [DOI] [PubMed] [Google Scholar]
- Fast DK, Conry J, Loock CA. Identifying fetal alcohol syndrome among youth in the criminal justice system. Journal of Developmental & Behavioral Pediatrics. 1999;20:370–372. doi: 10.1097/00004703-199910000-00012. [DOI] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. Journal of Pediatric Psychology. 2006;31:116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Jones-Gotman M, Zatorre RJ, Cendes F, Olivier A, Andermann E, McMackin D, Stauton H, Siegel AM, Wieser HG. Contribution of medial versus lateral temporal-lobe structures to human odour identification. Brain. 1997;120(10):1845–1856. doi: 10.1093/brain/120.10.1845. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Mulvaney S, Halverson PT. Learning following prenatal alcohol exposure: performance on verbal and visual multitrial tasks. Archives of Clinical Neuropsychology. 2003;18:3–47. [PubMed] [Google Scholar]
- Korkman M, Autti-Ramo I, Koivulchto H, Granstrom M. Neuropsychological effects at early school age of fetal alcohol exposure of varying duration. Child Neuropsychology. 1998;4(3):199–212. [Google Scholar]
- Larsson M, Semb H, Winblad B, Amberla K, Wahlund L. Odor identification in normal aging and early Alzheimer's disease: Effects of retrieval support. Neuropsychology. 1999;13(1):47–53. doi: 10.1037//0894-4105.13.1.47. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C. Brain Microstructure Is Related to Math Ability in Children With Fetal Alcohol Spectrum Disorder. Alcoholism: Clinical and Experimental Research. 2010;34:354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychological Review. 2011;120:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: Regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcoholism: Clinical and Experimental Research. 1999;23(4):726–734. doi: 10.1111/j.1530-0277.1999.tb04176.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20:361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Research & Health. 2011;34(1):51–55. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan T, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1996;20(6):1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2010;34(9):1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Research and Health. 2001;25(3):185–191. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McGee CL, Bjorkquist OA, Riley EP, Mattson SN. Impaired language performance in young children with heavy prenatal alcohol exposure. Neurotoxicology and Teratology. 2009;31:71–75. doi: 10.1016/j.ntt.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Carrierfenster K, Mooney SM, Youngentob SL. Gestational ethanol exposure alters the behavioral response to ethanol odor and the expression of neurotransmission genes in the olfactory bulb of adolescent rats. Brain Research. 2009;1252:105–116. doi: 10.1016/j.brainres.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. Olfactory functional testing: Sensitivity and specificity for Alzheimer's disease. Drug Development Research. 2002;56(123):123–131. [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Annals of the New York Academy of Sciences. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Murphy C, Anderson JA, Markison S. Psychophysical assessment of chemosensory disorders in clinical populations. In: Kurihara K, Suzuki N, Ogawa H, editors. Olfaction and taste XI. 1994. pp. 609–613. [Google Scholar]
- Murphy C, Jernigan T, Fennema-Notestine C. Left hippocampal volume loss in Alzheimer's disease is reflected in performance on odor identification: A structural MRI study. Journal of the International Neuropsychological Society. 2003;9(3):459–471. doi: 10.1017/S1355617703930116. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. Journal of the American Medical Association JAMA. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Murthy P, Kudlur S, George S, Mathew G. A clinical overview of Fetal Alcohol Syndrome. Addictive Disorders and their Treatment. 2009;8(1):1–12. [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O'Connor MJ, Narr KL, Sowell ER. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Human Brain Mapping. 2012;33(4):920–937. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sliger M, Lander T, Murphy C. Effects of the ApoE ε4 allele on olfactory function in Down Syndrome. Journal of Alzheimer's Disease. 2004;6(4):397–402. doi: 10.3233/jad-2004-6407. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: Effects of heavy prenatal alcohol exposure. Neurology. 2001;57:234–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O'Connor MJ, Bookheimer SY. Mapping white matter integrity and neurobehavioral correlates in children with Fetal Alcohol Spectrum Disorders. The Journal of Neuroscience. 2008;28(6):1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Final Report: Understanding the Occurrence of Secondary Disabilities in Clients with Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Effects (FAE) University Of Washington Publication Services; Seattle,WA: 1996. [Google Scholar]
- Streissguth AP, Barr HM, Olson HC, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a populationbased prospective study. Alcoholism: Clinical and Experimental Research. 1994;18:248–254. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure effects on child IQ and learning problems at age 7 1/2 years. Alcoholism: Clinical and Experimental Research. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2008;14:119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Bennett DA. Odor identification and mortality in old age. Chemical Senses. 2011;36:63–67. doi: 10.1093/chemse/bjq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Sheehe PR, Molina JC, Spear NE, Youngentob LM. Experience-induced fetal plasticity: The effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behavioral Neuroscience. 2007;121(6):1293–1305. doi: 10.1037/0735-7044.121.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]