Abstract
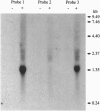
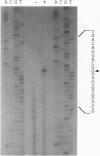
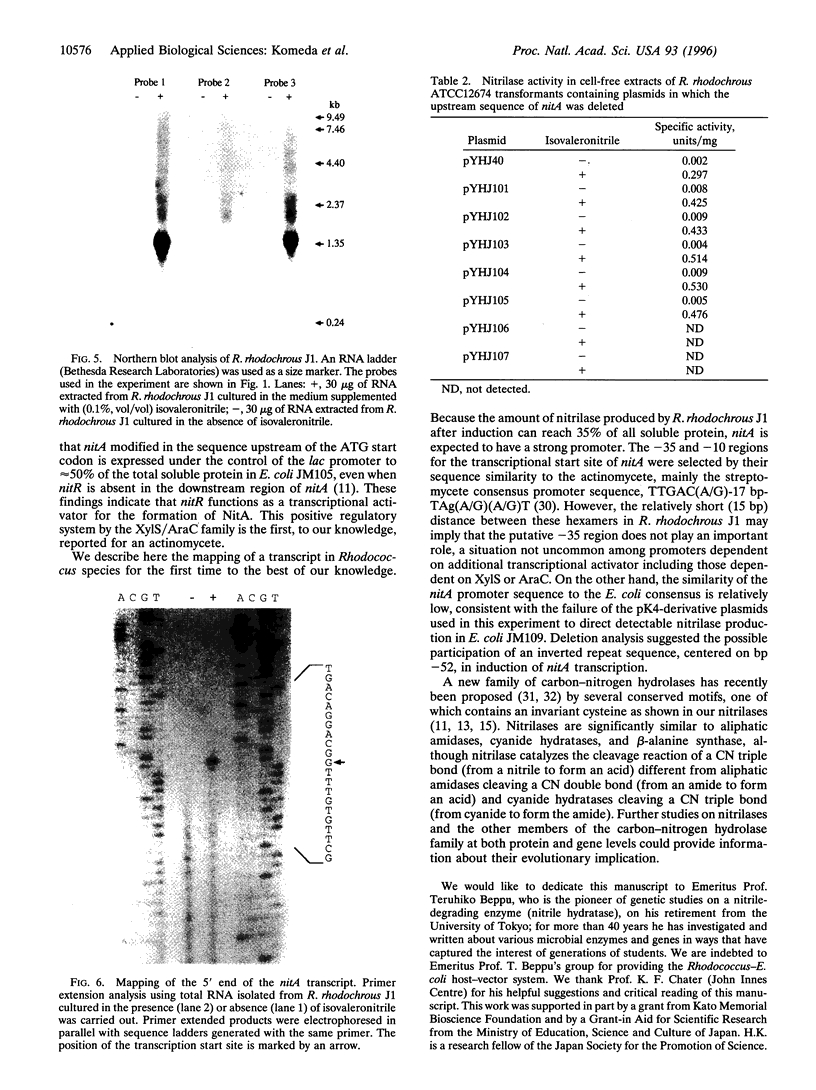
The 1.4-kb downstream region from a nitrilase gene (nitA) of an actinomycete Rhodococcus rhodochrous J1, which is industrially in use, was found to be required for the isovaleronitrile-dependent induction of nitrilase synthesis in experiments using a Rhodococcus-Escherichia coli shuttle vector pK4 in a Rhodococcus strain. Sequence analysis of the 1.4-kb region revealed the existence of an open reading frame (nitR) of 957 bp, which would encode a protein with a molecular mass of 35,100. Deletion of the central and 3'-terminal portion of nitR resulted in the complete loss of nitrilase activity, demonstrating that nitR codes for a transcriptional positive regulator in nitA expression. The deduced amino acid sequence of nitR showed similarity to a positive regulator family including XylS from Pseudomonas putida and AraC from E. coli. By Northern blot analysis, the 1.4-kb transcripts for nitA were detected in R. rhodochrous J1 cells cultured in the presence of isovaleronitrile, but not those cultured in the absence of isovaleronitrile. The transcriptional start site for nitA was mapped to a C residue located 26 bp upstream of its translational start site. Deletion analysis to define the nitA promoter region suggested the possible participation of an inverted repeat sequence, centered on base pair -52, in induction of nitA transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartel B., Fink G. R. Differential regulation of an auxin-producing nitrilase gene family in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6649–6653. doi: 10.1073/pnas.91.14.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartling D., Seedorf M., Mithöfer A., Weiler E. W. Cloning and expression of an Arabidopsis nitrilase which can convert indole-3-acetonitrile to the plant hormone, indole-3-acetic acid. Eur J Biochem. 1992 Apr 1;205(1):417–424. doi: 10.1111/j.1432-1033.1992.tb16795.x. [DOI] [PubMed] [Google Scholar]
- Bartling D., Seedorf M., Schmidt R. C., Weiler E. W. Molecular characterization of two cloned nitrilases from Arabidopsis thaliana: key enzymes in biosynthesis of the plant hormone indole-3-acetic acid. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6021–6025. doi: 10.1073/pnas.91.13.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Koonin E. V. A new family of carbon-nitrogen hydrolases. Protein Sci. 1994 Aug;3(8):1344–1346. doi: 10.1002/pro.5560030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Finnerty W. R. The biology and genetics of the genus Rhodococcus. Annu Rev Microbiol. 1992;46:193–218. doi: 10.1146/annurev.mi.46.100192.001205. [DOI] [PubMed] [Google Scholar]
- Gallegos M. T., Michán C., Ramos J. L. The XylS/AraC family of regulators. Nucleic Acids Res. 1993 Feb 25;21(4):807–810. doi: 10.1093/nar/21.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Nishiyama M., Yu F., Watanabe I., Horinouchi S., Beppu T. Development of a host-vector system in a Rhodococcus strain and its use for expression of the cloned nitrile hydratase gene cluster. J Gen Microbiol. 1992 May;138(5):1003–1010. doi: 10.1099/00221287-138-5-1003. [DOI] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Nucleotide sequence of the regulatory gene xylS on the Pseudomonas putida TOL plasmid and identification of the protein product. Gene. 1986;44(2-3):235–242. doi: 10.1016/0378-1119(86)90187-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Izui H., Nagasawa T., Yamada H. Nitrilase in biosynthesis of the plant hormone indole-3-acetic acid from indole-3-acetonitrile: cloning of the Alcaligenes gene and site-directed mutagenesis of cysteine residues. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):247–251. doi: 10.1073/pnas.90.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Komeda H., Yanaka N., Nagasawa T., Yamada H. Nitrilase from Rhodococcus rhodochrous J1. Sequencing and overexpression of the gene and identification of an essential cysteine residue. J Biol Chem. 1992 Oct 15;267(29):20746–20751. [PubMed] [Google Scholar]
- Kobayashi M., Nagasawa T., Yamada H. Enzymatic synthesis of acrylamide: a success story not yet over. Trends Biotechnol. 1992 Nov;10(11):402–408. doi: 10.1016/0167-7799(92)90283-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Nagasawa T., Yamada H. Nitrilase of Rhodococcus rhodochrous J1. Purification and characterization. Eur J Biochem. 1989 Jun 15;182(2):349–356. doi: 10.1111/j.1432-1033.1989.tb14837.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Suzuki T., Fujita T., Masuda M., Shimizu S. Occurrence of enzymes involved in biosynthesis of indole-3-acetic acid from indole-3-acetonitrile in plant-associated bacteria, Agrobacterium and Rhizobium. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):714–718. doi: 10.1073/pnas.92.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Yanaka N., Nagasawa T., Yamada H. Primary structure of an aliphatic nitrile-degrading enzyme, aliphatic nitrilase, from Rhodococcus rhodochrous K22 and expression of its gene and identification of its active site residue. Biochemistry. 1992 Sep 22;31(37):9000–9007. doi: 10.1021/bi00152a042. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Yanaka N., Nagasawa T., Yamada H. Purification and characterization of a novel nitrilase of Rhodococcus rhodochrous K22 that acts on aliphatic nitriles. J Bacteriol. 1990 Sep;172(9):4807–4815. doi: 10.1128/jb.172.9.4807-4815.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Mauger J., Yamada H. A novel nitrilase, arylacetonitrilase, of Alcaligenes faecalis JM3. Purification and characterization. Eur J Biochem. 1990 Dec 27;194(3):765–772. doi: 10.1111/j.1432-1033.1990.tb19467.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Takeuchi K., Yamada H. Occurrence of a cobalt-induced and cobalt-containing nitrile hydratase in Rhodococcus rhodochrous J1. Biochem Biophys Res Commun. 1988 Sep 15;155(2):1008–1016. doi: 10.1016/s0006-291x(88)80597-7. [DOI] [PubMed] [Google Scholar]
- Nakai R., Horinouchi S., Beppu T. Cloning and nucleotide sequence of a cellulase gene, casA, from an alkalophilic Streptomyces strain. Gene. 1988 May 30;65(2):229–238. doi: 10.1016/0378-1119(88)90459-3. [DOI] [PubMed] [Google Scholar]
- Novo C., Tata R., Clemente A., Brown P. R. Pseudomonas aeruginosa aliphatic amidase is related to the nitrilase/cyanide hydratase enzyme family and Cys166 is predicted to be the active site nucleophile of the catalytic mechanism. FEBS Lett. 1995 Jul 3;367(3):275–279. doi: 10.1016/0014-5793(95)00585-w. [DOI] [PubMed] [Google Scholar]
- Ramos J. L., Rojo F., Zhou L., Timmis K. N. A family of positive regulators related to the Pseudomonas putida TOL plasmid XylS and the Escherichia coli AraC activators. Nucleic Acids Res. 1990 Apr 25;18(8):2149–2152. doi: 10.1093/nar/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker D. M., Malyj L. D., McBride K. E. Purification and properties of a nitrilase specific for the herbicide bromoxynil and corresponding nucleotide sequence analysis of the bxn gene. J Biol Chem. 1988 May 5;263(13):6310–6314. [PubMed] [Google Scholar]
- Strohl W. R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992 Mar 11;20(5):961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. G., Lee N., Fowler A. V. The araC gene of Escherichia coli: transcriptional and translational start-points and complete nucleotide sequence. Gene. 1980 Dec;12(3-4):179–190. doi: 10.1016/0378-1119(80)90100-6. [DOI] [PubMed] [Google Scholar]