Abstract
Background
Daily left prefrontal repetitive transcranial magnetic stimulation (rTMS) over several weeks is an FDA approved treatment for major depression. Although rTMS is generally safe when administered using the FDA guidelines, there are a number of side effects that can make it difficult for patients to complete a course of rTMS. Many patients report that rTMS is painful, although patients appear to accommodate to the initial painfulness 1. The reduction in pain is hypothesized to be due to prefrontal stimulation and is not solely explained by accommodation to the stimulation.
Methods
In a recent 4-site randomized controlled trial (using an active electrical sham stimulation system) investigating the antidepressant effects of daily left dorsolateral prefrontal rTMS (Optimization of TMS, or OPT-TMS), the procedural painfulness of TMS was assessed before and after each treatment session. Computerized visual analog scale ratings were gathered before and after each TMS session in the OPT-TMS trial. Stimulation was delivered with an iron core figure-8 coil (Neuronetics) with the following parameters: 10 Hz, 120% MT (EMG-defined), 4 sec pulse train, 26 second inter-train interval, 3000 pulses per session, one 37.5 minute session per day. After each session, procedural pain (pain at the beginning of the TMS session, pain toward the middle, and pain toward then end of the session) ratings were collected at all 4 sites. From the 199 patients randomized, we had usable data from 142 subjects for the initial 15 TMS sessions (double-blind phase) delivered over three weeks (142 × 2 × 15 = 4260 rating sessions).
Results
The painfulness of real TMS was initially higher than that of the active sham condition. Over the 15 treatment sessions, subjective reports of the painfulness of rTMS (during the beginning, middle and end of the session) decreased significantly 37% from baseline in those receiving active TMS, with no change in painfulness in those receiving sham. This reduction, although greatest in the first few days, continued steadily over the three weeks. Overall, there was a decay rate of 1.56 VAS points per session in subjective painfulness of the procedure in those receiving active TMS.
Discussion
The procedural pain of left, prefrontal rTMS decreases over time, independently of other emotional changes, and only in those receiving active TMS. These data suggest that actual TMS stimulation of prefrontal cortex may be related to the reduction in pain, and that it is not a non-specific accommodation to pain. This painfulness reduction softly corresponds with later clinical outcome. Further work is needed to better understand this phenomenon and whether acute within-session or over-time painfulness changes might be used as short-term biomarkers of antidepressant response.
Introduction
Daily left dorsolateral prefrontal rTMS for several weeks is a new acute treatment for depression. However, some preliminary data and anecdotal reports suggest that this treatment may be painful for some patients. Recently, we found in an open label study of TMS for depression, that the painfulness of TMS stimulation decreases over time.2 The exact cause of the focal pain of prefrontal rTMS is not known, but some have reasoned that the magnetic pulses activate nociceptors in the scalp, periosteum, and maybe meninges under the coil. 3 Because prefrontal rTMS for depression is painful for some, and requires daily visits for several weeks, we initially hypothesized that there would be high dropout rates in clinical trials. In fact, the dropout rate has been very low. For example, in recent multisite trials, the dropout rate at 3 or 4 weeks after randomization was only 7 – 8%4, 5, lower than in antidepressant medication trials.
There is accumulating evidence that stimulation of the left dorsolateral prefrontal cortex with TMS is associated with analgesic effects in healthy adults undergoing experimental pain methods 6, in patients with chronic pain 7, and in patients with postoperative pain 8,9. The role of the left prefrontal cortex in pain perception is unclear, but there is some evidence indicating that its activation is negatively correlated with pain unpleasantness ratings suggesting a possible governing role of the prefrontal cortex over the affective dimension of pain. 10
To date, no one has examined whether the reduction in painfulness of the TMS procedure is a specific effect, linked only to active TMS, or is a non-specific effect of repeatedly being exposed to a noxious stimuli (general accommodation).
Methods
Study Design
We analyzed data from the recently completed NIMH-funded Optimization of TMS for the Treatment of Depression (OPT-TMS) study. This was a 4 year, 4 site study of double-blinded, randomized, sham-controlled daily left prefrontal rTMS as an acute clinical treatment for major depression.5 199 moderately depressed adult subjects were initially enrolled in the study. Visual Analog Rating Scale data were available for 142 participants (68 in the real TMS group and 74 sham).
Phase I of the study employed the double-blind, randomized, sham-controlled use of daily prefrontal rTMS to determine the efficacy and safety of rTMS in the treatment of depression. The first three weeks were double-blind, using a newly developed active electrical sham TMS system (similar to transcutaneous electrical nerve stimulation; TENS) that delivered a small electrical scalp stimulation to those receiving sham TMS, and thereby also controlled for facial twitching, scalp discomfort and noise differences (see 5 and 11, 12 between real and sham stimulation. In the trial, this system was an effective mask, and patients, raters and treaters were not able to distinguish active from sham TMS.
Study Device Description and rTMS Treatment Session Procedures
rTMS was delivered using the Neuronetics Model 2100 Therapy System investigational device. Each site used three magnetic coils, identical in weight, external appearance and acoustic properties when actively pulsed. One coil was unblinded and labeled ‘active’, and was used to determine motor thresholds (MT). The remaining two coils were distinguishable only by external labels as ‘coil B’ or ‘coil C’, with one being the active treatment coil while the other was a sham coil. The sham coils contained a magnetic shield which limited the magnetic energy reaching the cortex to 10% or less of that of the active coil, but nevertheless allowed the active and sham coils to have the identical appearance, placement and similar but not identical acoustic properties. Triplets of coils were periodically rotated from the central core at MUSC across the four clinical sites once each during the trial to reduce the possibility that inadvertent unmasking would result in knowledge of the true nature of the “B” and “C” coils. On 4 occasions (3 Emory, 1 NYSPI), if an administrator encountered equipment problems with the TMS coils, the sham and active coils were replaced at that site and the TMS administrator was changed until that patient completed treatment. This procedure ensured that the TMS administrator was not accidentally unblinded. There were no instances when treaters were clearly unblinded and extremely confident of the patient randomization status.
The novel active sham condition consisted of the sham coil described above, noise dampening earphones for the patient and treater, and electrical pads inserted under the coil on the patient’s head. Coincident with the discharge of the sham coil, a small electrical pulse (5mA) was administered that mimicked the active rTMS sensation and also caused focal scalp twitching. 13, 14 Patients, treaters, local raters, offsite expert raters and all other study personnel were masked to coil functionality. The integrity of the mask was assessed immediately at Phase 1 exit. Patients, treaters and raters made “best guesses” as to the assignment to active or sham rTMS and indicated their confidence in this guess.
Treatment Parameters
Treatment was standardized at 120% magnetic field intensity relative to the patient’s EMG-determined resting motor threshold, with a repetition rate of 10 pulses per second (10 Hz), with a stimulus train duration of 4 seconds and an inter-train interval of 26 seconds. During the first week of the acute phase only, treatment intensity could be reduced to 110% for tolerability, but was then required to return to 120% from week-2 onward. A treatment session lasted for 37.5 minutes (75 trains) for a total of 3000 magnetic pulses.
Subjects who complained of painfulness were offered the insertion of a simple sheet of packing foam. This could be folded to create 4 layers, but no more. Subjects rested in a semi-reclined chair with their head supported by a head-holder frame. The coil was positioned 5 cm anterior and in a parasagittal line from the location that maximized movement in the right hand flexor digiti indici (as measured by EMG). 15–17 Baseline MRI scans were performed on all subjects, and approximately 1/3 of subjects had the coil positioned 6 cm forward (rather than 5 cm) based on MRI analysis of where the 5cm-rule resulted in placement, and whether this was over prefrontal, or premotor, cortex. 18 Patients were instructed to rest quietly during the treatment, but not to fall asleep. Eyes could be open or closed.
Visual Analog Scales
All subjects completed a pre- and post-treatment custom-developed VAS questionnaire on a laptop computer running either MacOS or Windows. (software developed using REALbasic 5.5, REAL software, Austin TX) The questionnaire consisted of 15 pre-treatment questions and 27 post-treatment questions. They were asked to rate their experience of the following descriptors: happy, irritable, angry, excited, confused, calm, sad, anxious, nervous, bored, relaxed, tired, distracted, pain, and discomfort, and the visual analogue scores were converted to a scaling of 0 to 100 points. Included only in the post-treatment questionnaire were retrospective measures of pain, unpleasantness, tolerability, and discomfort for the beginning, middle and end of the treatment. Ratings of TMS-induced pain at the beginning, middle and end were the main factors examined in the present report.
Statistical Analyses
We analyzed these data using proc mixed in SAS (version 9.1). Individual subject intercepts and pain-rating-slopes over time were entered into the model as random effects at level-1 (see Singer 1999). Patient group assignment (real or sham) was our primary level-2 predictor. The estimation method was restricted maximum likelihood, the covariance structure was “unstructured” and the degrees of freedom method was Kenward-Roger.
Results
Procedural Pain over Time
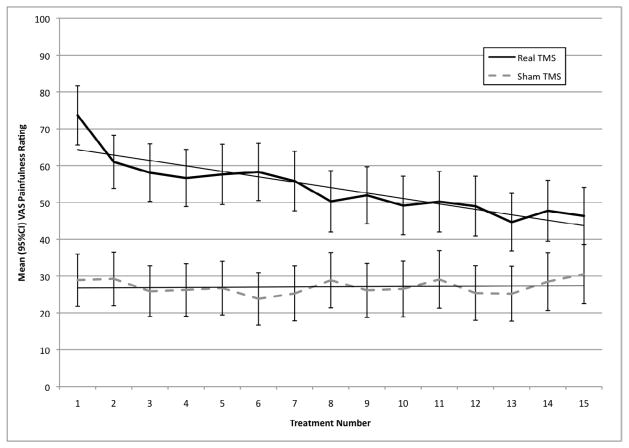
The mean painfulness of TMS at the beginning of the first session was 73.66 (SD=31.31) out of 100 in the real TMS group and 28.91 (SD=31.43) in the sham group. Findings from the two-way interaction model (group X time) found a significant difference between groups (real versus sham) with respect to mean pain ratings at the beginning of the TMS sessions (F(1,136)=60.50, p<.0001). The slope/time main effect was also significant (F(1,127)=23.39, p<.0001) and so was the group by slope/time interaction (F(1,127)=17.16, p<.0001), both after controlling for baseline differences between groups. In the sham TMS group, the slope of the TMS-painfulness ratings over time was not significantly different from zero (t(124)=0.51, ns), but in the real TMS group, the painfulness ratings decreased by 1.56 (SE=.35) VAS-points per treatment (t(127)=4.14, p=<.0001; see figure 1). The amount of pain at the beginning of the last TMS session during phase-I of the trial was 46.35 (SD=29.47) in the real group and 30.53 (SD=33.27). This represents a 37% reduction in the real TMS group and a 6% increase in the sham TMS group.
Figure 1.
Graph of painfulness changes over time (beginning, middle and end of treatment) separated by active and sham groups. Note that only in the active group is there a reduction over time. Note also that the two groups differed in their overall painfulness ratings.
Changes in Pain within a session, over time
Retrospective pain ratings decreased from the beginning to the middle, to the end of the TMS sessions in both the real group (mean pain at beginning=46.43 SD=31.92; middle=43.04 SD=30.53; and end=41.58 SD=31.13) and in the sham group (mean pain at beginning=33.58 SD=33.26; middle=30.33 SD=30.73; and end=30.22 SD=31.70), however the mean within-TMS-session change in pain ratings was significantly greater in the real TMS group than sham (t(133)=3.41, p=.0008) collapsing across all treatments. Over time, the amount of within-session change in procedural pain decreased by 1 VAS-point per session in the real group (t(128)=3.25, p=.002), and did not significantly change in the sham group (t(125)=0.44, ns).
Procedural Pain Reduction as a predictor of antidepressant clinical outcome
Among patients receiving real TMS during the 3-week double-blind phase, the slope of the TMS procedural pain (beginning of each TMS session) over time (per TMS session) significantly predicted percent change in HAM-D scores from the beginning to the end of phase-I of the trial (t(532)=2.70, p=.007). However the amount variability accounted for by the predictive model was only 4.38%. The slope of the TMS procedural pain ratings during the first 2-weeks of treatment significantly predicted change in HAM-D scores from the beginning to the end of phase-I of the trial (t(397)=2.38, p=.02) as well, however the pain slope during week-1 only of the trial did not predict clinical antidepressant change as measured by HAM-D (t(187)=0.97, ns). The amount of pain reduction within each TMS session was not a significant predictor of antidepressant outcome (t(816)=0.66, ns) nor was within-session change over time (t(805)=1.17, ns).
Discussion
In this well-studied multisite sample of medication free unipolar depressed patients, the painfulness of left prefrontal TMS diminished drastically (37%) over 3 weeks of treatment, but only in those receiving active TMS and not in those receiving a sham treatment. Moreover, the change in TMS procedural painfulness over time modestly predicted ultimate clinical response (percent reduction in HAM-D scores) at the end of the double-blind phase of the study. Unfortunately, given the substantial baseline differences in procedural pain ratings between the real and sham groups, regression to the mean cannot be ruled-out as a potential explanation for some of the observed effect (a major limitation of the present study) even though the baseline difference was controlled in the statistical analysis. The reason for this baseline difference may be that a standard mA intensity was applied for the sham system rather than individually titrating the sham intensity to match the subjective experience of real rTMS discomfort. Future studies should consider both approaches fort further study. Nonetheless, the fact that the slope of the procedural painfulness over time is a significant predictor of ultimate clinical response does add some credence to the notion that the change in painfulness is a brain modulated effect. Interestingly, the reduction in painfulness within-session was also more pronounced over time in the real TMS group compared to sham. This adds further credibility to the notion that analgesic effects of prefrontal rTMS are likely real and perhaps relevant to brain activity changes associated with anti-depressant effects.
The present findings replicate the changes seen in the open label phase of this trial and reported earlier demonstrating that the patients rate the painfulness of TMS as much less painful after 1–2 weeks of treatment. 2 These findings have some clinical implications. They suggest that for patients who have difficulty tolerating rTMS in the beginning, the discomfort is likely to decrease over time, and most drastically within the first week of treatment. While the reasons for this are still unclear, efforts to reassure patients early in treatment, and efforts to encourage their continuation despite some potential discomfort in the beginning may result in less treatment drop-out. greater sense of control, with a reduction in the valence of the paired stimulus.
Conclusion
We have found that the painfulness of prefrontal rTMS diminishes steadily over the course of 3 weeks in depressed patients undergoing a double-blind treatment trial and that this only occurs in patients getting active treatment. This accommodation likely contributes to the high rates of retention in rTMS depression treatment studies. Further studies are needed to understand the mechanisms behind this accommodation. Regardless of the mechanism, clinicians and researchers using rTMS should be aware of this accommodation in designing treatment studies and in working with individual patients within a trial who might find TMS initially painful.
Acknowledgments
Supported by the NIMH as the Optimization of TMS for Depression Trial, (OPT-TMS) involving grants 5R01MH069929 PI: AVERY, DAVID, 5R01MH069887 PI: GEORGE, MARK, 5R01MH069896 PI: GEORGE, MARK, 5R01MH069895 PI: LISANBY, SARAH, 5R01MH069886 PI: MCDONALD, WILLIAM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Conflicts of Interest: Full disclosures and listings for all authors are at the end of the manuscript. For the specific interests of this clinical trial, Dr. George, the study chair, has received no compensation from any TMS manufacturer for the past 5 years, and owns no equity stake in any device or pharmaceutical company. Following a competitive bid and request involving all known TMS manufacturers at the time, Neuronetics was selected and loaned the TMS device, head-holder and coils used in the trial and allowed the use of the safety IDE of their device, but has otherwise been uninvolved in trial conduct or analysis. The TMS sham equipment was purchased from the MECTA Corporation and the James Long Company.
Trial Registration: OPT-TMS depression trial, clinicaltrials.gov # NCT00149838 http://clinicaltrials.gov/ct2/show/NCT00149838?term=magnetic+brain+stimulation+depression&rank=1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson B, Kavanagh K, Borckardt J, et al. Decreasing procedural pain over time of left prefrontal rTMS for depression: Initial results from the open-label phase of a multisite trial (OPT-TMS) Brain Stimulation. 2009;2:88–92. doi: 10.1016/j.brs.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson BS, Kavanagh K, Borckardt JJ, et al. Decreasing procedural pain over time of left prefrontal rTMS for depression: initial results from the open-label phase of a multi-site trial (OPT-TMS) Brain Stimul. 2009;2(2):88–92. doi: 10.1016/j.brs.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borckardt JJ, Smith AR, Hutcheson K, et al. Reducing pain and unpleasantness during repetitive transcranial magnetic stimulation. J ECT. 2006;22(4):259–264. doi: 10.1097/01.yct.0000244248.40662.9a. [DOI] [PubMed] [Google Scholar]
- 4.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biological Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 5.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 6.Borckardt JJ, Smith AR, Reeves ST, et al. Fifteen minutes of left prefrontal repetitive transcranial magnetic stimulation acutely increases thermal pain thresholds in healthy adults. Pain Res Manag. 2007;12(4):287–290. doi: 10.1155/2007/741897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borckardt JJ, Smith AR, Reeves ST, et al. A pilot study investigating the effects of fast left prefrontal rTMS on chronic neuropathic pain. Pain Med. 2009;10(5):840–849. doi: 10.1111/j.1526-4637.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borckardt JJ, Weinstein M, Reeves ST, et al. Post-Operative Left Prefrontal Repetitive Transcranial Magnetic Stimulation (rTMS). Reduces Patient-Controlled Analgesia Use. Anesthesiology. 2006;105:557–562. doi: 10.1097/00000542-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Borckardt JJ, Reeves ST, Weinstein M, et al. Significant analgesic effects of one session of postoperative left prefrontal cortex repetitive transcranial magnetic stimulation: A replication study. Brain Stimulat. 2008;1(2):122–127. doi: 10.1016/j.brs.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(Pt 5):1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 11.Arana AB, Borckardt JJ, Ricci R, et al. Focal electrical stimulation as a sham control for repetitive transcranial magnetic stimulation: Does it truly mimic the cutaneous sensation and pain of active prefrontal repetitive transcranial magnetic stimulation? Brain Stimul. 2008;1(1):44–51. doi: 10.1016/j.brs.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borckardt JJ, Walker J, Branham RK, et al. Development and evaluation of a portable sham transcranial magnetic stimulation system. Brain Stimul. 2008;1(1):52–59. doi: 10.1016/j.brs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borckardt J, Walker J, Branham RK, et al. Development and Evaluation of a Portable Sham TMS System. Brain Stimulation: Basic, Translational and Clinical Studies in Neuromodulation. 2008;1(1):52–59. doi: 10.1016/j.brs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arana AB, Borckardt JJ, Ricci R, et al. Focal Electrical Stimulation as a Sham Control for rTMS: Does it truly mimic the cutaneous sensation and pain of active prefrontal rTMS? Brain Stimulation: Basic, Translational and Clinical Studies in Neuromodulation. 2008;1(1):44–51. doi: 10.1016/j.brs.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George MS, Wassermann EM, Kimbrell TA, et al. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry. 1997;154(12):1752–1756. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- 16.George MS, Wassermann EM, Williams WA, et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci. 1996;8(2):172–180. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- 17.George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 18.Herwig U, Padberg F, Unger J, et al. Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biological Psychiatry. 2001;50(1):58–61. doi: 10.1016/s0006-3223(01)01153-2. [DOI] [PubMed] [Google Scholar]