Abstract
OBJECTIVE
Gastric bypass surgery is an effective therapy for extreme obesity. However, substantial variability in weight loss outcomes exists that remains largely unexplained. Our objective was to determine whether any commonly collected pre-operative clinical variables were associated with weight loss following Roux-en-Y gastric bypass surgery.
DESIGN
The analysis was based on a prospectively recruited observational cohort of 2365 patients who underwent Roux-en-Y gastric bypass surgery from 2004-2009. Weight loss was stratified into three major phases, early (0-6 months), nadir, and long-term (>36 months). Multivariate regression models were constructed using a database of over 350 variables.
RESULTS
A total of 12-14 pre-operative variables were independently associated (p<0.05) with each of the temporal weight loss phases. Pre-operative variables associated with poorer nadir and long-term weight loss included: higher baseline BMI, higher pre-operative weight loss, iron deficiency, use of any diabetes medication, non-use of bupropion medication, no history of smoking, aged >50 years, and the presence of fibrosis on liver biopsy.
CONCLUSIONS
Several variables previously associated with poorer weight loss after RYGB surgery including age, baseline BMI, and type 2 diabetes were replicated. Several others suggest possible clinical interventions for post-operative management of RYGB patients to improve weight loss outcomes.
Keywords: Obesity, gastric bypass, weight loss
INTRODUCTION
Roux-en-Y gastric bypass (RYGB) surgery can induce a substantial weight loss that is associated with improvement in type 2 diabetes, decreased incidence of cancer, improved quality of life, and decreased mortality.1-6 Although the safety and potential efficacy of this procedure is well established,7 some patients may regain variable amounts of weight after a relatively short period of rapid weight loss and remain extremely obese, while others fail to lose significant weight despite the major anatomic and physiological effects from the RYGB surgical intervention.8-10 This has created an increasing emphasis on post-operative medical management, for which little empirical data exists. In addition, recent data from the 2009–2010 National Health and Nutrition Examination Survey (NHANES),11 using measured heights and weights, indicates that 15.5% of all US adults greater than 20 years of age have a BMI ≥ 35 kg/m2 and that 6.3% have a BMI ≥ 40 kg/m2 and therefore meet existing eligibility criteria for RYGB surgery.12 Thus, there are a substantial number of patients with extreme obesity for whom RYGB may be considered and a corresponding growing clinical need to provide evidence-based guidance on the selection of treatment modalities.
Previous studies have attempted to identify factors associated with weight loss outcomes.13, 14 A variety of socioeconomic, psychological, and biological variables have been analyzed.15 The clinical variable with the strongest effect is baseline BMI; the higher the BMI, the more likely the patient will lose less of a percentage of excess body weight relative to patients with lower initial BMIs. This effect is in part an artifact of measuring weight loss in relative rather than absolute terms.16, 17 However, patients with very extreme levels of obesity, i.e., “super-obesity”, may represent a different biological state than those with less severe obesity.18 Other factors, such as diabetes, psychological conditions, and limited physical activity, may also be associated with poorer weight loss outcomes after RYGB surgery. Most studies of pre-operative clinical predictors have analyzed only one or a few potential variables often in small populations, ranging up to analysis of 20 variables in 300 patients.13, 15 These studies have also had a relatively short length of follow-up, often only up to one year, and examined weight loss at a single time point.
We analyzed the association of more than 350 variables with the weight loss dynamics of more than 2000 patients over a follow-up period of more than 36 months stratified into three major phases, i.e., early weight loss, weight nadir, and long-term weight loss, in order to construct multivariate regression models.
MATERIALS AND METHODS
Study Population
All patients who entered the bariatric surgery program in the Center for Nutrition and Weight Management at Geisinger Clinic were consecutively offered participation in an IRB approved research program focused upon obesity. All study participants provided written informed consent. Patients who underwent RYGB gastric bypass surgery from January 1, 2004 through August 22, 2011 were included in the analysis. The bariatric surgery program consisted of a pre-operative program that typically lasted 6 to 12 months and included a diet-induced weight loss target of 10% of body weight (Supplementary Methods). Patients were scheduled for follow-up visits at the Geisinger Weight Management Clinic at approximately 1 week, 2 weeks, 2 months, 5 months, 8 months, and 12 months after RYGB surgery, and then every 6-12 months thereafter.
Study Variables
Data used for this study was obtained from several clinical sources and entered into an IRB approved database on RYGB patients enrolled in the obesity research program of the Geisinger Obesity Institute at Geisinger Clinic. The detailed methods for acquiring and storing these data are described elsewhere.19 Briefly, clinical data were extracted from an electronic health record (EHR) fed comprehensive enterprise-level data warehouse, the Clinical Decision Intelligence System (CDIS), which contained a variety of data from the EHR (EpicCare® EHR; Verona, WI). Additional self administrated survey data were obtained during the pre-operative period. The database also included results of intra-operative liver biopsy pathology analysis and pre-operative clinical ratings by dieticians to determine whether patients were prepared to make necessary dietary changes, and by psychologists to determine if patients were psychologically prepared for the RYGB surgery and lifestyle changes. Details regarding the data used for the study are provided in the Supplementary.
Post-operative weight measures were carefully reviewed to identify and remove implausible or inconsistent values as described previously.19 To evaluate weight loss after RYGB, weight change was quantified as percent of initial excess body weight lost (%EBWL). To calculate excess body weight (EBW), the weight at the visit occurring closest but before surgery (weightb) was compared to an ideal body weight of BMI = 25 kg/m2. The %EBWL at time t after surgery was calculated as:
where weightt was the weight measured at time t.
Statistical Analysis
Descriptive statistics of the study population were computed using means, standard deviation, and percentages, as appropriate. Quantile regression20 was used to estimate the overall median %EBWL after RYGB surgery. Weight loss measures were calculated for each patient within each of three post-surgery weight loss phases. A repeated measures regression model (using random effects to calculate slope and intercept for each patient) was used to estimate the %EBWL achieved at 6 months following surgery. The maximum weight loss achieved between 6 and 36 months after surgery was identified by selecting the lowest BMI from at least three available measurements. When this value was not the most recent measurement within this span, it was defined as the maximal %EBWL nadir. Patients whose most recent measurement in the 6 to 36 month period was the lowest were excluded from the analysis of weight nadir (a total of 25%, most of which were less than 36 months post-RYGB and had not yet reached weight nadir). The weight measure occurring after but closest to 36 months was used to evaluate long-term weight loss. For each weight loss phase, analyses were limited to the subset of the population with a qualifying weight loss outcome metric (see Supplementary Methods for more details).
Baseline regression models included a categorical variable for initial BMI (grouped as 35-39.9, 40-49.9, 50-59.9, and 60+ kg/m2). This variable was selected because baseline BMI is well known to be associated with degree of weight loss following RYGB surgery15 and may be correlated with some of the clinical variables. Each of the over 350 clinical variables (Supplementary Data) was included in a separate regression model to identify the subset that was significantly related to each temporal weight loss phase after accounting for baseline BMI using a p-value < 0.05 (Supplementary Data).
RESULTS
Demographics
The demographics of the initial study cohort, consisting of 2444 patients who had undergone RYGB surgery and had an initial BMI>35 kg/m2, are shown in Supplementary Table 1. The mean age was 46 years (range 18-74), 81% were female, 97% were Caucasian, and the mean baseline BMI was 49.6 kg/m2 (range 35-94.3). Patients with 4 or more weight measures after surgery were included in the analyses, with an average of 21 weight measures/patient and a range of 4-203.
Weight loss analysis
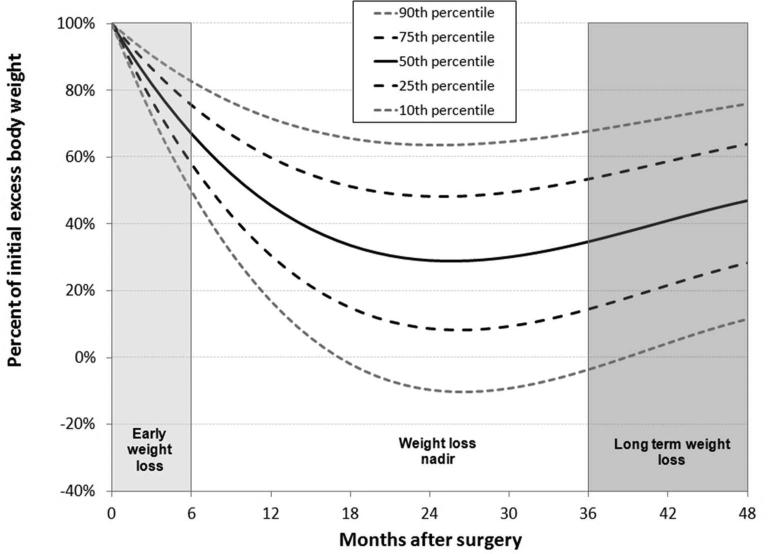
Many prior studies have analyzed only one or few distinct post-operative time points, e.g., 12 and/or 24 months. We18, 21 and others22 have used more advanced statistical methods to evaluate weight loss after surgery, including analysis of post-operative weight loss trajectories using linear mixed models21, 22 to model weight loss dynamics over time. We identified three distinct phases of post-operative weight loss; an initial steep weight loss followed by an extended period of more gradual weight loss in which a nadir or low point occurs transitioning to a period characterized by some degree of weight regain occurring at about 24 to 36 months and later (Figure 1). We divided the available weight loss data into these three weight loss phases and conducted an analysis of %EBWL as follows:
Early: The first 6 months after surgery where rapid weight loss is occurring
Nadir: The maximal %EBWL achieved that occurs between 6 and 36 months after surgery
Long-term: weight loss at 36 or more months after surgery
Figure 1.
Linear mixed model of the percent of initial excess body weight after RYGB surgery. Based upon the shape of the weight loss curve, the post-surgical weight loss was divided into three phases: early weight loss (0-6 months), weight loss nadir (maximum weight loss achieved), and long term weight loss (weight measured at 36+ months). The model was generated using 51,822 weight measurements from 2444 patients occurring between surgery and 60 months after RYGB surgery.
Of the 2444 patients, 2365 (97%) had 4 or more weight measures occurring in the first 6 months after surgery, 1369 (56%) had sufficient weight measurements to identify a weight loss nadir (at least 3 weight measures between 6 and 36 months after surgery, the last of which was not the lowest), and 857 of 1361 patients who were more than 36 months from surgery (60%) had at least one weight measure occurring 36 or more months after surgery. To determine whether the differences in data density affected the population substructures, we evaluated the demographic characteristics for each of these three weight loss groups (Supplementary Table S1), which were found to be similar to the overall population (P>0.05). The mean %EBWL at 6-months was 65% (Supplementary Table 2), the mean nadir was 77% %EBWL, and the mean %EBWL for the 36+ months follow-up was 61%. We found that the unadjusted correlation (r2) between %EBWL at 6-months and nadir was 0.761, between nadir and long-term weight loss was 0.852, and between early weight loss and 36+ months was 0.545 (Supplementary Figure S1).
Following initial univariate analysis of over 350 clinical variables (Supplementary methods), baseline BMI was the variable most strongly related to weight loss for each temporal phase (p<0.00001). We therefore attempted to control for baseline BMI through stratification into ranges of BMI, i.e., 35-39.9 kg/m2, 40-49.9 kg/m2, 50-59.9 kg/m2, and 60+ kg/m2, that roughly divided the population into quartiles and reflected thresholds for categorizing different degrees of obesity. We then repeated the analysis with baseline BMI accounted for in the models to identify the subset that would be brought forward for multivariate analyses. This resulted in the selection of 98, 69, and 44 variables (Supplementary Tables S3-S7) for the 6 month weight loss, weight loss nadir, and 36+ month weight loss phases, respectively. There were 17 variables that were common to all 3 weight loss phases (Supplementary Table S8). We then conducted analyses for each distinct weight loss phase.
6 month weight loss
Multiple linear regression analysis using the 98 variables brought forward from initial univariate analysis revealed 14 pre-operative variables (p-value<0.05) that were independently associated with lower early weight loss (Table 1). The magnitude of the effect (as measured using the parameter estimates from the multivariate linear regression) was largest for baseline BMI in which those with BMI<40 kg/m2 had 43.3% more %EBWL, pre-operative weight gain in which those who gained weight during the pre-operative period had 8.9% less %EBWL, and liver fibrosis in which those with a baseline BMI of less than 50 kg/m2 had 8.8% less %EBWL.
Table 1.
Clinical variables associated with 6 month weight loss (N=2365).
| Estimate | SE | p-value | |
|---|---|---|---|
| Intercept | 58.3 | 3.3 | |
| Baseline BMI | |||
| 35-39.9 | 43.3 | 1.6 | <.0001 |
| 40-49.9 | 23.1 | 1.2 | <.0001 |
| 50-59.9 | 8.9 | 1.1 | <.0001 |
| 60+ | Reference | ||
| Pre-operative weight loss | |||
| >0% gain | −8.9 | 1.0 | <.0001 |
| 0-5% loss | −6.3 | 1.0 | <.0001 |
| 5-10% loss | −6.5 | 1.0 | <.0001 |
| 10-19% loss | −5.0 | 0.9 | <.0001 |
| 20%+ loss | Reference | ||
| Surgical access | |||
| Open surgery | −5.3 | 0.7 | <.0001 |
| Laparoscopic surgery | Reference | ||
| Age | |||
| 18-39 | Reference | ||
| 40-49 | −2.0 | 0.8 | 0.0117 |
| 50-59 | −4.4 | 0.8 | <.0001 |
| 60+ | −7.4 | 1.1 | <.0001 |
| Waist circumference | |||
| <45 | 6.0 | 1.7 | 0.0004 |
| 45-49 | 2.8 | 1.2 | 0.0238 |
| 50-54 | 0.7 | 1.2 | 0.524 |
| 55-59 | −0.3 | 1.2 | 0.797 |
| 60+ | Reference | ||
| Time from baseline visit to surgery | |||
| >2 years | −3.8 | 1.4 | 0.0091 |
| Cholesterol HDL ratio | |||
| <4 | −1.8 | 0.8 | 0.0255 |
| 4-4.9 | 0.1 | 0.9 | 0.873 |
| 5+ | Reference | ||
| Smoking history | |||
| Yes | 2.3 | 0.7 | 0.0015 |
| Co-morbidity burden | |||
| Each additional ICD9 code | −0.3 | 0.1 | 0.0472 |
| Diabetes group | |||
| Any Insulin Sens Agent medication | −1.9 | 1.0 | 0.0462 |
| Public distress | |||
| Low distress score | −1.5 | 0.7 | 0.0352 |
| Anisocytosis (red blood cells of unequal size) | |||
| Red cell distribution width > 15% | −3.0 | 0.9 | 0.0011 |
| Certainty of commitment to weight loss program | |||
| Extremely certain (WLRQ2=5) | 2.5 | 1.0 | 0.0145 |
| Liver pathology and baseline BMI | |||
| No fibrosis | Reference | ||
| Any Fibrosis with baseline BMI<50 kg/m2 | −8.8 | 1.9 | <.0001 |
| Any Fibrosis with baseline BMI 50+ kg/m2 | 2.2 | 2.0 | 0.271 |
Weight nadir
Of the 69 univariate variables, a total of 12 clinical variables were independently associated (p<0.05) with higher weight nadir (Table 2). The magnitude of the effect was largest for the same variables as for 6 month weight loss but to different degrees; for baseline BMI those with BMI<40 kg/m2 had 40.8% more %EBWL, for those who gained weight during the pre-operative period had 10.7% less %EBWL, and those with liver fibrosis and a baseline BMI of less than 50 kg/m2 had 10.6% less %EBWL.
Table 2.
Clinical variables associated with weight loss nadir (N=1369).
| Estimate | SE | p-value | |
|---|---|---|---|
| Intercept | 75.1 | 3.3 | |
| Baseline BMI | |||
| 35-39.9 | 40.8 | 3.2 | <.0001 |
| 40-49.9 | 22.4 | 2.3 | <.0001 |
| 50-59.9 | 8.7 | 2.2 | <.0001 |
| 60+ | Reference | ||
| Pre-operative weight loss | |||
| >0% gain | −10.3 | 1.9 | <.0001 |
| 0-5% loss | −6.5 | 1.9 | 0.0007 |
| 5-10% loss | −7.0 | 1.9 | 0.0002 |
| 10-19% loss | −6.1 | 1.6 | 0.0002 |
| 20%+ loss | Reference | ||
| Diabetes group | |||
| No diabetes | Reference | ||
| Diabetes with HbA1c<9 | −4.4 | 1.3 | 0.0009 |
| Diabetes with HbA1c>=9 | −8.4 | 2.6 | 0.0012 |
| Age | |||
| 18-39 | Reference | ||
| 40-49 | −3.7 | 1.5 | 0.0142 |
| 50-59 | −5.0 | 1.6 | 0.0018 |
| 60+ | −8.6 | 2.1 | <.0001 |
| Surgical access | |||
| Open surgery | −3.2 | 1.2 | 0.0082 |
| Laparoscopic surgery | Reference | ||
| Waist circumference | |||
| <45 | 6.5 | 3.0 | 0.0309 |
| 45-49 | 0.4 | 2.3 | 0.860 |
| 50-54 | −1.7 | 2.1 | 0.429 |
| 55-59 | −1.7 | 2.1 | 0.416 |
| 60+ | Reference | ||
| Use of bupropion | |||
| Active use of medication | 6.2 | 2.0 | 0.0015 |
| Iron deficiency | |||
| Low Transferrin saturation (<15% men, <12% women) | −4.6 | 2.1 | 0.0296 |
| Cholesterol HDL ratio | |||
| <4 | −3.9 | 1.5 | 0.0107 |
| 4-4.9 | −4.3 | 1.6 | 0.0092 |
| 5+ | Reference | ||
| Motivated to lose weight (WLRQ1) | |||
| Extremely motivated compared to previous attempts | 5.1 | 2.0 | 0.0109 |
| Hypertension | |||
| Active diagnosis | −2.8 | 1.2 | 0.0200 |
| Liver pathology and baseline BMI | |||
| No fibrosis | Reference | ||
| Any Fibrosis with baseline BMI<50 kg/m2 | −10.6 | 3.3 | 0.0015 |
| Any Fibrosis with baseline BMI 50+ kg/m2 | −1.6 | 3.6 | 0.651 |
36+ month weight loss
A total of 12 of 44 univariate clinical variables were independently associated with (p<0.05) less long-term %EBWL at least 36 months after RYGB surgery (Table 3). The magnitude of the effect was largest for baseline BMI (40.9% more %EBWL for those with BMI<40 kg/m2), any fibrosis on liver biopsy (in those with a baseline BMI of less than 50 kg/m2, 13.4% less %EBWL), aged 50 years or older with open surgical access (9.5% less %EBWL), and non-users of bupropion medication (6.4% less %EBWL).
Table 3.
Clinical variables associated with 36+ month weight loss (N=857).
| Estimate | SE | p-value | |
|---|---|---|---|
| Intercept | 56.7 | 3.6 | |
| Baseline BMI | |||
| 35-39.9 | 40.9 | 4.3 | <.0001 |
| 40-49.9 | 16.4 | 2.9 | <.0001 |
| 50-59.9 | 3.5 | 2.9 | 0.230 |
| 60+ | Reference | ||
| Diabetes group | |||
| Any diabetes medication | −5.3 | 1.7 | 0.0024 |
| Use of bupropion | |||
| Active use of medication | 6.4 | 2.8 | 0.0244 |
| Smoking | |||
| History of smoking | 4.8 | 2.2 | 0.0300 |
| Age and surgical access | |||
| Age 50+ with Laparoscopic surgery | Reference | ||
| Age 50+ with Open surgery | −9.5 | 2.7 | 0.0004 |
| Age<50 with Laparoscopic surgery | −3.9 | 2.6 | 0.135 |
| Age<50 with Open surgery | −4.9 | 2.6 | 0.062 |
| Liver pathology and baseline BMI | |||
| No fibrosis | Reference | ||
| Any Fibrosis with baseline BMI<50 kg/m2 | −13.4 | 4.3 | 0.0020 |
| Any Fibrosis with baseline BMI 50+ kg/m2 | −0.1 | 4.6 | 0.974 |
We then used the multivariate regression results to develop equations to estimate %EBWL for each temporal weight loss phase (Supplementary data). The equation to determine the predicted amount of long-term %EBWL is:
Long-term %EBWL = 56.7 + 40.9*BMI35 + 16.4*BMI40 + 3.5*BMI50 – 5.3*DiabetesMed + 6.4*bupropion + 4.8*smoker – 9.5*age50*open – 3.9*age49*lap – 4.9*age49*open – 13.4*fibrosis*BMI35 – 13.4*fibrosis*BMI40 – 0.1*fibrosis*BMI50 – 0.1*fibrosis*BMI60.
where, BMI35 = 1 if baseline BMI 35-39.9, else = 0
BMI40 = 1 if baseline BMI 40-49.9, else = 0
BMI50 = 1 if baseline BMI 50-59.9, else = 0
BMI60 = 1 if baseline BMI 60+, else = 0
DiabetesMed = 1 if used diabetes med during pre-operative period, else = 0
bupropion = 1 if used bupropion during pre-operative period, else = 0
smoker = 1 if current smoker or had history of smoking, else = 0
age50 = 1 if age 50+, else = 0
age49 = 1 if age<50, else = 0
open = 1 if had open surgical approach, else = 0
lap = 1 if had laparoscopic surgical approach, else = 0
fibrosis = 1 if had any fibrosis on liver pathology, else = 0
For example, a 47 year old (age50 = 0; age49 = 1) non-smoker (smoker = 0) with a BMI of 56 (BMI50 = 1) who used diabetes medications (DiabetesMed=1) pre-operatively but not bupropion (bupropion = 0) who underwent laparoscopic RYGB (lap = 1; open = 0) and did not have fibrosis on liver biopsy (fibrosis = 0) would have the following predicted %EBWL:
Long-term %EBWL = 56.7 + 40.9*0 + 16.4*0 + 3.5*1 – 5.3*1 + 6.4*0 + 4.8*0 – 9.5*0*0 – 3.9*1*1 – 4.9*1*0 – 13.4*0*0 – 13.4*0*0 – 0.1*0*1 – 0.1*0*0.
Long-term %EBWL = 56.7 + 0 + 0 + 3.5 – 5.3+ 0 + 0 –0 – 3.9 – 0 – 0 – 0 – 0– 0.
Long-term %EBWL = 56.7 +3.5 – 5.3 – 3.9
Long-term %EBWL = 51.0
We also determined whether the model suggested an appropriate fit using a residual plot (Supplementary Figure S2). The plot indicates that the model is homoscedastic, i.e., that the variation in residuals was independent of the predicted value, and that the model is unbiased, i.e., the values of the residuals were independent of the predicted values.
DISCUSSION
The degree of weight loss that is achieved by patients who undergo RYGB surgery, even in the context of a highly standardized clinical program with excellent long-term follow up, may vary, suggesting that patient-specific factors may play a role in influencing weight loss outcomes. Previous studies have examined a wide variety of socioeconomic,20 psychological,12 procedural,16 and genetic factors18, 21 in an effort to identify variables which may influence the degree of post-operative weight loss. A recent systematic analysis focused on pre-operative BMI, pre-operative weight loss, eating disorders, and psychological factors/substance abuse.15 However, most of the available data has been based on small sample sizes with relatively short–term follow-up and analysis of single post-operative time points (e.g., 12 months). We studied a large cohort followed for over 3 years with a large database of clinical variables.
We found that baseline BMI was inversely associated with %EBWL at the early phase of weight loss as well as with weight nadir; the lower the BMI the more the %EBWL. This is consistent with data from over three dozen studies analyzed in a large systematic review.15 At ≥36 months of follow-up, however, we found that the relationship of %EBWL with BMI was not evident at BMI ≥50 kg/m2 (i.e., super-obesity), consistent with the large systematic review in which studies that analyzed only patients undergoing RYGB found no significant association between pre-operative BMI ≥50 kg/m2 and post-operative weight loss. We have previously observed that obesity-related common genetic variants were associated with poorer weight loss outcomes in patients with BMI <50 kg/m2, but not in patients with higher baseline BMIs.18 We therefore tested whether baseline BMI caused effect modification between %EBWL and the other statistically significant clinical factors that we found to be associated %EBWL at each of the three temporal weight loss phases. Hepatic fibrosis was the only variable that we found was significantly modified by initial BMI.
We used percentage of excess body weight (%EBWL) as the measure of weight loss, recommended as the standard metric.17 However, %EBWL is a relative measure that diminishes the significance of the absolute amount, i.e., pounds, of weight lost. BMI is directly correlated with health risks,23 thus the lower the BMI the less the risk. The disparity between %EBWL and other weight loss measures such as absolute weight loss is also likely magnified by the length of post-operative follow-up. The relatively short lengths of follow-up (i.e., 12 months) of many studies may not allow sufficient time for patients with higher BMIs to shed sufficient number of pounds to reach their weight nadir.
The majority of prior published studies have used only one or few distinct post-operative time points, e.g., 12 and/or 24 months. We18, 21 and others22 have used more advanced statistical methods to evaluate weight loss after surgery. Here we chose a hybrid approach and used longitudinal weight loss data to identify three distinct temporal phases of weight loss following RYGB surgery, including early weight loss (i.e. the first 6 months after surgery), weight loss nadir, and long-term weight loss (i.e. >36 months after surgery). This also allowed us to evaluate the relationship of short-term weight loss to long-term. We found that the unadjusted correlation (r2) between %EBWL at 6-months and 36+ months was 0.545. This also extends to pre-operative weight loss where we found a similar effect, with pre-operative weight loss significantly associated with 6 month and weight nadir outcomes (Table 2 and Table 3), but not with weight loss at >36 months. This is consistent with the results of systematic analyses15 which found that patients who lost more weight pre-operatively also lost more excess weight at 12 months following surgery, but not at longer follow-up periods. These data support the notion that long-term weight loss outcomes may be largely independent of short-term weight loss success.
We confirmed the association of type 2 diabetes with lower %EBWL that has been previously reported.13, 14, 24 We also found that the potentially related finding of liver fibrosis was related to lower %EBWL but only in patients with BMI <50 kg/m2. Liver fibrosis is part of the spectrum of non-alcoholic steatohepatitis, which has been related to insulin resistance.25 Our data are consistent with the finding that liver fibrosis measured non-invasively via the Fibrospect score II, which is comprised of plasma levels of alpha 2 macroglobulin, hyaluronic acid and tissue inhibitor of metalloprotease 2, was the only predictor of weight loss in study of a Hispanic RYGB population.26 Whether hepatic fibrosis is independent of the influence diabetes is not yet known.
We also found that waist circumference, cholesterol HDL ratio, and red cell distribution width (RDW) and iron deficiency were associated with both early weight loss and weight nadir. Waist circumference was highly correlated with BMI, but its lack of association at ≥36 months suggests a potential physiological effect from the presumed higher burden of metabolically active visceral fat. Dyslipidemia has been associated with lower weight loss following bariatric surgery.14 The association of iron deficiency (and its red blood cell correlate RDW) with less %EBWL suggests that this treatable condition should be carefully evaluated in patients undergoing RYGB, and perhaps more generally in patients undergoing weight loss interventions, especially pre-menopausal female patients in situations of decreased food and nutrient intake. Hypertension and less motivation to lose weight were specific to weight nadir. The individuals who have the highest level of motivation to lose weight appear to reach the lowest weight nadir. The role of hypertension is not clear.
Two other clinical observations related to long-term weight loss were a history of smoking and the pre-operative use of buproprion. Smoking history has been associated with an increased risk of serious complications (life threatening and/or associated with lasting disability) within 30 days of bariatric surgery.27 In patients presenting for bariatric surgery, previous attempts to quit smoking were associated with substantial weight gain.28 Buproprion was originally developed to treat depression29 but was found to be effective for smoking cessation30 and has recently been used for the treatment of obesity.31, 32 The association of these two clinical factors with increased weight loss following RYGB suggests that common pathways may be involved in their mechanism. Bupropion is a dopamine and norepinephrine reuptake antagonist and a putative stimulator of melanocortin pathways.33 Nicotine has been found to decrease food intake and body weight in mice via the hypothalamic melanocortin system.34 Melanocortin-4 receptor variants have been associated with weight loss outcomes after RYGB,35 further implicating this pathway underlying the molecular mechanism of variability in weight loss response.
Strengths of this study include the standardization of the RYGB surgical technique and the pre- and post-operative management program, large numbers of patients, and high rate and length of post-operative follow-up. However, several limitations are evident. The design was not randomized and the sex distribution was skewed toward women, characteristic of a bariatric surgery cohort. We only analyzed RYGB and not other weight loss interventions, and %EBWL was the only a post-operative outcome. RYGB has multiple and pleiotropic effects on a number of conditions, particularly type 2 diabetes, dyslipidemia, hypertension, and others. Despite the breadth and depth of the clinical data used in our analyses, we did not have variables outside of clinical standard of care. This includes information on physical activity. Physical activity is a major factor in energy expenditure and may play a significant role in post-RYGB weight loss 30. We did not have data available from either self-reported measures of physical activity, or from objective monitors such as pedometers, accelerometers, or other devices. Given the potential importance of physical activity in weight loss outcomes, future studies will need to be performed to gather such data.Despite these limitations, these data suggest that pre-operative data may be used for the early recognition of clinical factors that adversely impact post-RYGB weight loss. The limited access to surgical treatment for eligible extremely obese patients, the ever-expanding list of procedure options, and the wide range of weight loss outcomes mandates improved patient selection as well as better resource allocation for peri-operative medical management. The identification of the clinical factors which adversely impact post-RYGB weight loss will allow for the implementation of specific therapeutic strategies and clinical trials designed to address the underlying basis of unfavorable outcomes.
CONCLUSION
In summary, the early, nadir, and long-term weight loss phases following RYGB surgery were associated with different sets of easily measured pre-operative clinical variables. The variables associated with less %EBWL at least 36 months after RYGB surgery were higher baseline BMI, pre-operative use of any diabetes medication, non-use of bupropion medication, no history of smoking, aged greater than 50 years, and the presence of fibrosis on liver biopsy. These data suggest that specific therapeutic strategies may be designed to address the factors associated with unfavorable outcomes.
Supplementary Material
What is already known about this subject?
Baseline BMI, type 2 diabetes, certain psychological conditions, and limited physical activity have been associated with poorer weight loss outcomes usually defined by a single time point after RYGB surgery.
Most studies of pre-operative clinical predictors have analyzed only one or a few variables often in small populations with limited lengths of follow-up.
What does this study add?
We analyzed weight loss by temporal phase.
We identified additional novel clinical variables affecting weight loss outcomes.
We used a large number of clinical variables, a large population, and a long post-operative follow-up period.
ACKNOWLEDGMENTS
We gratefully acknowledge the extraordinary cooperation and support of the patients enrolled in the Geisinger Bariatric surgery program without which these studies would not have been possible.
Funding/support: This work was supported by funds from Geisinger Clinic, the Weis Center for Research, the Geisinger Obesity Institute, and NIH grants DK072488 (GSG, CDS, GA, and XC), DK088231 (GSG), and DK091601 (GSG) from the NIH.
Footnotes
Author's contributions: Drs. Still and Gerhard and Mr. Wood had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Still, Wood, Benotti, Argyropoulos, and Gerhard.
Acquisition of data: Wood, Chu, Manney, Strodel, Petrick, Gabrielsen, Still, and Seiler.
Statistical analysis and interpretation: Gerhard, Still, Wood, and Benotti.
Drafting of the manuscript: Benotti, Gerhard, Argyropoulos, Mirshahi, Still, and Wood.
Critical revision of the manuscript for important intellectual content: Benotti, Gerhard, Argyropoulos, Mirshahi, Strodel, Petrick, Gabrielsen, Still,
Obtaining funding: Gerhard, Argyropoulos, and Still
Administrative, technical or material support: Yung, Manney, Seiler
Study supervision: Gerhard, Still
CONFLICT OF INTEREST
Financial disclosures: Dr. Still receives grant and consulting support from Ethicon-Endosurgery. Dr. Petrick has educational grants from Covidien and Ethicon-Endosurgery.
REFERENCES
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom CD. Systematic review of bariatric surgery. JAMA : the journal of the American Medical Association. 2005;293:1726. doi: 10.1001/jama.293.14.1726-b. author reply. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. The New England journal of medicine. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 4.Clegg A, Colquitt J, Sidhu M, Royle P, Walker A. Clinical and cost effectiveness of surgery for morbid obesity: a systematic review and economic evaluation. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27:1167–77. doi: 10.1038/sj.ijo.0802394. [DOI] [PubMed] [Google Scholar]
- 5.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. The New England journal of medicine. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 6.Ali MR, Fuller WD, Choi MP, Wolfe BM. Bariatric surgical outcomes. The Surgical clinics of North America. 2005;85:835–52. vii. doi: 10.1016/j.suc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Dixon JB, Straznicky NE, Lambert EA, Schlaich MP, Lambert GW. Surgical approaches to the treatment of obesity. Nature reviews. Gastroenterology & hepatology. 2011;8:429–37. doi: 10.1038/nrgastro.2011.112. [DOI] [PubMed] [Google Scholar]
- 8.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Annals of surgery. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. discussion 50-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Annals of surgery. 2006;244:734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Annals of internal medicine. 2005;142:547–59. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA : the journal of the American Medical Association. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 12.Services CfMaM Services DoHH, editor. National Coverage Determination (NCD) for BARIATRIC SURGERY for Treatment of Morbid Obesity. 2009. 2009.
- 13.Hatoum IJ, Stein HK, Merrifield BF, Kaplan LM. Capacity for physical activity predicts weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring. 2009;17:92–9. doi: 10.1038/oby.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junior WS, do Amaral JL, Nonino-Borges CB. Factors related to weight loss up to 4 years after bariatric surgery. Obesity surgery. 2011;21:1724–30. doi: 10.1007/s11695-011-0420-3. [DOI] [PubMed] [Google Scholar]
- 15.Livhits M, Mercado C, Yermilov I, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obesity surgery. 2012;22:70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]
- 16.van de Laar A, de Caluwe L, Dillemans B. Relative outcome measures for bariatric surgery. Evidence against excess weight loss and excess body mass index loss from a series of laparoscopic Roux-en-Y gastric bypass patients. Obesity surgery. 2011;21:763–7. doi: 10.1007/s11695-010-0347-0. [DOI] [PubMed] [Google Scholar]
- 17.Bray GA, Bouchard C, Church TS, et al. Is it time to change the way we report and discuss weight loss? Obesity (Silver Spring) 2009;17:619–21. doi: 10.1038/oby.2008.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Still CD, Wood GC, Chu X, et al. High allelic burden of four obesity SNPs is associated with poorer weight loss outcomes following gastric bypass surgery. Obesity (Silver Spring) 2011;19:1676–83. doi: 10.1038/oby.2011.3. [DOI] [PubMed] [Google Scholar]
- 19.Wood G, Chu X, Manney C, et al. An electronic health record-enabled obesity database. BMC Bioinformatics and Medical Decision MAking. 2012 doi: 10.1186/1472-6947-12-45. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenker R, Bassett GW. Regression quantiles. Econometrica. 1978;46:33–50. [Google Scholar]
- 21.Matzko ME, Argyropoulos G, Wood GC, et al. Association of Ghrelin Receptor Promoter Polymorphisms with Weight Loss Following Roux-en-Y Gastric Bypass Surgery. Obesity surgery. 2012;22:783–90. doi: 10.1007/s11695-012-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dallal RM, Quebbemann BB, Hunt LH, Braitman LE. Analysis of weight loss after bariatric surgery using mixed-effects linear modeling. Obesity surgery. 2009;19:732–7. doi: 10.1007/s11695-009-9816-8. [DOI] [PubMed] [Google Scholar]
- 23.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melton GB, Steele KE, Schweitzer MA, Lidor AO, Magnuson TH. Suboptimal weight loss after gastric bypass surgery: correlation of demographics, comorbidities, and insurance status with outcomes. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2008;12:250–5. doi: 10.1007/s11605-007-0427-1. [DOI] [PubMed] [Google Scholar]
- 25.Cassie S, Menezes C, Birch DW, Shi X, Karmali S. Effect of preoperative weight loss in bariatric surgical patients: a systematic review. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2011;7:760–7. doi: 10.1016/j.soard.2011.08.011. discussion 7. [DOI] [PubMed] [Google Scholar]
- 26.Guajardo-Salinas GE, Hilmy A, Martinez-Ugarte ML. Predictors of weight loss and effectiveness of Roux-en-Y gastric bypass in the morbidly obese Hispano-American population. Obesity surgery. 2008;18:1369–75. doi: 10.1007/s11695-008-9461-7. [DOI] [PubMed] [Google Scholar]
- 27.Finks JF, Kole KL, Yenumula PR, et al. Predicting risk for serious complications with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Annals of surgery. 2011;254:633–40. doi: 10.1097/SLA.0b013e318230058c. [DOI] [PubMed] [Google Scholar]
- 28.Forbush S, Nof L, Echternach J, Hill C, Rainey J. Influence of activity levels and energy intake on percent excess weight loss after Roux-en-Y gastric bypass. Obesity surgery. 2011;21:1731–8. doi: 10.1007/s11695-011-0450-x. [DOI] [PubMed] [Google Scholar]
- 29.Dhillon S, Yang LP, Curran MP. Bupropion: a review of its use in the management of major depressive disorder. Drugs. 2008;68:653–89. doi: 10.2165/00003495-200868050-00011. [DOI] [PubMed] [Google Scholar]
- 30.King WC, Bond DS. The importance of preoperative and postoperative physical activity counseling in bariatric surgery. Exercise and sport sciences reviews. 2013;41:26–35. doi: 10.1097/JES.0b013e31826444e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Billes SK, Greenway FL. Combination therapy with naltrexone and bupropion for obesity. Expert Opin Pharmacother. 2011;12:1813–26. doi: 10.1517/14656566.2011.591382. [DOI] [PubMed] [Google Scholar]
- 32.Plodkowski RA, Nguyen Q, Sundaram U, Nguyen L, Chau DL, St Jeor S. Bupropion and naltrexone: a review of their use individually and in combination for the treatment of obesity. Expert Opin Pharmacother. 2009;10:1069–81. doi: 10.1517/14656560902775750. [DOI] [PubMed] [Google Scholar]
- 33.Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009;17:30–9. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 34.Mineur YS, Abizaid A, Rao Y, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–2. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirshahi UL, Still CD, Masker KK, Gerhard GS, Carey DJ, Mirshahi T. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96:E2088–96. doi: 10.1210/jc.2011-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.