Abstract
Chemotherapy-induced amenorrhea (CIA) often occurs in pre- and peri-menopausal BC patients, and while cancer/chemotherapy and abrupt estrogen loss have separately been shown to affect cognition and brain function, studies of the cognitive effects of CIA are equivocal, and its effects on brain function are unknown. Functional MRI (fMRI) during a working memory task was used to prospectively assess the pattern of brain activation and deactivation prior to and one month after chemotherapy in BC patients who experienced CIA (n=9), post-menopausal BC patients undergoing chemotherapy (n=9), and pre- and post-menopausal healthy controls (n=6 each). Neurocognitive testing was also performed at both time points. Repeated measures general linear models were used to assess statistical significance, and age was a covariate in all analyses. We observed a group-by-time interaction in the combined magnitudes of brain activation and deactivation (p = 0.006): the CIA group increased in magnitude from baseline to post-treatment while other groups maintained similar levels over time. Further, the change in brain activity magnitude in CIA was strongly correlated with change in processing speed neurocognitive testing score (r=0.837 p=0.005), suggesting this increase in brain activity reflects effective cognitive compensation. Our results demonstrate prospectively that the pattern of change in brain activity from pre- to post-chemotherapy varies according to pre-treatment menopausal status. Cognitive correlates add to the potential clinical significance of these findings. These findings have implications for risk appraisal and development of prevention or treatment strategies for cognitive changes in CIA.
Keywords: breast cancer, chemotherapy, amenorrhea, functional MRI
Introduction
Cancer and its treatments have been linked to cognitive dysfunction, particularly in the executive function, working memory, processing speed, verbal, and visuospatial domains (Jansen et al. 2005; Jim et al. 2012). Approximately 80% of pre- or peri-menopausal breast cancer (BC) patients undergoing current widely used chemotherapy (CTx) regimens (cyclophosphamide and doxorubicin, with or without a taxane) experience chemotherapy-induced amenorrhea (CIA) in the months immediately following CTx (Petrek et al. 2006; Minisini et al. 2009; Swain et al. 2009; Swain et al. 2010). CIA results from disruption of normal ovarian follicular maturation, leading to markedly decreased systemic estrogen levels (Warne et al. 1973), and is associated with increased survival (Walshe et al. 2006; Swain et al. 2010). As abrupt estrogen loss in pre-menopausal women has been linked to cognitive dysfunction (Vearncombe and Pachana 2009), it is plausible that CIA may lead to increased detrimental effects of CTx compared to women who undergo CTx but not CIA (usually BC patients post-menopausal before CTx). Indeed, prospective studies have shown decline or failure to improve with practice in multiple cognitive domains in patients undergoing CIA compared to patients undergoing CTx but not amenorrhea (Jenkins et al. 2006; Vearncombe et al. 2011), although other studies found no such effect (Schagen et al. 2006; Hermelink et al. 2007; Hermelink et al. 2008). Timing of measurements appears to play a role.
Prospective functional neuroimaging has the power to observe, in the face of a neural insult such as CTx or estrogen loss, how the brain might compensate (in the context of maintained cognitive performance), or fail to adapt (in the context of decreased performance). We recently showed pre-treatment frontal hyperactivation in BC during a working memory task, with a decrease in activation in this region one month post-CTx accompanied by decreased working memory performance (McDonald et al. 2012). Performance and activation returned to higher levels one year later. The neural effects of abrupt estrogen loss in pre-menopausal women have been studied prospectively with gonadotropin hormone releasing hormone (GnRH) agonists. These studies generally show that estrogen ablation is associated with reversible decreased task-related activation (Berman et al. 1997; Craig et al. 2007; Craig et al. 2008; Craig et al. 2008). However, the neural effects of CIA remain unclear.
The aim of this study was to prospectively measure global changes in working memory-related activation and deactivation, before cancer treatment and one month post-CTx completion. During a cognitive task, brain activation increases in “task-positive network” regions, while task-induced deactivation occurs in the anatomical regions of the “default mode network” (DMN) in a reallocation of neural resources (Fox et al. 2005). Both activation and deactivation are important in cognition, and both are affected by normal aging as well as pathological conditions. While activation and deactivation occur in complementary brain regions during a particular task, they can be differentially affected by pathological processes. Drawing on participants in our previous prospective fMRI study of BC patients (McDonald et al. 2012), we examined working memory-related activation and deactivation in BC patients who underwent CIA (i.e., pre- or perimenopausal patients) and patients who were post-menopausal at CTx initiation. Pre- and post-menopausal healthy control (HC) groups were imaged at yoked intervals. We hypothesized that the CIA profile of activation and deactivation during a working memory task would be distinct from that of HC or post-menopausal CTx, and that these changes over time would be correlated with changes seen in neurocognitive performance.
Methods
Participants
Appropriate cases were selected from our previous fMRI study (McDonald et al. 2012) who fit the following categories: post-menopausal BC patients undergoing CTx (n=9), pre- or peri-menopausal BC patients undergoing CTx (n=9), pre- and post-menopausal HC (n=6 each). Scans and neuropsychological measures were collected post-surgery but prior to any other cancer treatment (baseline; BL) and one month post-CTx completion (M1) or yoked intervals for HC.
Patients had stage I, II, or IIIA BC treated with standard-dose AC or AC-T CTx regimens (Table 1). Exclusion criteria included prior cancer or cancer treatment and medical, neurological, and psychiatric risk factors known to affect brain structure or function, as detailed previously (McDonald et al. 2010). Depressive symptoms and anxiety were assessed with the Center for Epidemiologic Studies-Depression Scale (CES-D) (Radloff 1977) and the State-Trait Anxiety Inventory-State subscale (STAI-S) (Spielberger 1983).
Table 1.
Demographics and cancer treatment information
| Chemotherapy- induced amenorrhea (n=9) |
Healthy control pre- menopausal (n=6) |
Chemotherapy post- menopausal (n=9) |
Healthy control post- menopausal (n=6) |
||
|---|---|---|---|---|---|
| Age (years)** | 45.3±5.8 (range 35–55) | 44.8±4.0 (range 37–48) | 58.7±4.4 (range 53–69) | 55.2±4.0 (range 50–59) | |
| Education (years) | 14.8±1.8 | 15.8±1.3 | 15.2±3.3 | 15.8±2.0 | |
| Handedness (R:L,A) | 8:1 | 4:2 | 8:1 | 6:0 | |
| Full Scale IQ Estimate (Barona et al. 1984) | 110.5±6.8 | 113.8±3.0 | 112.7±6.6 | 114.8±2.9 | |
| CES-D | BL | 8.2±9.0 | 7.6±6.1 | 8.0±7.4 | 2.3±1.6 |
| M1 | 11.0±11.5 | 7.2±5.8 | 9.8±9.2 | 4.2±4.8 | |
| STAI-S | BL | 34.1±14.0 | 28.6±8.6 | 29.0±9.1 | 25.2±7.5 |
| M1 | 35.7±18.2 | 28.0±5.3 | 28.4±8.8 | 27.5±11.9 | |
| Psychiatric Medications | BL | 2 (1 buspirone and desipramine, 1 zolpidem) | 0 | 2 (1 venlafaxine and buspirone, 1 fluoxetine) | 0 |
| M1 | 3 (1 buspirone and desipramine, 1 zolpidem, 1 sertraline) | 0 | 2 (1 venlafaxine and buspirone, 1 fluoxetine) | 0 | |
| BL-M1 interval (days) | 153±45 | 167±36 | 181±78 | 206±51 | |
| Cancer stage | 2 stage I 5 stage II 2 stage IIIa |
2 stage I 7 stage II |
|||
| Chemotherapy regimen | 7 AC-T, 1 AC-T plus trastuzumab, 1 AC | 7 AC-T, 2 AC | |||
| Hormonal medication | BL | 1 estradiol (for perimenopausal symptoms) | 0 | 0 | 4 hormone replacement therapy |
| M1 | 2 tamoxifen | 0 | 1 tamoxifen, 1 aromatase inhibitor | 2 hormone replacement therapy | |
| Menstrual Status | |||||
| Periods regular throughout study | 0 | 6 | 0 | 0 | |
| Post-menopausal (>6 months) throughout study | 0 | 0 | 9 | 6 | |
| Periods irregular at BL, amenorrhea ≥6 months at M1 | 3 | 0 | 0 | 0 | |
| Periods regular at BL, amenorrhea ≥6 months at M1 | 4 | 0 | 0 | 0 | |
p<0.001 overall ANOVA; in post-hoc Tukey tests, both pre-/peri-menopaual groups less than both postmenopausal groups; both pre-/peri- and post-menopausal groups not different from each other
CES-D: Center for Epidemiologic Studies-Depression Scale (Radloff 1977)
STAI-S: State-Trait Anxiety Inventory-State subscale (Spielberger 1983)
BL: baseline
M1: one month post-chemotherapy completion or yoked interval
AC: Doxorubicin and cyclophosphamide
AC-T: Doxorubicin, cyclophosphamide, and a taxane
Written informed consent was obtained according to the Declaration of Helsinki under a protocol approved by the Dartmouth College Committee for the Protection of Human Subjects.
MRI Scans
Scans were acquired on a 1.5T GE Signa LX scanner with echospeed gradients and standard head coil. A gradient-echo, echo-planar sequence provided whole brain coverage for fMRI: TR=2500ms, TE=40ms, FOV=24cm, NEX=1, 29 interleaved 5mm thick contiguous sagittal slices, yielding a 64×64 matrix with 3.75mm2 in-plane resolution. Structural scans were acquired to rule out incidental pathology and for the previously reported gray matter density analysis (McDonald et al. 2010).
An auditory-verbal “N-back” task was used as in our previous study (McDonald et al. 2012). During scanning participants heard a string of consonant letters (except L, W, and Y) presented one every three seconds. Task conditions were 0-, 1-, 2-, and 3-back, in a blocked design. For each consonant participants used a button press device to signify whether the current letter was a match (i.e., was the same as the designated target or the letter presented 1, 2, or 3 back in the sequence) or a non-match. Each condition was presented in 27-second epochs preceded by three seconds of instruction (e.g., “the match is one back”). The four experimental conditions were each presented three times in pseudo-random order for a total of 12 task blocks. Participants rehearsed a practice version of the task prior to scanning to ensure comprehension of task demands. Stimuli were presented through an MRI compatible headphone system, and programmed in Presentation software (Neurobehavioral Systems, Inc., Albany, CA), which recorded response accuracy and reaction times.
Using raw scan data, spatial realignment using a six parameter model was performed in SPM5. Realignment parameters were entered as covariates at the subject level, and volumes were normalized into MNI space, resampled to 2mm3 voxels, and smoothed to a FWHM of 8mm. Statistical parametric mapping on a voxel-by-voxel basis was conducted using a general linear model (GLM) approach. Contrast images comparing pairs of working memory load conditions (e.g., 3-back > 0-back) were created for each subject.
To derive numerical values for activation and deactivation during the 3-back task, all 3-back > 0-back contrast images were entered into a one-sample t-test in SPM5. Maps were created for activation (“task-positive network”; contrast vector 1; i.e., 3-back > 0-back) and deactivation (“default mode network”; contrast vector −1; i.e., 0-back > 3-back) at pcrit = 0.05, cluster minimum size (k) = 10 voxels. MarsBar v. 0.42 (Brett et al. 2002) was used to extract mean values for each participant’s activation and deactivation in the appropriate anatomical regions at both time points. Our imaging analyses used three dependent measures: total activation, total deactivation, and the summed magnitudes of activation and deactivation (as a measure of overall neural change in response to the task).
Neurocognitive Testing Domains
Raw neurocognitive test scores were normalized using the mean and standard deviation of the larger HC group from which HC subjects for the current study were drawn (demographic data regarding the larger cohort can be found in (McDonald et al. 2010)). Alternate test forms were used at each study visit where possible. Domain scores were created for each subject by averaging the z-scores of the included tests, similar to our prior cognitive studies (Ahles et al. 2008; Ahles et al. 2010), and were then adjusted for age using the entire sample of the current study. The verbal domain was calculated using the Wide Range Achievement Test (WRAT-3) reading score (Wilkinson 1993), the Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary score (The Psychological Corporation 1999), and the Delis-Kaplan Executive Function System (D-KEFS) Verbal Fluency Test phonemic and semantic trial scores (Delis et al. 2001). The verbal memory domain consisted of the California Verbal Learning Test (CVLT or CVLT-II) total and delayed free recall scores (Delis et al. 2000; Delis et al. 1987) and the Craft story immediate and delayed recall scores (Craft et al. 1996). The visual memory domain was the Brown Learning Test initial learning trials and delayed recall score (Brown et al. 2007). The working memory score consisted of the Rao Paced Auditory Serial Addition Test (PASAT) 2 and 3 second trial totals (Fischer et al. 2001). The processing speed score comprised the WAIS-III Digit Symbol-Coding score (The Psychological Corporation 1997), the left- and right-hand grooved pegboard times (Lafayette Instrument 1989), and completion times from D-KEFS Trail Making Test trials 1–5 and the D-KEFS Color-Word Interference Test color naming, word reading, and color-word interference trials. The sorting domain was the D-KEFS Sorting Test number of correct sorts and the free sorting and recognition trial description scores. The distractibility domain was the Gordon Continuous Performance Task (CPT) distractibility trial total correct and false positive scores, while the reaction time domain was the average of the CPT distractibility and vigilance trial reaction times. The global score was the average of all the above domains as well as the WASI Block Design score.
Statistical Analyses
One-way ANOVA with Tukey post-hoc tests were used to examine between-group effects of age, education, IQ estimates, and BL-M1 interval, while chi-square examined handedness distribution. Experimental measures, including fMRI activation and deactivation, neuropsychological tests, 3-back task performance and reaction times, and depression and anxiety scores were assessed using a repeated measures general linear model in SPSS 19 with factors of group and time. Age covariates were used in imaging analyses, while neuropsychological measures were pre-adjusted for age. Group-by-time interactions from these analyses were examined, as well as main effects of group and time. Absolute values of activation and deactivation were summed at each time point to create a measure of total change in neural activity and analyzed using the same repeated measures model. Pearson correlations were used to assess relationships between changes in imaging variables and neuropsychological values.
Results
Demographics, psychological state, hormonal status and cancer treatment data are provided in Table 1. As expected, groups differed in age, with both post-menopausal groups significantly older than both pre-menopausal groups at baseline (overall ANOVA p<0.001; post-hoc Tukey tests p<0.05)
Education, handedness, estimated IQ, and BL-M1 interval did not differ between groups. CES-D and STAI-S mean scores were well below clinical thresholds and did not show main effects of group or time or group-by-time interactions. Several cancer patients were using psychiatric medications at each study visit, with little change in medication regimens between BL and M1. Cancer stage and CTx regimens were similar between groups.
All post-menopausal HC and CTx participants reported amenorrhea >6 months at study entry, and all pre-menopausal HC had regular periods at study entry (Table 1). In the CIA group, 4 patients reported regular periods and 3 patients reported irregular periods at study entry, and all of these reported amenorrhea >6 months related to CTx at M1. Menstrual status was unavailable for 2 CIA patients, but they were placed in this group based on their ages of 44 and 45 at study entry, as all HC under 50 in this cohort were pre-menopausal. For hormonal medications, several post-menopausal patients reported using hormonal replacement therapy at each visit (4 at BL, 2 at M1). One 55-year-old CIA participant (the oldest in this group) was using estrogen for perimenopausal symptoms at BL but had stopped at M1. No cancer patients were using antiestrogen treatments at BL, but 2 patients in each cancer group had started these treatments at M1.
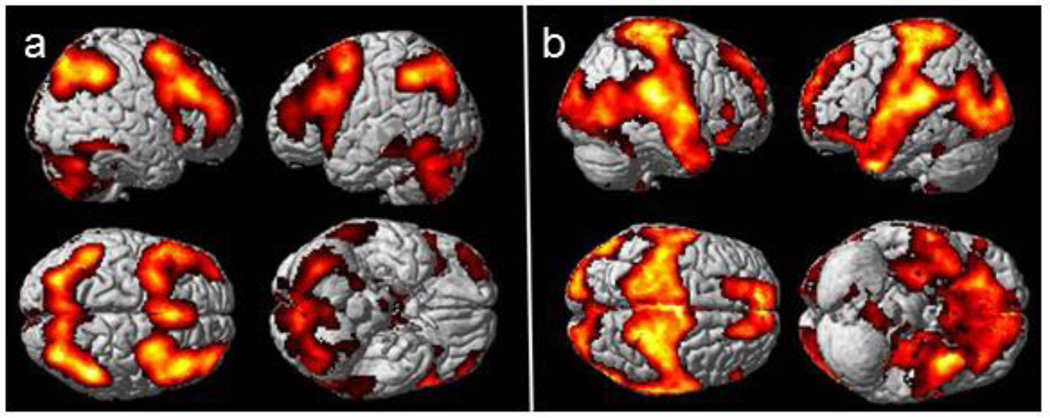
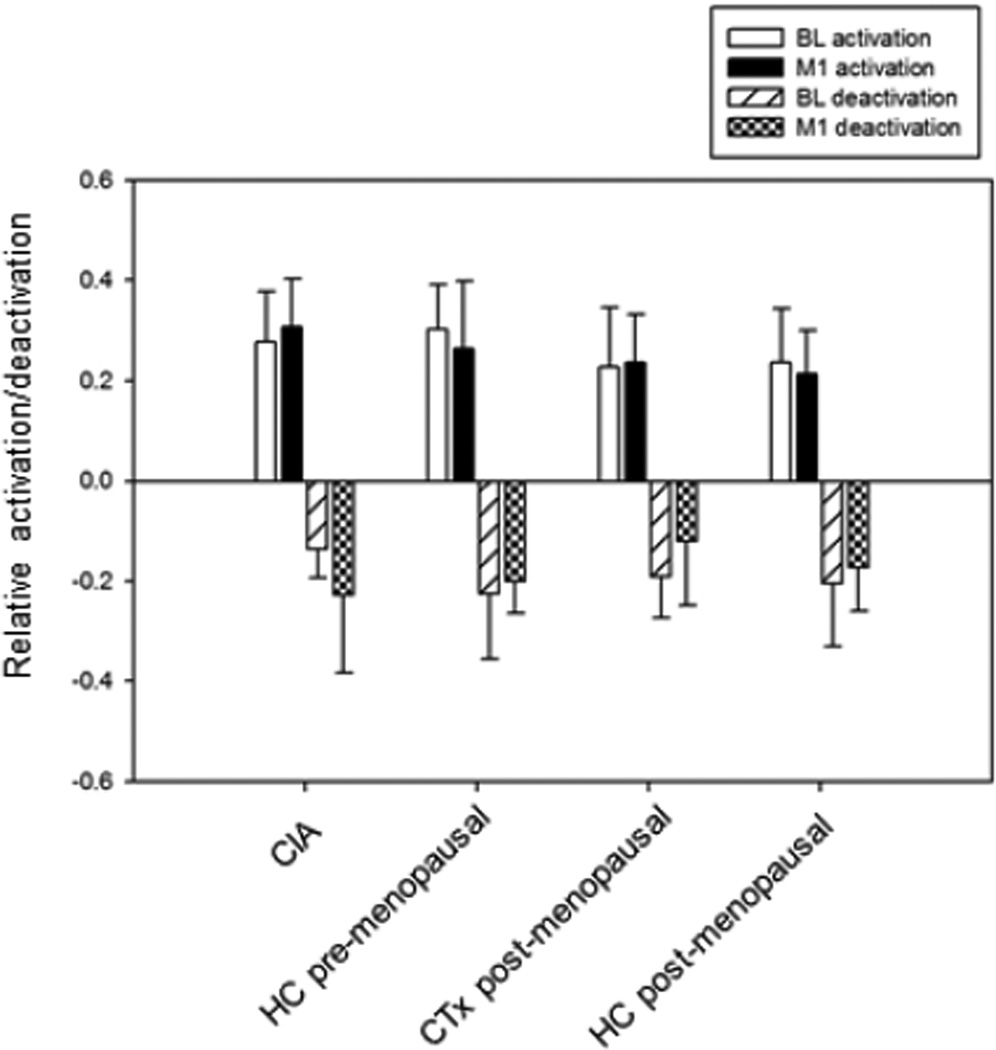
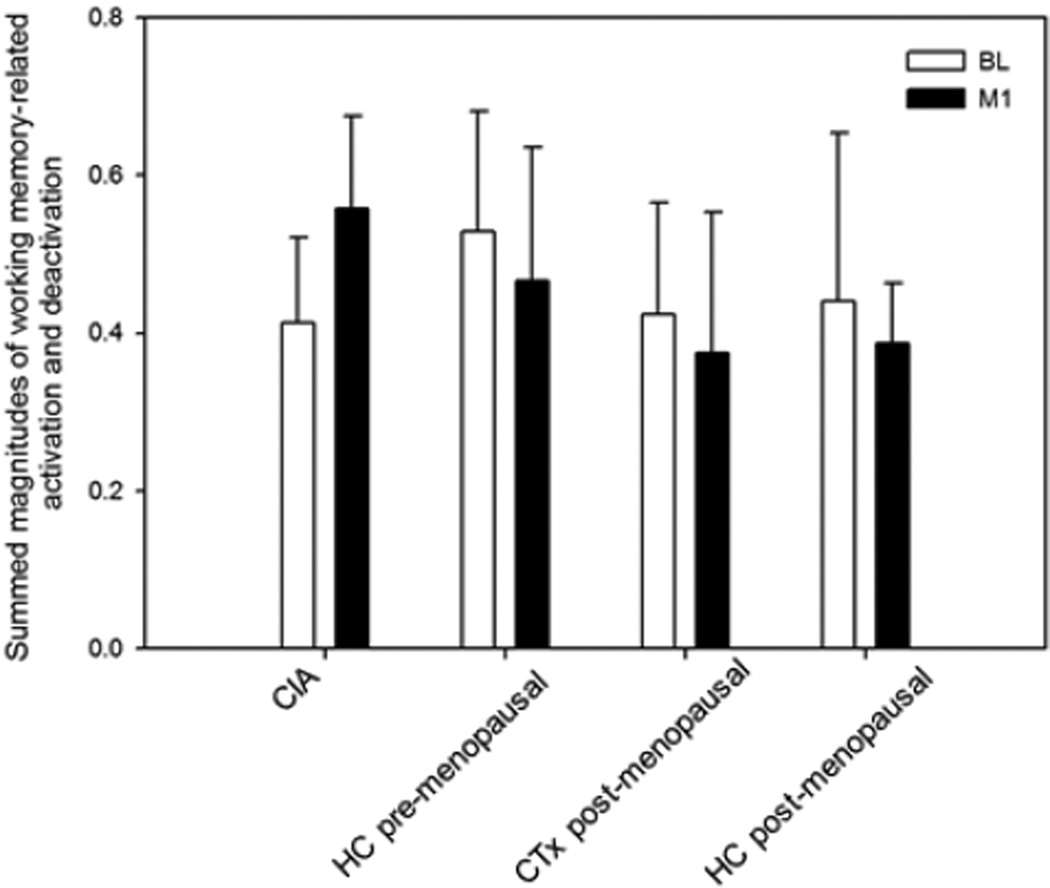
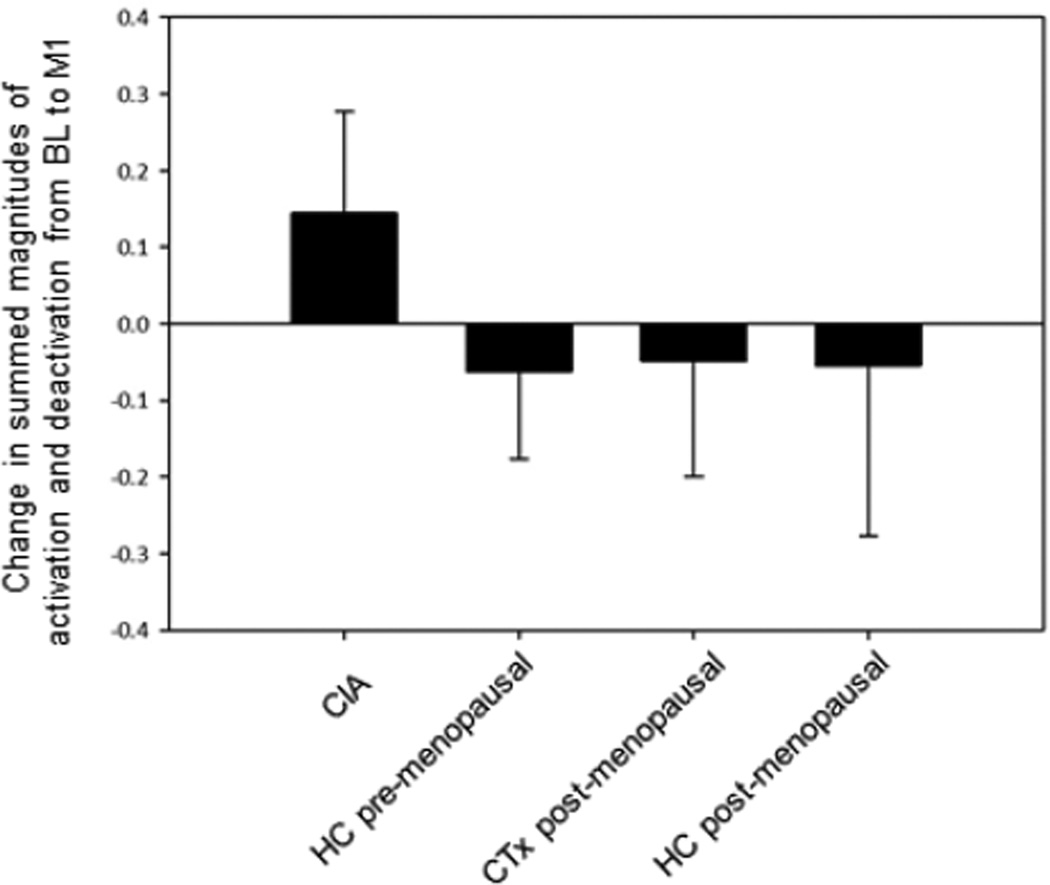
Figure 1 depicts the activation and deactivation regions of interest used to derive values for each participant at BL and M1. Figure 2a shows mean values of activation and deactivation for each group at BL and M1. Repeated measures ANOVA with an age covariate did not show any significant main effects or interactions for either activation or deactivation. Repeated measures ANOVA on the summed magnitudes of activation and deactivation for the four groups with an age covariate revealed a significant group-by-time interaction (p=0.006). In contrast to the other groups, the CIA group showed an increase in the summed magnitudes of activation and deactivation from BL to M1 (Figures 2b, 2c), and this change was statistically significant (p=0.011). There was also a significant effect of time (p=0.046), with an overall pattern of increased magnitudes from BL to M1 and a time-by-age interaction (p=0.047) in which younger participants tended to increase in magnitude over time (driven by the CIA group).
Figure 1.
Working memory-related (a) activation (3-back > 0-back contrast) and (b) deactivation (0-back > 3-back contrast) regions of interest derived from main effects of all groups’ baseline and one month post-chemotherapy scans; pcrit=0.05, minimum cluster extent (k)=10.
Figure 2.
(a) Working memory-related total activation and deactivation at baseline (BL) and 1 month post-chemotherapy (M1) (arbitrary units; mean±SD). (b) Summed magnitudes of activation and deactivation at BL and M1; group-by-time interaction is statistically significant (p=0.006), with only the CIA group showing change over time (p=0.011). (c) Change between BL and M1 in summed magnitudes of activation and deactivation.
In-scanner 3-back task performance and reaction times did not differ between groups or over time (Table 2).
Table 2.
Neurocognitive testing and in-scanner task results
| Chemotherapy -induced amenorrhea (n=9) |
Healthy control pre- menopausal (n=6) |
Chemotherapy post- menopausal (n=9) |
Healthy control post- menopausal (n=6) |
||
|---|---|---|---|---|---|
| Neurocognitive Testing Domains | |||||
| Verbal^ | BL | −0.339±;0.837 | 0.211±0.581 | 0.173±1.074 | −0.264±0.976 |
| M1 | −0.230±0.722 | 0.370±0.662 | 0.029±0.956 | −0.029±0.723 | |
| Verbal Memory | BL | −0.271±1.029 | −0.335±0.723 | −0.345±0.932 | 0.380±0.767 |
| M1 | −0.311±0.881 | −0.092±0.495 | −0.198±0.893 | 0.297±1.011 | |
| Visual Memory**^ | BL | −0.241±1.42 | 0.179±0.607 | 0.222±0.549 | 0.145±1.336 |
| M1 | 0.584±1.548 | 0.848±0.778 | 0.135±0.618 | 1.071±1.615 | |
| Working Memory** | BL | 0.125±0.965 | 0.019±0.290 | 0.778±0.718 | 0.123±0.977 |
| M1 | 0.659±1.157 | 0.421±0.585 | 1.091±0.920 | 0.312±0.670 | |
| Processing Speed* | BL | 0.123±0.940 | 0.132±0.415 | 0.061±0.405 | 0.138±0.401 |
| M1 | 0.292±0.981 | 0.355±0.384 | 0.064±0.638 | 0.255±0.232 | |
| Sorting** | BL | −0.598±0.878 | −0.035±0.912 | −0.360±0.988 | 0.135±0.827 |
| M1 | −0.011±1.079 | 0.613±0.659 | −0.431±1.168 | 0.566±1.345 | |
| Distractibility** | BL | −1.276±1.940 | −0.430±0.750 | −0.475±2.213 | 0.468±1.342 |
| M1 | −1.808±2.296 | −0.438±0.651 | −1.318±3.702 | 0.812±0.531 | |
| Reaction Time** | BL | 0.319±0.701 | −0.446±1.173 | 0.021±0.788 | 0.113±0.710 |
| M1 | 0.552±0.508 | −0.097±1.318 | 0.291±0.637 | 0.407±0.628 | |
| Global** | BL | −0.320±0.760 | −0.079±0.518 | −0.076±0.713 | 0.190±0.710 |
| M1 | −0.102±0.863 | 0.240±0.439 | −0.096±0.982 | 0.447±0.569 | |
| In-scanner 3-back task | |||||
| 3-back score (% corrected for guessing) | BL | 65.3±27.3 | 74.4±7.8 | 62.1±24.6 | 78.5±16.1 |
| M1 | 65.3±27.3 | 74.3±12.7 | 63.2±25.0 | 67.1±12.7 | |
| Reaction time (seconds) | BL | 1.20±0.31 | 1.01±0.13 | 1.01±0.20 | 1.15±0.10 |
| M1 | 1.19±0.34 | 0.97±0.10 | 1.09±0.15 | 1.16±0.08 | |
main effect of time p≤0.01
main effect of time p≤0.05
group-by-time interaction trend p<0.10
Group means of neuropsychological testing domains are shown in Table 2. A main effect of time was present for most domains at p ≤0.01. This reflected an overall tendency toward improved performance at M1, likely due to practice effects. Main effect of group was not statistically significant in any domain. Interestingly, trends toward group-by-time interactions were present in the verbal (p=0.073) and visual memory (p=0.098) domains. In both of these domains, all groups showed improved performance from BL to M1 except for the CTx post-menopausal group, whose performance decreased between sessions.
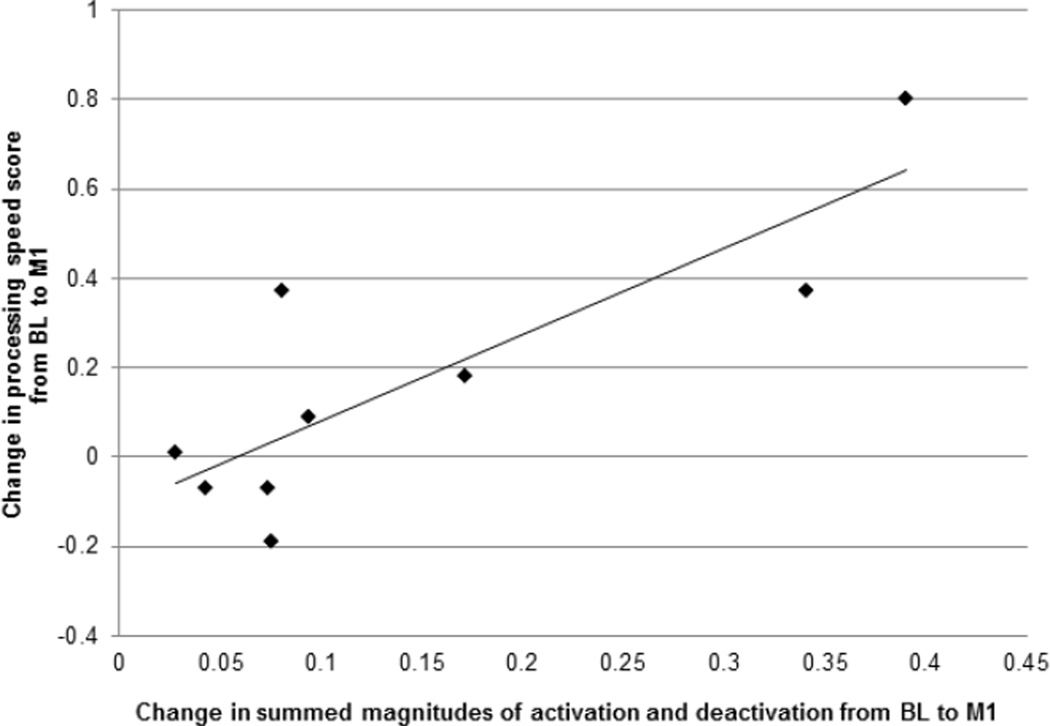
To assess the functional significance of the increased magnitude of neural activity in CIA, we tested for correlations between the activity change in this group and change in neuropsychological domain scores. A significant positive correlation emerged in the processing speed domain (r=0.837, p=0.005, survives Bonferroni threshold for 9 domains (p=0.045 after correction); Figure 3). The positive valence indicates that increased magnitude of neural activation/deactivation between BL and M1 is associated with improvement in processing speed scores. No other CIA activity change-neurocognitive domain correlations were statistically significant.
Figure 3.
Correlation of change in summed magnitude of activation and deactivation with change in processing speed neuropsychological testing domain scores from baseline to one month post-chemotherapy in chemotherapy-induced amenorrhea (CIA) (r=0.837, p=0.045 after Bonferroni correction for 9 neuropsychological domains).
Discussion
Our results demonstrate prospectively that the pattern of change in brain activity from pre- to post-CTx varies according to pre-treatment menopausal status. BC patients who underwent CIA showed increased magnitudes of activation and deactivation from pre- to post-CTx, while CTx post-menopausal and both HC groups did not. In the context of maintained 3-back task performance, this may indicate effective compensatory neural activity in the CIA group. Age, an inevitable between-group confound in this type of analysis, was included as a covariate, suggesting that these effects are due to CIA itself. We found no evidence for the negative cognitive effect of CIA that has been observed in some studies (Jenkins et al. 2006; Vearncombe et al. 2011) but not others (Schagen et al. 2006; Hermelink et al. 2007; Hermelink et al. 2008), although multiple studies of women in this demographic who undergo abrupt loss of ovarian function via surgical menopause suggest that larger samples of CIA patients may well demonstrate this effect (Henderson and Sherwin 2007; Vearncombe and Pachana 2009).
Relationships between brain changes and behavioral measures increase the potential clinical significance of our findings. CIA-related change in neural activity was highly correlated with improvement in neurocognitive processing speed scores from BL to M1, suggesting that this adaptation is functional. Processing speed has been shown prospectively to be the domain most sensitive to chemotherapy-related change (Ahles et al. 2010), in addition to being one of several domains most implicated in cancer- and treatment-related cognitive dysfunction (Jansen et al. 2005),
The current results are consistent with the interpretation that the neural stress of CIA’s abrupt decrease in estrogen, in addition to CTx itself, requires alterations in brain activity to maintain cognitive function. In light of evidence that both activation (Nagel et al. 2011) and deactivation (McKiernan et al. 2003) increase parametrically with task demand, these results suggest that after CTx, the CIA brain responds to the same 3-back task as if it is more difficult. A similar phenomenon of effective compensatory activation has been observed in mild cognitive impairment (MCI) in older adults: in the early, higher functioning stages of MCI, patients show hippocampal and prefrontal hyperactivation during memory tasks compared to cognitively normal older adults, while activation decreases in later stages of MCI as cognitive functioning declines (Clement and Belleville 2010; O'Brien et al. 2010).
Interestingly, in neuropsychological testing, the post-menopausal CTx group was unable to maintain performance in the post-CTx visit, while in all other groups scores improved between BL and M1 in the verbal and visual memory domains (in non-significant trends for group-by-time interactions). These domains involve different neural circuitry than the working memory scanner task and have not been especially previously implicated in CTx-related cognitive dysfunction, but the lack of increase in neural activity in the post-menopausal CTx group may be related to this decreased neurocognitive performance post-treatment. As estrogen loss reduces neuroplasticity, and longer duration of estrogen deprivation (i.e., more time after menopause) is associated with worse outcomes (Brinton 2009), the lack of change in brain activity may be related to reduced neuroplastic adaptation to CTx in the post-menopausal brain. This result is also consistent with a recent longitudinal study (Ahles et al. 2010) which found that older age was related to increased risk for cognitive dysfunction after CTx, particularly in the context of processing speed domain and lower cognitive reserve (although no effects of pre-treatment menopausal status were observed). Follow-up of cognitive results in larger cohorts is warranted.
While there are no neuroimaging studies of CIA with which to compare the current results, neuroimaging studies of both cancer/CTx and estrogen suppression in pre-menopausal women do exist. In cancer and CTx, BC patients have shown pre-treatment frontal hyperactivation (Cimprich et al. 2010; McDonald et al. 2012) that is attenuated one month post-CTx but remains hyperactive one year later (McDonald et al. 2012). Task performance tended to decrease along with the attenuation of hyperactivation at one month and return to higher levels one year later (McDonald et al. 2012). Studies of longer-term survivors with a history of CTx have demonstrated lower activation in task-related regions (de Ruiter et al. 2011; Kesler et al. 2011). Prospective studies of pre-menopausal women who undergo abrupt estrogen suppression with gonadotropin releasing hormone (GnRH) agonists have shown globally attenuated task-related blood flow on PET in the context of maintained task performance (Berman et al. 1997), and decreased frontal fMRI activation during memory encoding with a trend toward worse performance on the subsequent recognition test (Craig et al. 2007). It is notable that in the GnRH agonist studies, estrogen is still being suppressed at the time of scanning, while at M1 in the current study, ovarian function may be starting to resume after CIA and several patients were using tamoxifen, which also affects estrogen function. All of the above studies measured regionally specific task-related activation, while the current study focuses on global levels of activation and deactivation, so direct comparison is difficult.
The effects of cancer and CTx on cognition and brain activity likely involve multiple biological pathways (Ahles and Saykin 2007), and the present results contribute to a better understanding of one key pathway, hormonal changes. Possible mechanisms for CTx-related cognitive dysfunction, including oxidative stress and genetic factors, overlap with those thought to be involved in the deleterious effects of estrogen loss (Stearns et al. 2006; Ahles and Saykin 2007; Henderson and Brinton 2010), suggesting possible compounding effects.
This study has several strengths. To our knowledge, this is the first neuroimaging study of CIA. The study is prospective, increasing confidence in attribution of brain effects to CIA. Two groups of HC were employed to match the CIA and post-menopausal CTx groups. Our analyses employed either an age covariate or data pre-adjusted for age, minimizing the effects of this potential confound. Comprehensive neuropsychological testing was conducted in order to determine domain-specific effects of treatment. Finally, the strong correlation in CIA between changes in neural activity and processing speed is promising for the behavioral relevance of these results.
This study also has several limitations. First, the sample sizes are modest, commensurate with the difficulty of recruiting BC patients prospectively for a time-consuming study. Pre- and peri-menopausal BC patients were included in the CIA group, potentially increasing variability. We relied on self-reported menstrual status at each session rather than hormone levels; future work correlating cognitive and imaging variables with hormone levels would be informative. Several of our BC patients used antidepressants and post-CTx anti-estrogen therapy, which is difficult to avoid in this population, and several post-menopausal HC participants were using hormone replacement therapy. Larger studies testing the neural effects of anti-estrogen treatments as well as previous hormone replacement therapy in the BC population will be informative. Finally, we report here objective neuropsychcological testing results; assessment of self-reported cognitive complaints would also be interesting in the context of CIA, as increased cognitive complaints have been reported in a range of patients undergoing breast cancer treatment (Pullens et al. 2010).
Subsequent studies should follow the effects of CIA longitudinally. While 80% of pre- and peri-menopausal BC patients experience CIA in the months immediately following CTx (Petrek et al. 2006; Minisini et al. 2009; Swain et al. 2009; Swain et al. 2010), only 20–60% remain amenorrheic 6–12 months later (Minisini et al. 2009; Sukumvanich et al. 2010; Ganz et al. 2011). Complicating matters, tamoxifen has been shown to potentiate CIA (Ganz et al. 2011), and post-menopausal CTx comparison groups will likely be using aromatase inhibitors, which may have different neural effects than tamoxifen. While estrogen is neurotrophic and neuroprotective in vitro, the effects of estrogen loss (and replacement) on the human brain are complicated and controversial (Turgeon et al. 2006; Sukumvanich et al. 2010), and CIA is no exception. Despite these complications, it will be important to determine the neural effects of CIA throughout survivorship. Other types of fMRI analyses (voxelwise, resting state, etc.) will also be informative, as estrogen influences both structural and functional connectivity (Peper et al. 2011). While BC patients cannot be treated with any agent that is an estrogen agonist to breast tissue, future intervention with other agents, such as specially designed “neuro-SERMs” (Zhao et al. 2005; Doncarlos et al. 2009), which might be estrogen antagonists to breast and uterus and estrogen agonists to brain and bone, may prove beneficial. The current findings have implications for risk appraisal and development of prevention or intervention strategies for cognitive changes in CIA.
Acknowledgements
This work was supported by the National Cancer Institute and National Institute on Aging at the National Institutes of Health (grant numbers R01 CA101318, R01 CA087845, R25 CA117865, and F30 AG 039959) and the Indiana Economic Development Corporation (grant number 87884).
References
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole B, Hanscom BS, Mulrooney TJ, Schwartz G, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve. Journal of Clinical Oncology. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94(16):8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox: presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. NeuroImage. 2002;16(2) [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30(4):212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FC, Roth RM, Saykin AJ, Beverly-Gibson G. A new measure of visual location learning and memory: development and psychometric properties for the Brown Location Test (BLT) Clin Neuropsychol. 2007;21(5):811–825. doi: 10.1080/13854040600878777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, Berman MG, Hayes DF, Noll DC, Peltier S, Welsh RC. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32(3):324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- Clement F, Belleville S. Compensation and disease severity on the memory-related activations in mild cognitive impairment. Biological Psychiatry. 2010;68(10):894–902. doi: 10.1016/j.biopsych.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging. 1996;17(1):123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Picchioni MM, Brammer M, Giampietro V, Rymer J, McGuire PK, Maki PM, Murphy DG. A study of visuospatial working memory pre- and post-Gonadotropin Hormone Releasing Hormone agonists (GnRHa) in young women. Horm Behav. 2008;54(1):47–59. doi: 10.1016/j.yhbeh.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Maki PM, Murphy DG, Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Maki PM, Murphy DGM. Reversibility of the effects of acute ovarian hormone suppression on verbal memory and prefrontal function in pre-menopausal women. Psychoneuroendocrinology. 2008;33(10):1426–1431. doi: 10.1016/j.psyneuen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Rymer J, Cutter WJ, Brammer M, Giampietro V, Wickham H, Maki PM, Murphy DG. Gonadotropin hormone releasing hormone agonists alter prefrontal function during verbal encoding in young women. Psychoneuroendocrinology. 2007;32(8–10):1116–1127. doi: 10.1016/j.psyneuen.2007.09.009. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Human Brain Mapping. 2011;32(8):1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan executive function system. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Californial verbal learning test, 2nd ed. adult version manual. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Doncarlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009;34(Suppl 1):S113–S122. doi: 10.1016/j.psyneuen.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JS, Jak AJ, Kniker JE, Rudick RA. Administration and Scoring Manual for the Multiple Sclerosis Functional Composite Measure (MSFC) National Multiple Sclerosis Society; 2001. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Land SR, Geyer CE, Cecchini RS, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff JA, Vogel VG, Erban JK, Livingston RB, Perez EA, Mamounas EP, Wolmark N, Swain SM. Menstrual History and Quality-of-Life Outcomes in Women With Node-Positive Breast Cancer Treated With Adjuvant Therapy on the NSABP B-30 Trial. Journal of Clinical Oncology. 2011;29(9):1110–1116. doi: 10.1200/JCO.2010.29.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14(3 Pt 2):572–579. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K, Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer. 2008;113(9):2431–2439. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, Munzel K. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109(9):1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J, Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104(10):2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, Shah E, Stein R, Whitehead S, Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94(6):828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim HSL, Phillips KM, Chait S, Faul LA, Popa MA, Lee Y-H, Hussin MG, Jacobsen PB, Small BJ. Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated With Standard-Dose Chemotherapy. Journal of Clinical Oncology. 2012;30(29):3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, O'Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68(11):1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafayette Instrument. Grooved pegboard: instruction/owner's manual. Lafayette, IN: Lafayette Instrument; 1989. [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in Brain Activation during Working Memory Processing Associated with Breast Cancer and Treatment: A Prospective Functional MRI Study. Journal of Clincal Oncology. 2012 doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Minisini AM, Menis J, Valent F, Andreetta C, Alessi B, Pascoletti G, Piga A, Fasola G, Puglisi F. Determinants of recovery from amenorrhea in premenopausal breast cancer patients receiving adjuvant chemotherapy in the taxane era. Anticancer Drugs. 2009;20(6):503–507. doi: 10.1097/CAD.0b013e3283243df3. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Backman L, Lindenberger U, Heekeren HR. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci. 2011;23(8):2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- O'Brien JL, O'Keefe KM, Laviolette PS, Deluca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74(24):1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RCW, Pol HEH, van Honk J. Sex steroids and connectivity in the human brain: A review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, Sukumvanich P. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24(7):1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology. 2010;19(11):1127–1138. doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS, Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FSAM. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. Journal of the National Cancer Institute. 2006;98(23):1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- Stearns V, Schneider B, Henry NL, Hayes DF, Flockhart DA. Breast cancer treatment and ovarian failure: risk factors and emerging genetic determinants. Nat Rev Cancer. 2006;6(11):886–893. doi: 10.1038/nrc1992. [DOI] [PubMed] [Google Scholar]
- Sukumvanich P, Case LD, Van Zee K, Singletary SE, Paskett ED, Petrek JA, Naftalis E, Naughton MJ. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer. 2010;116(13):3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- Swain SM, Jeong JH, Geyer CE, Jr, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff J, Vogel VG, Erban JK, Rastogi P, Livingston RB, Perez EA, Mamounas EP, Land SR, Ganz PA, Wolmark N. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Land SR, Ritter MW, Costantino JP, Cecchini RS, Mamounas EP, Wolmark N, Ganz PA. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113(2):315–320. doi: 10.1007/s10549-008-9937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation. WAIS-III Wechsler memory scale, 3rd ed., WMS Wechsler memory scale 3rd ed., Updated technical manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- The Psychological Corporation. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27(6):575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- Vearncombe KJ, Pachana NA. Is cognitive functioning detrimentally affected after early, induced menopause? Menopause. 2009;16(1):188–198. doi: 10.1097/gme.0b013e3181775eb4. [DOI] [PubMed] [Google Scholar]
- Vearncombe KJ, Rolfe M, Andrew B, Pachana NA, Wright M, Beadle G. Cognitive effects of chemotherapy-induced menopause in breast cancer. Clin Neuropsychol. 2011;25(8):1295–1313. doi: 10.1080/13854046.2011.631586. [DOI] [PubMed] [Google Scholar]
- Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24(36):5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- Warne GL, Fairley KF, Hobbs JB, Martin FI. Cyclophosphamide-induced ovarian failure. N Engl J Med. 1973;289(22):1159–1162. doi: 10.1056/NEJM197311292892202. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test (WRAT3): Administration Manual. Wilmington, DE: Wide Range, Inc.; 1993. [Google Scholar]
- Zhao L, O'Neill K, Diaz Brinton R. Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. [Review] [208 refs] Brain Research Brain Research Reviews. 2005;49(3):472–493. doi: 10.1016/j.brainresrev.2005.01.009. [DOI] [PubMed] [Google Scholar]