Abstract
Background
Delayed entry of blood culture bottles is inevitable when microbiological laboratories do not operate for 24 hr. There are few studies reported for prestorage of these bottles. The growth dynamics of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa were investigated with respect to various preincubation conditions.
Methods
Fifteen or 150 colony-forming units (CFU) of bacteria were inoculated into standard aerobic or anaerobic blood culture bottles. Bottles were preincubated at 25℃ or 37℃ for 0, 2, 4, 8, 12, 24, or 48 hr. The time to detection (TTD) then was monitored using the BacT/Alert 3D system (bioMerieux Inc., USA).
Results
Significant difference in TTD was observed following preincubation for 8 hr at 25℃ vs. 4 hr at 37℃ for S. aureus, 4 hr at 25℃ vs. 4 hr at 37℃ for E. coli, 12 hr at 25℃ vs. 4 hr at 37℃ for P. aeruginosa, compared to no preincubation (P<0.005). TTD values did not vary significantly with bacterial CFU or with aerobic or anaerobic bottle type. The BacT/Alert 3D system returned false negatives following preincubation of P. aeruginosa for 48 hr at 25℃ or 24 hr at 37℃.
Conclusions
TTD was mainly affected by preincubation temperature and duration rather than by input CFU quantity or bottle type for the 3 experimental bacteria.
Keywords: Blood culture, Detection, Storage, Preincubation
INTRODUCTION
The recent introduction of continuous blood culture monitoring systems has enabled early detection of bacteremia. CLSI guidelines for blood culture procedures recommend transporting samples within 2 hr [1]; however, microbiological laboratories that are unable to operate continuously (for example at nights and weekends) may opt for delayed insertion of blood culture bottles into monitoring systems, thereby increasing the time from blood collection to the detection of microorganisms in bacteremia patients [2]. The time to detection (TTD) is defined as the period from the insertion of a blood culture bottle into a monitoring instrument to the detection of microorganisms. It may be possible to reduce the TTD by storing culture bottles at 37℃; this could lead to shorter turnaround times for bacteremia diagnoses.
Staphylococcus aureus and Escherichia coli are the most common pathogens causing sepsis. Sepsis resulting from Pseudomonas aeruginosa infections may cause serious complications among immunologically compromised patients. Knowledge is lacking regarding the growth dynamics of these microorganisms as a function of preincubation conditions. We investigated the effect of blood culture bottle pre-storage on the TTD by using these bacteria monitored with the BacT/Alert 3D system (bioMerieux Inc., Durham, NC, USA).
METHODS
The effects of preincubation of blood culture bottles under aerobic or anaerobic conditions were investigated using S. aureus (ATCC 29213), E. coli (ATCC 25922), and P. aeruginosa (ATCC 27853) inocula at concentrations of 150 colony-forming units (CFU)/mL or 15 CFU/mL in brain-heart infusion broth. Experiments were conducted in triplicate on separate days. For 15 CFU/mL input concentrations, the observed mean CFU values were 14 for S. aureus (12, 15, and 16 CFU), 16 for E. coli (14, 17, and 18 CFU), and 17 for P. aeruginosa (13, 17, and 20 CFU). For S. aureus and E. coli, 1 mL of each preparation was inoculated into standard aerobic (SA) and standard anaerobic (SN) bottles (bioMerieux Inc.), whereas for P. aeruginosa, 1 mL of preparation was inoculated into aerobic bottles only. Bottles were preincubated at 25℃ or 37℃ for 0, 2, 4, 8, 12, 24, or 48 hr. Subsequently, bottles were inserted to the BacT/Alert 3D system until a positive signal was detected. TTD values were compared across bacterial concentrations, preincubation times, bottle types, and storage temperatures.
The Mann-Whitney U test was used for nonparametric analyses of mean TTD differences between each preincubation time and direct insertion into the BacT/Alert 3D system (0 hr preincubation). Bonferroni correction (P=0.05/6=0.0083) was applied to adjust for multiple comparisons between each preincubation time and direct entry. The Kruskal-Wallis test was used to examine differences in the mean TTD values across all preincubation times. All statistical analyses were two-sided and were performed using IBM SPSS v.20.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
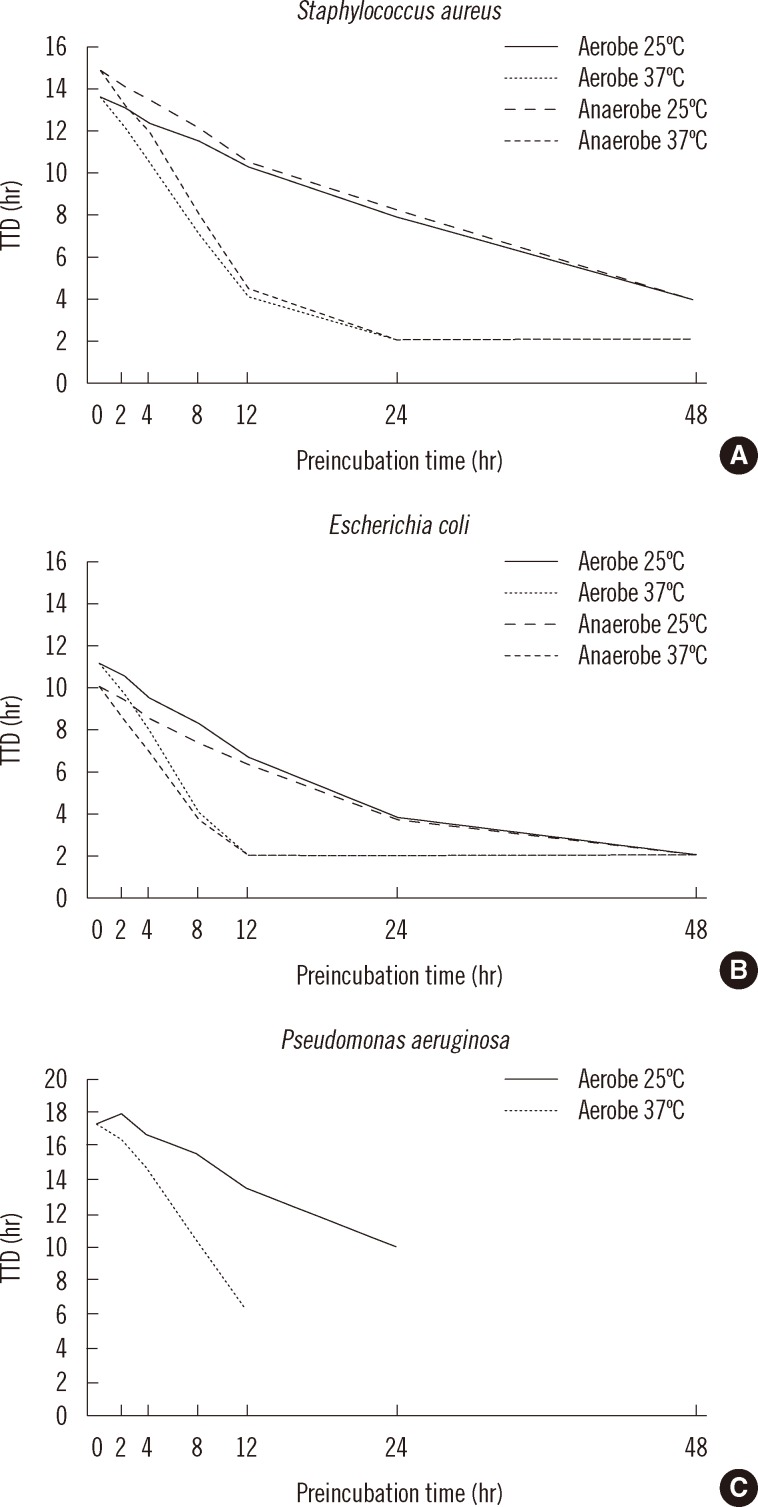
The TTD values for triplicate growth experiments under various storage conditions were consistent. The different input concentrations of S. aureus (150 CFU/mL vs. 15 CFU/mL) resulted in similar TTD values under aerobic conditions (12.6 hr vs. 13.7 hr, respectively, P=0.10) and under anaerobic conditions (13.4 hr vs. 14.9 hr, respectively, P=0.10). Significant differences in TTD values were detected after 8 hr of preincubation at 25℃ and 4 hr of preincubation at 37℃, in both aerobic and anaerobic bottles compared with immediate insertion (0 hr preincubation) (P=0.002, all comparisons) (Fig. 1A).
Fig. 1.
TTD values for (A) Staphylococcus aureus, (B) Escherichia coli, and (C) Pseudomonas aeruginosa preincubated in aerobic and anaerobic blood culture bottles at 25℃ or 37℃ prior to insertion into the BacT/Alert 3D system. False negatives were detected after 48 hr of prestorage at 25℃ and after 24 hr of preincubation at 37℃ for P. aeruginosa. Bacterial input concentration was 15 colony-forming units/mL.
Abbreviation: TTD, time to detection.
Although E. coli grew faster than S. aureus, the growth dynamics of the 2 species displayed a similar pattern. In contrast to S. aureus, the TTD values for E. coli decreased in anaerobic bottles compared with aerobic bottles (9.9 hr vs. 11.0 hr at 150 CFU/mL input), but the difference was not statistically significant. Significant differences in TTD values were observed following 4 hr of preincubation at 25℃ in both aerobic and anaerobic bottles (P<0.004) or at 37℃ under both aerobic and anaerobic conditions (P=0.002) compared with direct insertion of bottles (Fig. 1B).
P. aeruginosa exhibited a different TTD pattern from those of S. aureus and E. coli. No positive signals were detected for bottles preincubated for 48 hr at 25℃ or for 24 hr at 37℃ (Fig. 1C). Significant differences in TTD values were observed after a 12 hr preincubation at 25℃ or a 4 hr preincubation at 37℃ under aerobic condition compared to immediate insertion of the bottles (both P=0.004) (Fig. 1C). The dynamic patterns of TTD at 150 CFU/mL were similar to those at 15 CFU/mL for the 3 bacterial species (data not shown).
DISCUSSION
S. aureus, E. coli, and P. aeruginosa are the 3 most important pathogens detected in patients with sepsis. Typical bacterial concentrations identified during a bacteremic episode are in the range of 1-10 CFU/mL [3]; we examined input concentrations of both 150 CFU/mL and 15 CFU/mL. Although our simulated bacteremia model did not correspond directly to sepsis concentrations, it allows for inferences regarding bacterial growth dynamics at different storage temperatures, bacterial concentrations, preincubation times, and bottle types (aerobic or anaerobic). For each input concentration, 42 blood culture bottles were assayed (2 bottle types×7 time points×3 replicates). Although a 10-fold difference existed between input concentrations, there was no significant differences in TTD values for all of the 3 bacterial strains included in this study. Storage temperature and preincubation time were the major factors influencing TTD.
The bottles containing S. aureus and E. coli cultures preincubated at 37℃ for 24-48 hr were accurately detected using the BacT/Alert 3D system. However, bottles containing P. aeruginosa preincubated for 48 hr at 25℃ or for 24 hr at 37℃ returned false-negative results. TTD values corresponding to fully-grown S. aureus and E. coli were generally less than 2 hr. P. aeruginosa exhibited the lowest growth rate, whereas E. coli showed the highest growth rate. Our TTD results exhibited similar patterns to those observed in a previous study using FAN bottles containing 15 CFU/mL input bacteria [4]. We assumed that the transport of bottles and insertion into monitoring instruments would require a turnaround time of 2-4 hr during working hours and a turnaround time of 8-24 hr otherwise. A storage duration of 48 hr is unlikely, but may occur with transfer to a reference laboratory. Significant growth enhancements were observed beginning with the 4 hr preincubation at 37℃ and the 4-8 hr preincubation at 25℃ for S. aureus and E. coli, suggesting that bottle storage at 37℃ could lower the TTD values for these bacteria when entering is delayed. van der Velden et al. [5] reported a decrease in TTD with preincubation at 37℃ in a clinical setting. Kerremans et al. [6] recommended the installation of continuously monitoring blood culture incubators outside of microbiological laboratories not offering 24 hr service to reduce turnaround time and accelerate antibiotic switching.
However, a study examining preincubation effects on 15 clinical isolates inoculated into 468 bottles reported that false-negative results were more common for bottles stored at 37℃ (21 false negatives) compared with room temperature (11 false negatives) [7]. Sautter et al. [7] proposed the definition of "delayed entry" for bottles that are held for more than 24 hr at 4℃ or room temperature, or for more than 12 hr at 37℃, because false-negative results were more prominent after these preincubation times. Our findings obtained using P. aeruginosa were consistent with these results. P. aeruginosa was more affected by delayed entry than S. aureus or E. coli. It appears that bottles should be inserted into the instruments within 24 hr, when pre-stored at room temperature, or within 12 hr, when preincubated at 37℃, to avoid false-negative results for P. aeruginosa obtained using the BacT/Alert 3D system. In the experiments using S. aureus and E. coli, no detection failure was noted, even with a 48 hr preincubation period, in contrast to the observation in a previous study [8]. This may be due to the fact that we used only 1 strain of each bacterium.
Klaerner et al. [9] reported a failure of the BacT/Alert system to detect non-fermentative Gram-negative bacteria (for example, P. aeruginosa and Acinetobacter baumannii) when FAN bottles were preincubated for 8 hr at 36℃. A study examining P. aeruginosa (<7 CFU/mL) growth in BacT/Alert FA-medium reported several false negatives following preincubation at 36℃ for 24 hr [10]. Notably, previous reports regarding the effect of delayed entry or preincubation on TTD have involved various storage temperatures, bacterial input concentrations, incubation times, and types of bottles/instruments. Very few studies have used standard bottles (SA and SN) in the BacT/Alert 3D system or monitored TTDs continuously according to preincubation times.
Our study had several limitations. First, we used only 3 ATCC bacterial strains. Data derived from these bacteria will be different from those of clinical isolates; therefore, further evaluation should be performed with more diverse clinical isolates. In addition, human blood should have been added to the medium to produce conditions more similar to clinical blood culture. Second, the input bacterial concentrations used here (15-150 CFU/mL) exceeded those typical of bacteremic patients; this may have biased TTD dynamics in comparison to sepsis patients.
In conclusion, TTD values for the ATCC strains of S. aureus, E. coli, and P. aeruginosa were profoundly affected by changes in preincubation temperature and storage times at input concentrations of 15 or 150 CFU/mL. In contrast, quantity of bacterial inocula or aerobic/anaerobic bottle type did not significantly affect TTD dynamics for these bacteria.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education, Science, and Technology (2011-0008757). The authors thank Dr. Rock-Bum Kim (Medical School, Dong-A University) for his excellent assistance with statistical analyses.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Clinical and Laboratory Standards Institute. Principles and procedures for blood cultures; approved guidline. CLSI document M47-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. [Google Scholar]
- 2.Saito T, Iinuma Y, Takakura S, Nagao M, Matsushima A, Shirano M, et al. Delayed insertion of blood culture bottles into automated continuously monitoring blood culture systems increases the time from blood sample collection to the detection of microorganisms in bacteremic patients. J Infect Chemother. 2009;15:49–53. doi: 10.1007/s10156-008-0664-6. [DOI] [PubMed] [Google Scholar]
- 3.Reimer LG, Wilson ML, Weinstein MP. Update on detection of bacteremia and fungemia. Clin Microbiol Rev. 1997;10:444–465. doi: 10.1128/cmr.10.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viganò EF, Vasconi E, Agrappi C, Clerici P. Use of simulated blood cultures for time to detection comparison between BacT/ALERT and BACTEC 9240 blood culture systems. Diagn Microbiol Infect Dis. 2002;44:235–240. doi: 10.1016/s0732-8893(02)00451-0. [DOI] [PubMed] [Google Scholar]
- 5.van der Velden LB, Vos FJ, Mouton JW, Sturm PD. Clinical impact of preincubation of blood cultures at 37℃. J Clin Microbiol. 2011;49:275–280. doi: 10.1128/JCM.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerremans JJ, van der Bij AK, Goessens W, Verbrugh HA, Vos MC. Immediate incubation of blood cultures outside routine laboratory hours of operation accelerates antibiotic switching. J Clin Microbiol. 2009;47:3520–3523. doi: 10.1128/JCM.01092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sautter RL, Bills AR, Lang DL, Ruschell G, Heiter BJ, Bourbeau PP. Effects of delayed-entry conditions on the recovery and detection of microorganisms from BacT/ALERT and BACTEC blood culture bottles. J Clin Microbiol. 2006;44:1245–1249. doi: 10.1128/JCM.44.4.1245-1249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akan OA, Yildiz E. Comparison of the effect of delayed entry into 2 different blood culture systems (BACTEC 9240 and BacT/ALERT 3D) on culture positivity. Diagn Microbiol Infect Dis. 2006;54:193–196. doi: 10.1016/j.diagmicrobio.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Klaerner HG, Eschenbach U, Kamereck K, Lehn N, Wagner H, Miethke T. Failure of an automated blood culture system to detect nonfermentative gram-negative bacteria. J Clin Microbiol. 2000;38:1036–1041. doi: 10.1128/jcm.38.3.1036-1041.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seegmüller I, Eschenbach U, Kamereck K, Miethke T. Sensitivity of the BacT/ALERT FA-medium for detection of Pseudomonas aeruginosa in pre-incubated blood cultures and its temperature-dependence. J Med Microbiol. 2004;53:869–874. doi: 10.1099/jmm.0.45533-0. [DOI] [PubMed] [Google Scholar]