Abstract
Background
Clarithromycin, amoxicillin, metronidazole, tetracycline, and levofloxacin have been commonly used for the eradication of Helicobacter pylori. We compared the change in antibiotic resistance of H. pylori strains during two separate periods and investigated the effect of antibiotic resistance on H. pylori eradication.
Methods
H. pylori strains were isolated from 71 patients between 2009 and 2010 and from 94 patients between 2011 and 2012. The distribution of minimal inhibitory concentration (MIC) of 5 antibiotics was assessed using the agar dilution method, and H. pylori eradication based on the antimicrobial susceptibility of the isolates was investigated retrospectively.
Results
Antibiotic resistance rate against clarithromycin, amoxicillin, tetracycline, metronidazole, and levofloxacin for the 2009-2010 isolates were 7.0% (5/71), 2.8% (2/71), 0% (0/71), 45.1% (32/71), and 26.8% (19/71), respectively, and for the 2011-2012 isolates were 16.0% (15/94), 2.1% (2/94), 0% (0/94), 56.3% (53/94), and 22.3% (21/94), respectively. Multi-drug resistance for 2 or more antibiotics increased slightly from 16.9% (12/71) in the 2009-2010 isolates to 23.4% (22/94) in the 2011-2012 isolates. In follow-up testing of 66 patients, first-line treatment successfully eradicated H. pylori in 50 patients (75.8%) and failed in 4 of 7 patients (57.1%) in a clarithromycin-resistant and amoxicillin-susceptible group.
Conclusions
We observed an increase in resistance to clarithromycin and an overall increase in multi-drug resistance during the 2 study periods. The effectiveness of the eradication regimen was low with combinations of clarithromycin and amoxicillin, particularly in the clarithromycin-resistant group. Thus, eradication of H. pylori depends upon periodic monitoring of antimicrobial susceptibility.
Keywords: Helicobacter pylori, Antibiotic resistance, Eradication
INTRODUCTION
Eradication of gastric colonies of Helicobacter pylori helps heal gastritis and peptic ulcer disease and has beneficial effects on the regression of atrophic gastritis and the prevention of distal gastric cancer [1, 2]. Triple therapy using a proton pump inhibitor (PPI) with clarithromycin and amoxicillin or metronidazole is recommended as the first-line treatment regimen for H. pylori eradication. If it fails, bismuth-containing quadruple therapy, which involves inclusion of additional antibiotics to the first-line treatment regimen is used [3, 4]. The increase in clarithromycin resistance in Korea is considered to be closely related to the decrease of eradication rate in first-line therapy. According to recent data, clarithromycin resistance sharply increased from 16.7% to 38.5% from 2003 through 2009, and eradication rates have decreased by 77-87% since 2003 [4-6]; these rates are inclusive of regional and institutional differences.
Although regular antibiotic resistance monitoring is important in the clinical setting, the labor-intensive and time-consuming nature of H. pylori isolation from clinical samples complicates comparative antibiotic susceptibility testing. In this study, we investigated H. pylori antibiotic resistance and its effect on eradication rates in a single center in Korea between 2009-2010 and 2011-2012.
METHODS
1. Patients
H. pylori strains were isolated from 71 patients with H. pylori infections from July 2009 to December 2010 and from 94 patients from June 2011 through December 2012 at the Yongin Severance Hospital of Yonsei University, Korea. Of these patients, 66 (clinical characteristics listed in Table 1) had previously undergone eradication treatments, including week-long first-line treatment with PPI (pantoprazole or esomeprazole 30 mg, bid), amoxicillin (2,250 mg, tid), clarithromycin (1,000 mg, bid). First-line therapy failed in 16 patients, and they were subjected to second-line treatment with PPI (30 mg, bid), bismuth (300 mg, bid), metronidazole (2,250 mg, bid), and tetracycline (1,000 mg, qid). Eradication of H. pylori was verified by a negative result in a 13C-urea breath test (Isotechnika, Alberta, Canada) after at least 4 weeks of drug administration.
Table 1.
Clinical characteristics of patients with a history of eradication treatments
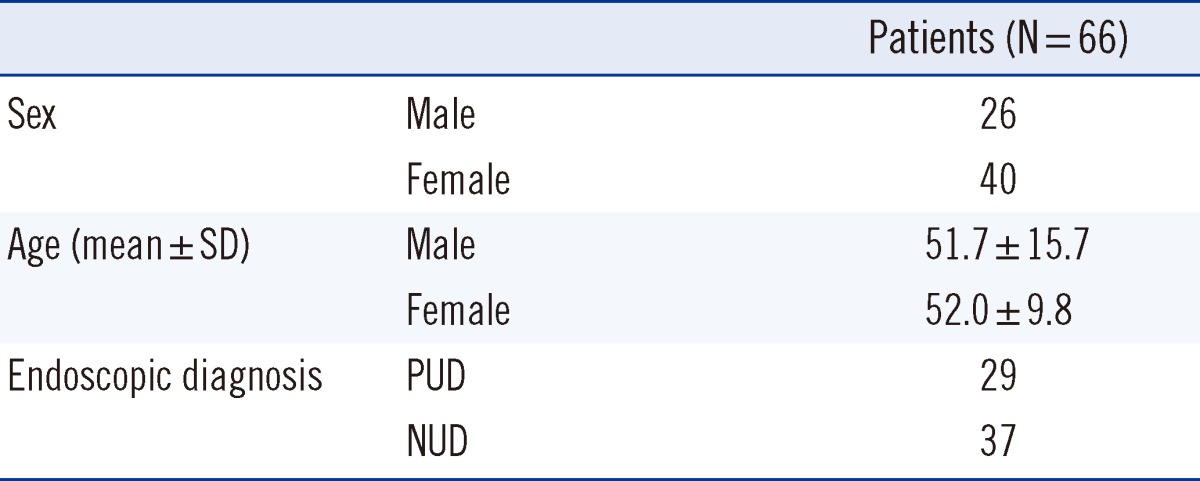
Values are presented as number or mean±SD.
Abbreviations: PUD, peptic ulcer disease; NUD, non ulcer disease.
This study was conducted retrospectively to follow up the results of eradication of H. pylori on the basis of antimicrobial susceptibility of the isolates, and it did not interfere with patient management decisions. The study was approved by the Institutional Review Board of Yonsei University College of Medicine (No. 4-2011-0508). Written informed consent was provided by all patients at the time of their first visit to the hospital.
2. H. pylori culture
The culture medium used in this study was composed of Brucella broth (BBL, Sparks, MD, USA) containing 1.2% agar, 10% bovine serum, and selected antibiotics (Oxoid Limited, Hampshire, England) (10 µg/mL vancomycin, 5 µg/mL trimethoprim, 5 µg/mL cefsulodin, and 5 µg/mL amphotericin B). Completely minced gastric biopsy specimens were incubated under 10% CO2, 5% O2, and 100% humidity at 37℃ for 3-5 days. Strains were identified as H. pylori by Gram staining; colony morphology analysis; and oxidase, catalase, and urease tests. The H. pylori ATCC 43504 strain was cultured as a standard using the same methods described above for quality control assessment.
3. Susceptibility tests
The minimal inhibitory concentrations (MICs) for clarithromycin (Sigma-Aldrich Co., St. Louis, MO, USA), amoxicillin (Sigma-Aldrich), tetracycline (Sigma-Aldrich), metronidazole (Sigma-Aldrich), and levofloxacin (Sigma-Aldrich) were determined using a slightly modified agar dilution method (using Brucella broth base containing 1.2% agar). Clarithromycin resistance was defined according to the CLSI-approved breakpoint (≥1 µg/mL) [7]. Isolates were defined as resistant to amoxicillin, tetracycline, metronidazole, and levofloxacin, when MICs were ≥1, ≥4, ≥8, and ≥1 µg/mL, respectively [8-10].
For H. pylori ATCC 43504, the MIC ranges for clarithromycin, amoxicillin, metronidazole, tetracycline, and levofloxacin were 0.016-0.125 µg/mL, 0.016-0.125 µg/mL, 64-256 µg/mL, 0.125-1 µg/mL, and 0.064-0.5 µg/mL, respectively.
4. Statistical analysis
Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences version 18.0; SPSS Ins., Chicago, IL, USA). Data of antibiotic resistance were analyzed using the student t test and Chi-square test. P<0.05 was considered statistically significant.
RESULTS
1. Antibiotic resistance of H. pylori
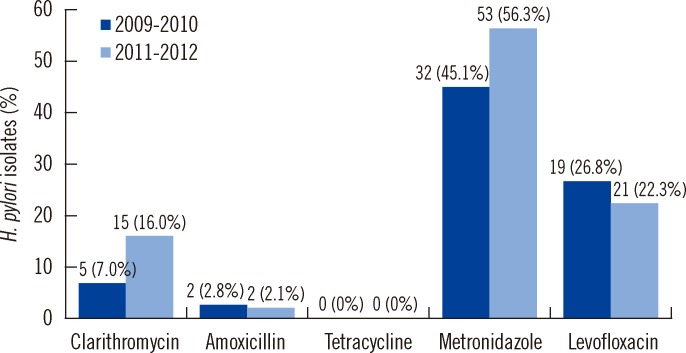
The antibiotic resistance rates for the isolates from the 2009-2010 group against clarithromycin, amoxicillin, tetracycline, metronidazole, and levofloxacin were 7.0% (5/71), 2.8% (2/71), 0% (0/71), 45.1% (32/71), and 26.8% (19/71), respectively, and those for the isolates from the 2011-2012 group were 16.0% (15/94), 2.1% (2/94), 0% (0/94), 56.3% (53/94), and 22.3% (21/94), respectively. The rate of H. pylori resistance to clarithromycin and metronidazole increased from 7.0% to 16.0% and from 45.1% to 56.3% for the 2 periods, respectively (Fig. 1), although the increase was not statistically significant.
Fig. 1.
Antibiotic resistance in H. pylori strains isolated during 2009-2010 and 2011-2012. Increased antibiotic resistance was notable in clarithromycin and metronidazole, although there was no statistical difference.
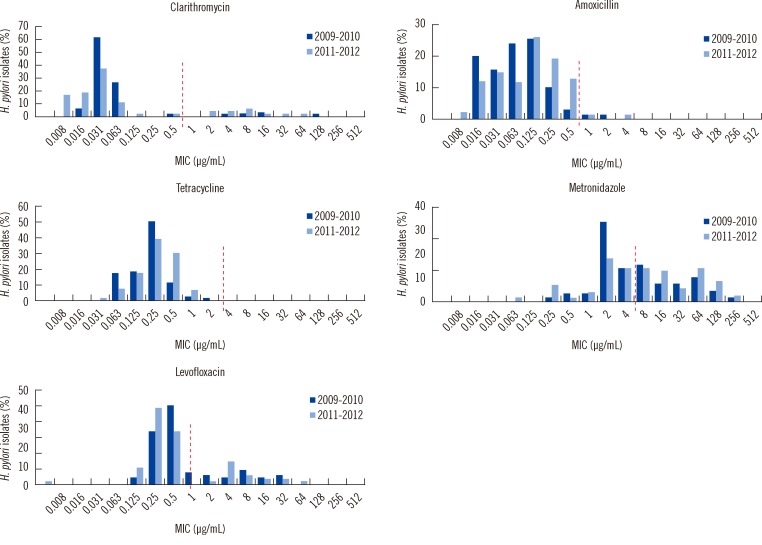
When the MIC distribution profiles for the 2 study periods were compared, the MICs for clarithromycin showed notable differences between the susceptible and resistant strains (Fig. 2). The MIC of the clarithromycin-susceptible strains was less than 0.125 µg/mL. Resistance to tetracycline was not detected in any strain (based on a cut-off of ≥ 4 µg/mL). The MIC range of tetracycline was 0.031-2 µg/mL. The MIC of metronidazole varied widely (8-256 µg/mL). Multi-drug resistance for 2 or more antibiotics was more frequent in the isolates from 2011-2012 (23.4%, 22/94) than in the isolates from 2009-2010 (16.9%, 12/71), but there was no statistical significance (P<0.082). Only 1 strain exhibited multi-drug resistance to clarithromycin, amoxicillin, metronidazole, and levofloxacin (Table 2).
Fig. 2.
Distribution of minimal inhibitory concentration (MIC) for Helicobacter pylori isolates. The dotted line indicates the break point for each antibiotic.
Table 2.
Comparison of multi-drug resistance over 2 time periods*
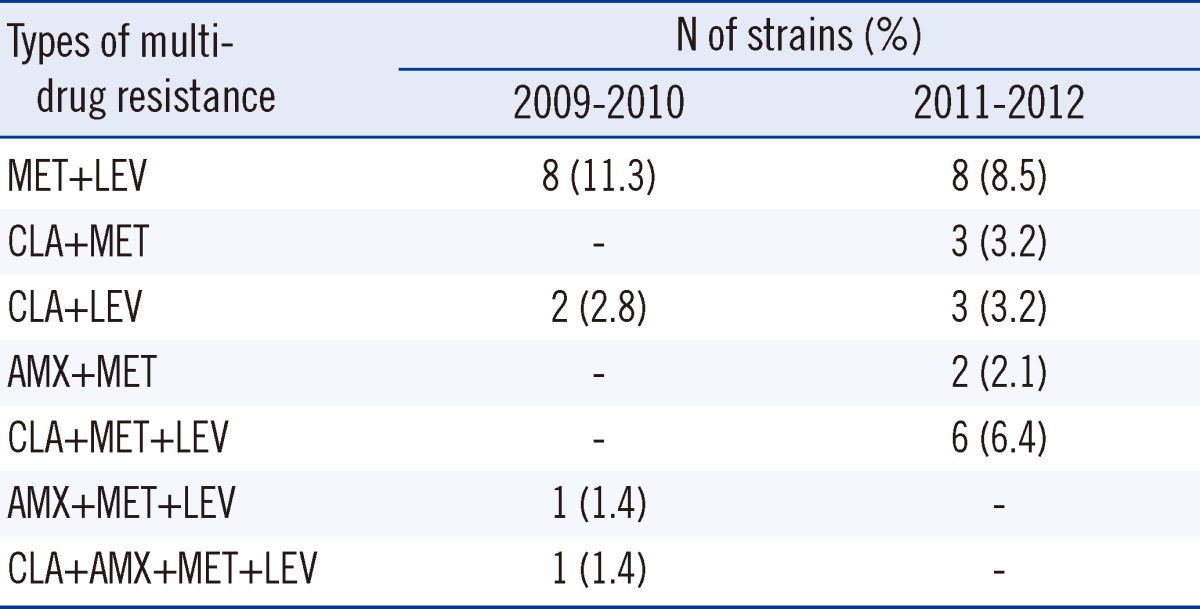
*Isolates were defined as resistant to clarithromycin (CLA), amoxicillin (AMX), metronidazole (MET), and levofloxacin (LEV), when the MICs were ≥1, ≥1, ≥8 and ≥1 µg/mL, respectively.
2. Effect of antibiotic resistance on H. pylori eradication rates
Of the 165 patients studied during the 2009-2012 period, 66 patients who were subjected to the first-line therapy were followed up for H. pylori eradication after treatment. Among these 66 patients, no significant differences were found with respect to sex, age, and endoscopic diagnosis. Eradication of H. pylori was successful in 50 of these 66 patients (75.8%). The effects of antibiotic resistance on H. pylori eradication rates are shown in Table 3. Eradication rates were 79.3% (46/58) for the clarithromycin-susceptible and amoxicillin-susceptible strains, and 100% (1/1) for the clarithromycin-susceptible and amoxicillin-resistant strains. A significant difference was observed between the eradication rates for the clarithromycin-resistant (42.9%, 3/7) and the clarithromycin-sensitive (79.7%, 47/59) strains (P<0.001) .
Table 3.
Effects of antibiotic resistance on Helicobacter pylori eradication
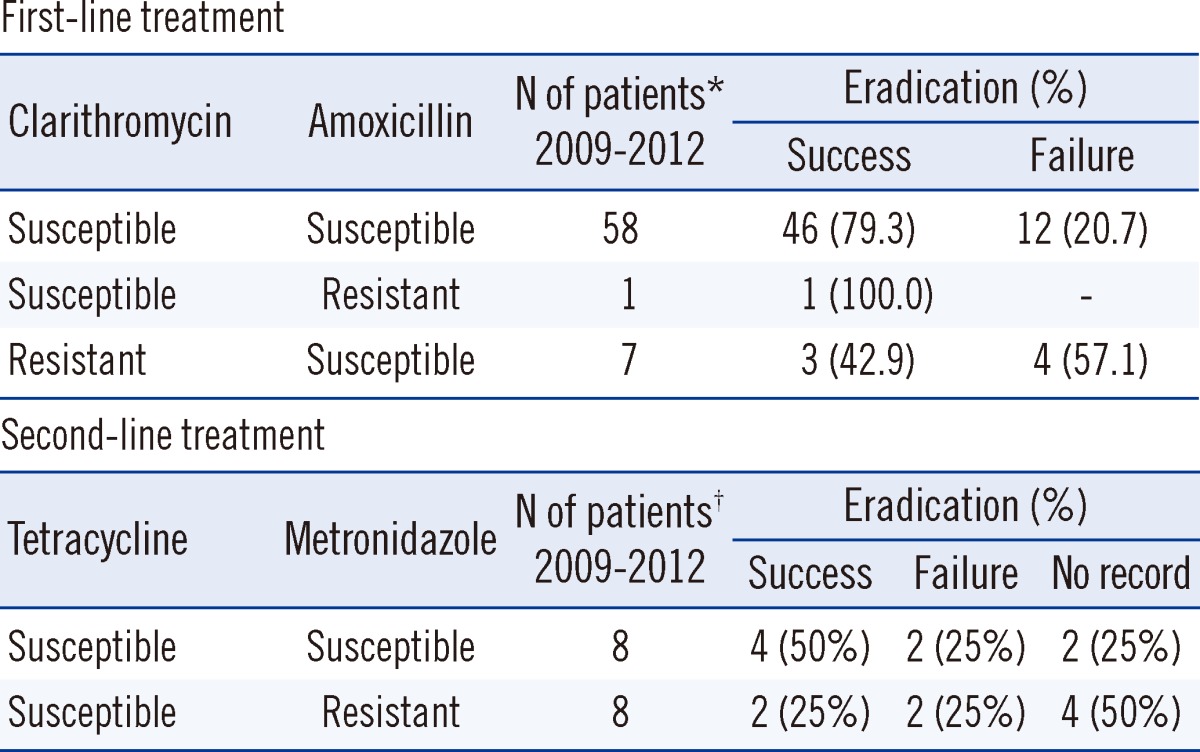
Isolates were defined as resistant to clarithromycin, amoxicillin, tetracycline and metronidazole when the MICs were ≥1, ≥1, ≥4 and ≥8 µg/mL, respectively. An eradication regimen included *first-line treatment with PPI, clarithromycin, and amoxicillin, and †second-line treatment with bismuth, tetracycline, and metronidazole.
Second-line therapy was prescribed for the 16 patients in whom first-line therapy failed. The eradication rates for the tetracycline-susceptible and metronidazole-susceptible strains and the tetracycline-susceptible and metronidazole-resistant strains were 50.0% (4/8) and 25.0% (2/8), respectively (P<0.32).
DISCUSSION
Recently, H. pylori eradication rates of 70-95% have been reported [4-6]. Failure of eradication may be attributed to increase in antibiotic resistance associated with problems in patient compliance, such as difficulties in taking drugs, or side effects [11-13]. In this study, the antimicrobial susceptibility test was conducted for H. pylori strains isolated from a single center over 2 periods, followed by examination of the factors affecting failure.
Clarithromycin resistance rates increased from 7.0% in the 2009-2010 patient group to 16.0% in the 2011-2012 patient group. These rates are slightly lower than that reported in a previous study, which showed that the overall frequency of clarithromycin-resistant H. pylori in 2008 was 21.6% [14]. This discrepancy is conceivably attributable to regional differences in the location of the studies. The primary factor influencing clarithromycin resistance is known to be the A2142-4 point mutation in the 23S rRNA [14-17].
Amoxicillin resistance rates decreased slightly from 2.8% (2009-2011) to 2.1% (2011-2012). Resistance to tetracycline was not detected in any strain when the cut-off was set at ≥ 4 µg/mL, and the MICs were as low as 0.031-2 µg/mL. Recently, tetracycline resistance rates of 0-36% have been reported. However, as with clarithromycin resistance, the differences could be due to regional differences [18, 19]. Metronidazole resistance rates were higher than those for all other antibiotics, ranging from 45.1% in the 2009-2010 group to 56.3% in the 2011-2012 group, and the MIC of metronidazole was the highest among all the studied antibiotics (8-256 µg/mL). Levofloxacin resistance rates decreased slightly from 26.8% in 2009-2010 to 23.3% in 2011-2012. This finding is consistent with that reported in a previous domestic study (resistance rates decreased from 26.3% to 22.5%) [18, 19]. The continuous increase in levofloxacin resistance warrants the use of rescue therapy based on the results of antimicrobial susceptibility tests. Although differences in resistance rates to the 5 antibiotics in the 2 study periods failed to reach statistical significance, increases in the resistance to clarithromycin and metronidazole were identified. Moreover, multi-drug resistance for 2 or more antibiotics increased from 16.9% (12/71) in 2009-2010 group to 23.4% (22/94) in the 2011-2012 group, but there was no statistical significance (P<0.082).
The overall eradication rate in patients who received first-line therapy with clarithromycin and amoxicillin was 75.8% (50/66), ranging from 78.1% in 2009-2010 to 73.5% in 2011-2012 (data not shown). The eradication rate for clarithromycin-resistant strains (42.9%, 3/7) was significantly lower than that for the clarithromycin-susceptible strains (79.7%, 47/59) (P<0.001). These results indicate that resistance to clarithromycin is a critical factor in the effectiveness of eradication with the first-line regimen.
Complete eradication rate was only 79.3% in strains susceptible to both clarithromycin and amoxicillin with first-line therapy (Table 3). Thus failure rate of 20.7% may be attributable to problems with patient compliance; however, a more extensive follow-up survey is needed to confirm it. Eradication was successful in 6 of 16 patients, who received second-line therapy including tetracycline and metronidazole; second-line therapy failed in 4 patients, and data were unavailable for the remaining 6 patients. Previous treatment histories of the 16 patients in the second-line treatment group were as follows: clarithromycin treatment for eradication of H. pylori, 2 patients; treatment for liver cirrhosis, 1 patient; and poor compliance, 1 patient. Specific histories were unavailable for the remaining 12 patients. Although failure of eradication is generally linked to antibiotic resistance, increase in antibiotic resistance does not always correlate with decrease in eradication rates; therefore, further studies are required to identify other factors affecting eradication rates.
In conclusion, the effectiveness of eradication using first-line therapy with clarithromycin and amoxicillin decreased, especially in the clarithromycin-resistant group, and clarithromycin resistance was considered crucial for the eradication of H. pylori. This result suggested that eradication of H. pylori is greatly dependent on periodic monitoring of antimicrobial susceptibility, which is necessary for selection of an appropriate antibiotic regimen.
Acknowledgements
This work was supported by the Cooperative Research Program (Project No. PJ907017042012 and PJ907017022012), Rural Development Administration, Republic of Korea.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Sugiyama T, Sakaki N, Kozawa H, Sato R, Fujioka T, Satoh K, et al. Sensitivity of biopsy site in evaluating regression of gastric atrophy after Helicobacter pylori eradication treatment. Aliment Pharmacol Ther. 2002;16(Suppl 2):187–190. doi: 10.1046/j.1365-2036.16.s2.17.x. [DOI] [PubMed] [Google Scholar]
- 2.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009;54:269–278. doi: 10.4166/kjg.2009.54.5.269. [DOI] [PubMed] [Google Scholar]
- 5.Hwang TJ, Kim N, Kim HB, Lee BH, Nam RH, Park JH, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010;44:536–543. doi: 10.1097/MCG.0b013e3181d04592. [DOI] [PubMed] [Google Scholar]
- 6.Choi YS, Cheon JH, Lee JY, Kim SG, Kim JS, Kim N, et al. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006;48:156–161. [PubMed] [Google Scholar]
- 7.Na HS, Hong SJ, Yoon HJ, Maeng JH, Ko BM, Jung IS, et al. Eradication rate of first-line and second-line therapy for Helicobacter pylori infection, and reinfection rate after successful eradication. Korean J Gastroenterol. 2007;50:170–175. [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Eighteenth informational supplement, M2-A9, M7-A7, and M100-S18. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 9.Glupczynski Y, Mégraud F, Lopez-Brea M, Andersen LP. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 2001;20:820–823. doi: 10.1007/s100960100611. [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Kim JS, Kim N, Jung HC, Song IS. Distribution of fluoroquinolone MICs in Helicobacter pylori strains from Korean patients. J Antimicrob Chemother. 2005;56:965–967. doi: 10.1093/jac/dki334. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Kim JS, Jung HC, Kim N, Song IS. Antibiotic resistance of Helicobacter pylori isolated from Korean patients in 2003. Korean J Gastroenterol. 2004;44:126–135. [PubMed] [Google Scholar]
- 12.Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001;47:459–461. doi: 10.1093/jac/47.4.459. [DOI] [PubMed] [Google Scholar]
- 13.Eun CS, Han DS, Park JY, Jeon YC, Hahm JS, Kim KS, et al. Changing pattern of antimicrobial resistance of Helicobacter pylori in Korean patients with peptic ulcer diseases. J Gastroenterol. 2003;38:436–441. doi: 10.1007/s00535-002-1079-2. [DOI] [PubMed] [Google Scholar]
- 14.Lee HK, Chae HS, Kang JO, Lee MK, Sung HS, Kim MN, et al. Multicenter study for the frequency of 23S rRNA point mutations associated with clarithromycin resistance in Helicobacter pylori in Korea. Korean J Clin Microbiol. 2008;11:84–89. [Google Scholar]
- 15.Queiroz DM, Dani R, Silva LD, Santos A, Moreira LS, Rocha GA, et al. Factors associated with treatment failure of Helicobacter pylori infection in a developing country. J Clin Gastroenterol. 2002;35:315–320. doi: 10.1097/00004836-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kim N, Kim JM, Kim CH, Park YS, Lee DH, Kim JS, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006;40:683–687. doi: 10.1097/00004836-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Versalovic J, Shortridge D, Kibler K, Griffy MV, Beyer J, Flamm RK, et al. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MH, Kim HS, Park SY, Park CH, Choi SK, Rew JS. Analysis of antimicrobial resistance of Helicobacter pylori in Gwangju, Chonnam Provinces. Korean J Helicobacter Up Gastrointest Res. 2012;12:82–87. [Google Scholar]
- 19.Kim JY, Kim NY, Kim SJ, Baik GH, Kim GH, Kim JM, et al. Regional difference of antibiotic resistance of Helicobacter pylori strains in Korea. Korean J Gastroenterol. 2011;57:221–229. doi: 10.4166/kjg.2011.57.4.221. [DOI] [PubMed] [Google Scholar]