Abstract
Background
Hydrogen sulfide (H2S) has been shown to induce angiogenesis in in vitro models and to promote vessel growth in the setting of hind-limb ischemia. The goal of the present study was to determine the therapeutic potential of a stable, long-acting H2S donor, diallyl trisulfide (DATS), in a model of pressure-overload heart failure and to assess the effects of chronic H2S therapy on myocardial vascular density and angiogenesis.
Methods and Results
Transverse aortic constriction (TAC) was performed in mice (C57BL/6J, 8-10 weeks of age). Mice received either vehicle or DATS (200 μg/kg) starting 24 hours after TAC and were followed for 12 weeks using echocardiography. H2S therapy with DATS improved left ventricular remodeling and preserved LV function in the setting of TAC. H2S therapy also increased the expression of the pro-angiogenic factor, vascular endothelial cell growth factor, while decreasing the angiogenesis inhibitor, angiostatin. Further studies revealed that H2S therapy increased the expression of the proliferation marker, Ki67, as well as increased the phosphorylation of endothelial nitric oxide synthase and increased the bioavailability of nitric oxide. Importantly, these changes were associated with an increase in vascular density within the H2S-treated hearts.
Conclusions
These results suggest that H2S therapy attenuates LV remodeling and dysfunction in the setting of heart failure by creating a pro-angiogenic environment for the growth of new vessels.
Keywords: H2S donor, endothelial nitric oxide synthase, nitric oxide, angiogenesis, diallyl trisulfide
Heart failure is a heterogeneous syndrome that can result from a number of common disease stimuli, including, but not limited to long-standing hypertension, myocardial infarction or ischemia associated with coronary artery disease.1,2 The prevalence of heart failure has increased dramatically as modern therapies have reduced the in-hospital mortality of acute myocardial infarction.2 In the United States, it has become the most common discharge diagnosis in patients aged 65 years or older and the primary cause of readmission within 60 days of discharge.3 Current treatments for heart failure are woefully inadequate, and the availability of hearts for transplantation is severely limited.2 Therefore, adjunct pharmacotherapies designed to coincide with the standard means of care are needed to decrease the extent of injury leading to the development of heart failure.
For centuries, the consumption of garlic has been recognized for its health benefits.4 For instance, garlic has been associated with reducing cardiovascular risk and diabetes, stimulating the immune system, protecting against infection, and inducing anti-cancer effects.5 The organosulfur compounds, such as diallyl trisulfide (DATS), found in garlic are considered to be responsible for its pharmacological activity. Recent evidence indicates that DATS mediates the vasoactive properties of garlic via the sustained release of hydrogen sulfide (H2S).4 H2S is a gaseous signaling molecule with a diverse physiological profile.6 In the heart, treatment with exogenous H2S or modulation of the endogenous production of H2S through the cardiac-specific overexpression of the H2S-generating enzyme, cystathionine γ-lyase (CSE), protects against acute myocardial ischemia-reperfusion (I/R) injury and heart failure by attenuating oxidative stress, inhibiting apoptosis, and reducing inflammation.7,8 In contrast, pharmacological inhibition or genetic deficiency of CSE results in vascular dysfunction9 and an exacerbation of myocardial injury.10 Furthermore, DATS administration was recently shown to reduce infarct size and improve contractile function following acute myocardial I/R injury by restoring cardiac H2S levels.11
Angiogenesis is a complex biological process characterized by extracellular matrix remodeling and changes in endothelial cell behavior that leads to increased growth, migration, and assembly into capillary structures.12 It remains an attractive therapeutic option for the treatment of heart failure. Recently, several in vitro studies indicate that H2S induces angiogenesis13,14 and there is evidence that H2S promotes vessel growth in a wound-healing model15 and in the setting of hind-limb ischemia.16 However, there is not any data regarding the ability of the H2S to induce vessel formation in the setting of heart failure. The goal of the present study was to determine the therapeutic potential of a stable, long-acting H2S donor, DATS, in a model of pressure-overload heart failure and to assess the effects of chronic H2S therapy on myocardial vascular density and angiogenesis.
Methods
Mice
Male C57BL/6J mice 8-10 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME). All experimental protocol were approved by the Institute for Animal Care and Use Committee at Emory University School of Medicine and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996), and with federal and state regulations.
DATS Preparation and administration
DATS (LKT Labs, St. Paul, MN) was maintained in sealed amber glass ampules and kept at −20°C until use. On the day of experimentation a fresh ampule of DATS was opened. 5 μl of DATS was diluted in 500 μl of 100% DMSO followed by further dilution in sterile saline to obtain the correct dosage to be delivered in a volume of 50 μl. The resulting concentration of DMSO in this dosage was 1%. Vehicle consisted of a solution of 1% DMSO in sterile saline. DATS (200 μg/kg) or Vehicle (1% DMSO) groups were injected intraperitoneally once per day for 12 weeks following TAC. This dose of DATS was selected on the basis of our previous experience investigating DATS in murine models of cardiac ischemia/reperfusion injury.11
Transverse Aortic Constriction (TAC) Protocol and Echocardiography
To create pressure overload, TAC procedure was performed in mice by placing a 7-0 silk suture around the aortic arch between the brachiocephalic trunk and the left carotid artery. The suture was ligated around a 27G blunt needle. The needle was immediately removed after ligation. At 2 days prior to TAC procedure, baseline transthoracic echocardiogram was performed using 30-MHz probe on a Vevo 2100 (Visualsonics) under anesthesia with isoflurane (0.25 to 0.50%) supplemented with 100% O2. Following TAC procedure, echocardiography was also performed in same manner for up to 12 weeks. To determine cardiac structure and function, intraventricular septal end diastolic dimension (IVSd), LV end diastolic dimension (LVEDD), LV end systolic dimension (LVESD), and LV ejection fraction (LVEF) were analyzed from M-mode images.
Histology
Hearts were collected at the indicated times, fixed in 10% buffered formalin, embedded in paraffin stained with Masson’s trichrome and Picrosirius Red.
Vascular density and cellular proliferation measurements
Angiogenic index measurements from frozen tissue sections were performed as previously described.16 Briefly hearts from the experimental groups were collected, dissected, and embedded in OCT freezing medium. Frozen tissue blocks were cut into 5 μm sections and slides fixed for staining. Slides were stained with anti-CD31 antibody to calculate an angiogenesis index or with anti-Ki67 antibody to determine cellular proliferation index. We also performed formalin fixed tissue immunohistochemistry using anti-vWF antibody staining to quantify the number of capillaries per unit area.
Western Blot Analysis
Myocardial tissue samples were taken homogenized and lysates were used for Western blot analysis as previously described.17
Measurement of Nitrite Levels
Nitrite analysis was performed as previously described.18
Measurement of Hydrogen Sulfide and Sulfane Sulfur
Hydrogen sulfide and sulfane sulfur levels were measured in heart and blood according to previously described methods.11
Statistical analysis
All data are expressed as mean ± SEM. Statistical significance was evaluated using unpaired Student t-test for comparison between 2 means and a 1-way ANOVA with a Tukey test as the posthoc analysis for comparison among 3 or more means by use of Prism 5 (GraphPad Software Inc). For the echocardiography data, a 2-way repeated measures ANOVA with a Bonferroni test as the posthoc analysis was used. The following comparisons were made separately: (1) baseline vs. post-baseline measurements at each time point for the DATS and vehicle groups and (2) DATS vs. vehicle measurements at each time point. The p-value for these evaluations was adjusted by applying the Bonferroni correction for multiple comparisons. A value of p<0.05 denoted statistical significance and p-values were two-sided.
Results
DATS therapy attenuates cardiac dysfunction following TAC
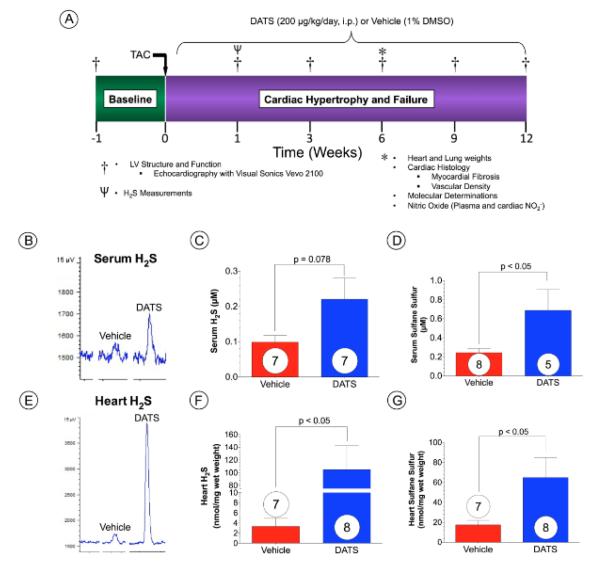
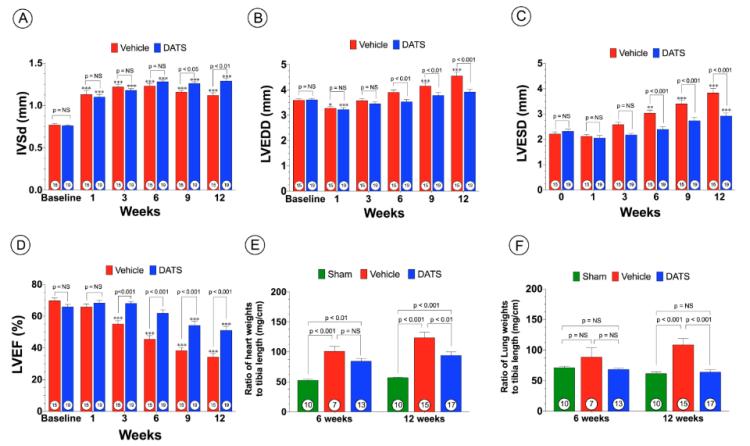
To investigate the effects of DATS, on pressure overload-induced cardiac hypertrophy and dysfunction, we performed TAC procedure in C57/BL6 mice and evaluated cardiac structure and function using 2-D echocardiography (Figure 1A). For these experiments Vehicle (1% DMSO) or DATS (200 μg/kg) was administered intraperitoneally (i.p.) daily starting at 24 hrs following TAC surgery. Analysis at 1 week following TAC revealed that DATS increased both circulating and cardiac free H2S and sulfane sulfur (bound sulfide) levels (Figure 1B-G). At 6 weeks following TAC, both Vehicle and DATS-treated animals displayed a similar degree of IVSd thickness (Figure 2A). However, administration of DATS significantly reduced LV cavity diameters, LVEDD and LVESD, in treated mice at 6 weeks up to 12 weeks following TAC when compared to Vehicle-treated mice (Figure 2B-C). In addition, DATS treatment improved cardiac function beginning at 3 weeks following TAC (Figure 2D) as evidenced by improved LVEF. In addition, DATS-treated mice displayed significantly less of an increase in heart weight/tibia length ratios and less pulmonary edema when compared to Vehicle-treated mice at 12 weeks following TAC (Figure 2E-F).
Figure 1. DATS Increases Sulfide Levels Following TAC.
(A) Mice (C57 BL6/J) were subjected to transverse aortic constriction (TAC) surgery and were studied for a period of 12 weeks following TAC. The H2S donor, Diallyl trisulfide (DATS), was initiated at a dose of 200 μg/kg (daily i.p. injection) at 24 hours following TAC surgery. Vehicle-treated mice were administered 1% DMSO. Baseline echocardiography was performed 1-week prior to TAC and thereafter at 1, 3, 6, 9, and 12 weeks following TAC. Additional mice were sacrificed at 6 weeks post TAC. Heart and lung tissue were collected for assessment of cardiac and lung weights, cardiac histology, and myocardial molecular determinations. In addition, blood samples were also collected for measurement of nitric oxide intermediates. Representative gas chromatograph peaks and summary data for (B-D) serum and (E-G) myocardial levels of free H2S and sulfane sulfur after 1 week of TAC in groups of mice injected daily with vehicle or DATS. Results are expressed as mean ± SEM. Numbers in bars represent sample size.
Figure 2. DATS Prevents Adverse Cardiac Remodeling and heart failure after TAC.
(A) Inter-ventricular septal wall thickness (IVSd, mm), (B) Left ventricular end-diastolic diameter (LVEDD, mm) (C) Left venricular end-systolic diameter (LVESD, mm) and (D) Left ventricular ejection fraction (LVEF, %) as determined by echocardiography at baseline and from 1 to 12 weeks of TAC. (E) Heart weights (mg/cm) and (F) lung weights (mg/cm) expressed as ratio of tibia length at 6 weeks following TAC. Results are expressed as mean ± SEM. *p<0.05, **p<0.01, and ***p<0.001 vs. Baseline.
DATS attenuates myocardial fibrosis after TAC
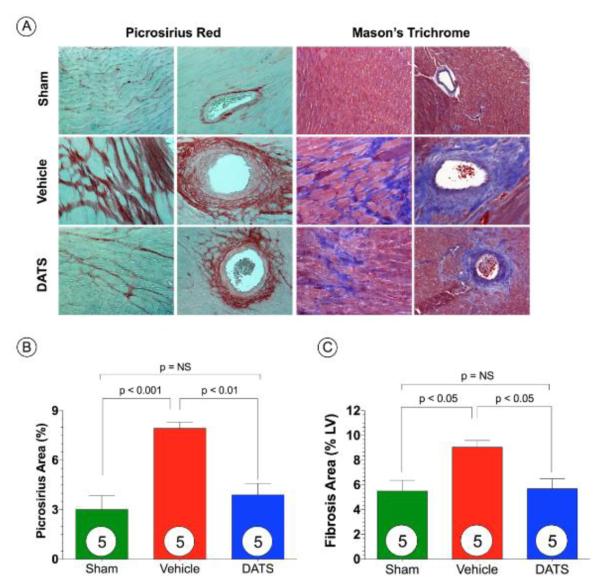
We investigated the extent of left ventricular fibrosis at 6 weeks following TAC (Figure 3). Histological analysis of Masson’s Trichrome and Picrosirius Red stained sections revealed extensive areas of intermuscular and perivascular fibrosis in hearts from Vehicle-treated mice (Figure 3B-C; p<0.001 for Picrosirius Red and p<0.05 for Masson’s Trichrome vs. Sham). Although fibrosis was evident in the sections taken from DATS-treated hearts, it was significantly less when compared to the Vehicle-treated hearts (p<0.01 for Picrosirius Red and p<0.05 for Masson’s Trichrome).
Figure 3. DATS Attenuates Myocardial Fibrosis after TAC.
(A) Representative photomicrographs of Picrosirius Red and Masson’s Trichrome stained heart sections depicting perivascular intermuscular fibrosis in hearts from Sham, Vehicle, and DATS treated mice at 6 weeks of TAC. Summary of fibrosis area as % of the LV calculated from (B) Picrosirius Red sections and (C) Masson’s Trichrome sections. Scale bar equals 50-μm. Results are expressed as mean ± SEM.
DATS augments myocardial angiogenic factors and vascular density after TAC
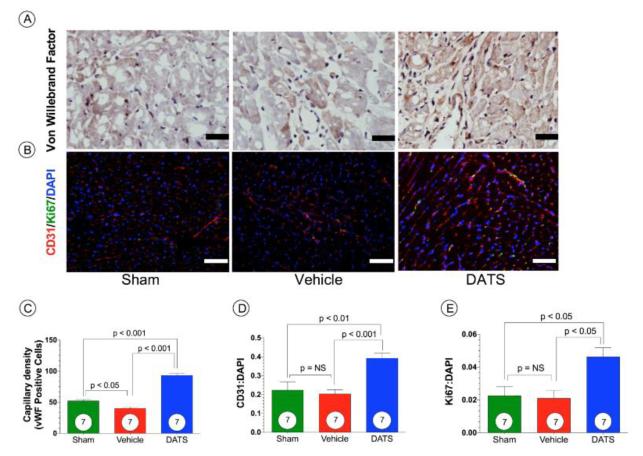
DATS significantly (p<0.001) increased myocardial CD31 protein levels compared to both Sham and Vehicle-treated mice (Figure 4A). At 6 weeks following DATS, treated mice exhibited significantly greater cardiac VEGF-A protein expression compared to Sham mice (Figure 4A; p<0.05). Moreover, DATS also significantly increased basic fibroblast growth factor (bFGF) protein levels in the myocardium as compared to Sham mice (Figure 4B; p<0.05). Furthermore, DATS significantly reduced angiostatin protein, a known endogenous inhibitor of angiogenesis (Figure 4C; p<0.001 vs. Sham). We also measured the capillary density and angiogenic index of the heart after TAC. Figure 5 shows that both the von Willebrand factor (vWF) capillary density (Figure 5A and 5C) and CD31:DAPI angiogenic index (Figure 5B and 5D) were increased by DATS therapy compared to Sham or Vehicle-treated groups. This increase in vascular density corresponds to an increase cellular proliferation (Ki67:DAPI ratio) of the DATS treatment group (Figure 5B and 5E). Together, these results clearly demonstrate that DATS therapy significantly increases vascular density and cell proliferation in heart after TAC.
Figure 4. DATS Augments Myocardial Angiogenic Factors.
(A) Representative immunoblots and densitometric analysis for PECAM-1 (CD31) and VEGF-A expression in hearts from Sham, Vehicle, and DATS-treated mice. Representative immunoblots and densitometric analysis for myocardial (B) basic fibroblast growth factor (bFGF) and (C) angiostatin expression in hearts from Sham, Vehicle, and DATS-treated mice. Horizontal solid lines define noncontiguous gel lanes. Results are expressed as mean ± SEM.
Figure 5. DATS Increases Vascular Density after TAC.
(A-B) Representative photomicrographs of von Willebrand factor (vWF), CD31, and Ki67 stained heart sections from Sham, Vehicle, and DATS treated mice at 6 weeks of TAC. Staining. (C) Summary of capillary density as determined by von Willebrand staining. (D) Angiogenesis index expressed as a ratio between CD31 and DAPI positive regions. (E) Cellular proliferation determined as the ratio between Ki67 and DAPI positive areas. Results are expressed as mean ± SEM.
DATS Augments eNOS-Nitric Oxide Signaling Following TAC
We next investigated whether DATS treatment modulated Akt phosphorylation in the heart following TAC (Figure 6). Total Akt expression was not different among all groups. However, a significant (p<0.05) increase in the phosphorylation of Akt at serine residue 473 (Akt-PSer473) was observed in hearts treated with DATS at 6 weeks following TAC compared to Sham mice. Nitric oxide (NO) generated from endothelial nitric oxide synthase (eNOS) is known to modulate vascular angiogenesis and promote vascular and myocardial cell cytoprotection during ischemic conditions.19 Activation of AMPK increases the phosphorylation and activity of eNOS.20 There were no differences in the phosphorylation of AMPK at threonine residue 172 (AMPK-PThr172) and total AMPK expression in the heart among all groups (Figure 6B). However, we observed a significant increase in the phosphorylation of eNOS at serine residue 1177 (eNOS-PSer1177; activation site) and a significant decrease in the phosphorylation of eNOS at threonine residue 495 (eNOS-PThr495; inhibition site) following treatment with DATS (Figure 6C) when compared to either Sham (p<0.05) or Vehicle mice (p<0.01). There were no differences in total eNOS expression in the heart among all groups. Furthermore, DATS treatment increased cardiac nitrite levels (Figure 6D) following TAC compared to Vehicle mice (p<0.01). Nitrite is an established biomarker for NO18 suggesting that eNOS activation resulted in increased NO bioavailability following DATS treatment.
Figure 6. DATS upregulates Akt phosphorylation and activates the eNOS-No pathway after TAC.
Representative immunoblots and densitometric analysis of (A) phosphorylated Akt at serine residue 473 (Akt-PSer473) and total Akt, (B) phosphorylated AMPK at threonine residue 172 (AMPK-PThr172) and total AMPK, and (C) phosphorylated eNOS at serine residue 1177 (eNOS-PSer1177), phosphorylated eNOS at threonine residue 495 (eNOS-PThr495), and total eNOS in hearts from Sham, Vehicle, and DATS-treated mice at 6 weeks of TAC. (D) Plasma and myocardial nitrite levels (μM) at 6 weeks following TAC. Results are expressed as mean ± SEM.
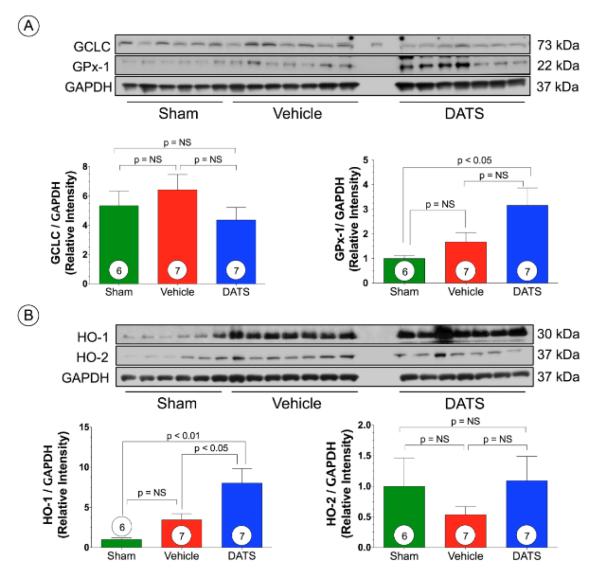
DATS increases GPx1 and HO-1 expression after TAC
We next investigated the effects of the administration of DATS following TAC on various antioxidants. We observed no significant difference in the protein levels of the glutathione subunit, glutamate-cysteine ligase (GCLC), or heme oxygenase 2 (HO-2) among all groups (Figure 7A). However, DATS significantly increased the expression of glutathione peroxidase (GPx-1) compared to Sham (p<0.05). We also observed a significant increase in HO-1 expression following TAC compared to Sham (p<0.01) and Vehicle-treated mice (p<0.05) (Figure 7B).
Figure 7. DATS upregulates GPx-1 and HO-1 after TAC.
Representative immunoblots and densitometric analysis of (A) glutamate-cysteine ligase (GCLC) and Glutathione Peroxidase 1 (GPx-1) and (B) heme oxygenase 1 (HO-1) and HO-2 in hearts from Sham, Vehicle, and DATS-treated mice at 6 weeks of TAC. Results are expressed as mean ± SEM.
Discussion
In response to myocardial injury, the LV undergoes morphological changes resulting in ventricular remolding that are initially considered adaptive. However, in response to sustained pathological stimuli such as increased pressure, LV remodeling becomes maladaptive leading to the development of heart failure.21 Moreover, the morphological and functional changes that accompany LV remodeling serve as predictors of morbidity and mortality22 The underlying mechanisms of LV remodeling include many biological reactions, such as cell death, inflammation, oxidative stress, and development of fibrosis.23 These reactive processes stimulate each other and advance from acute cellular reactions to chronic anatomical and functional changes. In the current study, chronic administration of DATS, an organosulfur compound that augments H2S levels4,11, provides protection against the adverse remodeling associated with TAC by increasing circulating and cardiac sulfide levels. Specifically, we found that DATS therapy attenuated LV dilatation and dysfunction, attenuated the development of perivascular and intermuscular fibrosis, and attenuated the development of cardiac hypertrophy. These findings are in concert with previous studies, which demonstrated that both exogenous and endogenously derived H2S exhibit potent cytoprotective effects in different models of heart failure.17,24 Together, these findings support recent experimental data demonstrating that H2S is an important mediator of both cell survival and remodeling in the heart following the induction of heart failure.
Recent studies indicate that the heart progresses rapidly from a compensatory hypertrophic state to a state of decompensated failure when vascular growth cannot keep pace with pathological myocyte growth.25 VEGF, a potent angiogenic cytokine, plays a central role in coronary vascular network growth under these conditions, as evidenced by the finding that a reduction in VEGF signaling contributes to the rapid progression from compensatory cardiac hypertrophy to failure.26 Recent evidence indicates that H2S is a strong promoter of angiogenesis.14,27 For instance, exposing cultured endothelial cells to H2S stimulates cell proliferation, migration, and tube formation.13 Additionally, H2S promotes angiogenesis in vivo, as evidenced by an increase in the neovascularization of implanted matrigel plugs in mice following treatment with sodium hydrosulfide.28 Finally, the proangiogenic effects of H2S are evident in models of chronic vascular disease, such as hind-limb ischemia.16 In the current study, H2S therapy increased the protein expression of VEGF after TAC. This was associated with increased vascular density in the treated hearts, as evidenced by the observed increase in the expression of vWF and CD31 and an increase in the proliferation marker, Ki67. H2S therapy also decreased the expression of angiostatin, an inhibitor of angiogenesis29, and increased the expression of bFGF, a factor that facilitates the formation of new blood vessels.30 NO has also been reported to play a role in mediating VEGF-induced angiogenesis.31 Although NO and H2S signaling have traditionally been considered to operate via distinct pathways, there is evidence of cross-talk between the two pathways. For instance, H2S therapy improves survival after cardiac arrest and cardiopulmonary resuscitation in an eNOS-dependent manner32 and provides cardioprotection against acute myocardial I/R injury by activating eNOS/NO.11 Additionally, Coletta and colleagues15 demonstrated an unexpected level of cooperation, as well as a mutual reliance between these signaling pathways to promote angiogenesis. Specifically, blocking either eNOS/NO or H2S markedly reduces the angiogenic effects of the other.15 In agreement with previous reports33,34, we observed a significant increase in the phosphorylation of eNOS (activation site) following H2S therapy. We also found that H2S increased cardiac nitrite levels, which indicates an encouragement of NO production following exogenous H2S administration. Taken together, this evidence suggests that H2S therapy does not simply prevent vessel dropout after TAC, but rather creates a pro-angiogenic environment for the growth of new vessels. Given that this correlates with the observed improvements in LV remodeling and function, the induction of angiogenesis via VEGF-NO signaling appears to play a major role in the cardioprotection afforded by H2S.
VEGF, in part, regulates multiple angiogenic cellular responses, including survival, migration, and differentiation through the activation of Akt signaling.26 While we did find that DATS therapy significantly increased Akt phosphorylation when compared to Sham mice, we only observed a non-significant trend between the DATS and Vehicle-treated mice. Based on these findings, there is not enough evidence to suggest a role for Akt in mediating the H2S-induced angiogenesis or cardioprotection. VEGF also regulates eNOS/NO signaling via PKC35, so further studies are needed to determine the exact mechanism by which H2S induces angiogenesis and cardioprotection.
There is considerable evidence to suggest that oxidative stress plays a prominent role in the development of LV remodeling during heart failure.23 The increased oxidative stress of the failing heart is a result of an increased production of reactive oxygen species and an overwhelmed antioxidant defense system. Therefore, the capacity of cardiac myocytes to maintain homeostasis during periods of oxidative stress resides in the ability to activate or induce protective enzymes.36 H2S has previously been shown to act either as a direct antioxidant and/or upregulate antioxidant defenses.37 Previously, we have reported that H2S attenuates oxidative stress in the heart by activating nuclear factor E2-related factor (Nrf2), a member of the NF-E2 family of nuclear basic leucine zipper transcription factors.8 Nrf2 regulates the gene expression of a number of enzymes that serve to detoxify pro-oxidative stressors38, such as GPx1 and HO-1, by binding to the antioxidant response element found in the gene’s promoter region.8 GPx1 is an antioxidant enzyme that plays a vital role in detoxifying hydrogen peroxide.39 In the heart, deficiency of GPx1 has been shown to exacerbate ischemic injury by promoting oxidative damage at the level of the mitochondria.39 Furthermore, deficiency of GPx1 accelerates cardiac hypertrophy and dysfunction in response to angiotensin-induced hypertension.40 In the current study, we report for the first time that the expression of GPx1 is significantly upregulated in the heart following H2S treatment, suggesting that it may contribute to the protective effects of H2S therapy in the setting of TAC. HO-1 is an inducible stress-response protein that imparts antioxidant and antiapoptotic effects by degrading pro-oxidant heme to carbon monoxide (CO) and biliverdin/bilirubin.41 In the heart, HO-1 has been shown to attenuate the effects of prohypertrophic ROS signals, thereby inhibiting LV hypertrophy and remodeling.42 Previously, we have shown that H2S upregulates HO-1 in a model of acute myocardial I/R injury.8 In the current study, we expand on these previous findings and demonstrate that H2S therapy increases HO-1 in a model of pressure-induced heart failure. The increase in HO-1 is especially notable given that it is associated with the production of CO, the third member of the gasotransmitter family. Although the levels of CO were not evaluated in the current study, we did find that H2S therapy increased NO levels. Therefore, it is intriguing to speculate that H2S therapy has the ability to increase the levels of the other two gasotransmitters. This suggests that the activation of one of the endogenously produced gases can lead to the activation of the other two. The specific role of H2S as it influences both NO and CO is worthy of further exploration, as the administration of one gasotransmitter than can have control over the others would likely prove to be clinically powerful, advantageous, and efficient.
Accumulating evidence indicates that H2S signals by modifying cysteine residues in proteins via a process termed sulfhydration.43 Sulfhydration is similar nitrosylation, which is the process by which NO modifies proteins.44 However, unlike nitrosylation, which often results in the inhibition of target protein activity, sulfhydration appears to enhance activity.45 For instance, the antiapoptotic actions of NFkB are dependent on H2S sulfhydrating its p65 subunit.46 In addition, H2S induces endothelial cell and smooth muscle cell hyperpolarization and vasorelaxation by sulfhydrating ATP-sensitive potassium channels.45 Finally, H2S has recently been shown to sulfhydrate Keap1, which results in the release and translocation of Nrf2 to the nucleus.47 Although the degree of protein sulhydration was not evaluated in the current study, it can be speculated that the observed improvements in LV remodeling and function are a result of this post-translational modification. Therefore, future studies are needed to evaluate specific protein targets to determine the full mechanism(s) by which H2S provides protection in the setting of heart failure.
The attractiveness of using H2S as a therapeutic strategy to treat heart failure stems from the ability of H2S to activate multiple protective pathways at the same time. In the current study, we provide evidence that H2S protects via a VEGF-eNOS-NO pro-angiogenic pathway and a GPx-1-HO-1 antioxidant pathway. In addition to our current findings, treatment with H2S could also limit the development of LV remodeling and dysfunction associated with heart failure through its ability to inhibit inflammation, modulate mitochondrial function, and inhibit apoptosis.7,8,17,24 While these protective pathways can be separated into distinct signaling cascades, there can be overlap between the signaling molecules. For example, H2S can provide antioxidant effects via an Nrf2-HO-1 pathway.8 However there is evidence that NO possesses antioxidant effects and can activate Nrf2 and HO-1.48,49 Moreover, activation of either of these antioxidant pathways could not only lead to a reduction of oxidative stress but could also contribute to a reduction in cell death and improvement in mitochondrial function. Together this would provide a more suitable environment for the VEGF-eNOS-NO pathway to induce the growth of new vessels. Given the diverse signaling profile of H2S, it is likely that all of these pathways work together and in parallel to prevent the adverse remodeling of heart failure.
In summary, this study provides novel evidence that chronic H2S therapy attenuates LV remodeling and preserves cardiac function in the setting of pressure-induced heart failure. Our data suggest that H2S mediates these protective effects by inducing angiogenesis via an increase in VEGF and eNOS/NO. Additionally, the current study indicates that H2S upregulates the endogenous antioxidants, GPx1 and HO-1. Together, these findings continue to support the emerging concept that treatment strategies aimed at increasing the levels of H2S may be of clinical importance in the treatment of heart failure.
Supplementary Material
Clinical Perspective.
Adjunct pharmacotherapies designed to coincide with the standard means of care are needed to decrease the extent of injury leading to the development of heart failure. In this regard, therapeutic strategies aimed at increasing the levels of the gaseous signaling molecule, hydrogen sulfide (H2S), have come to be a focus of interest given their ability to exert cytoprotective effects in various models of cardiac injury. In the current study, chronic administration of diallyl trisulfide (DATS), an organosulfur compound that augments H2S levels, provides protection against the adverse remodeling associated with TAC by increasing circulating and cardiac sulfide levels. Specifically, we found that DATS therapy attenuated LV dilatation and dysfunction, attenuated the development of perivascular and intermuscular fibrosis, and attenuated the development of cardiac hypertrophy. Our data indicated that DATS mediated these protective effects by inducing angiogenesis and alleviating oxidative stress. Together, these findings continue to support the emerging concept that treatment strategies aimed at increasing the levels of H2S may be of clinical importance in the treatment of heart failure.
Acknowledgements
We thank Marah Condit for her expert technical assistance during the course of these studies.
Sources of Funding This work was supported by grants from the National Heart, Lung, and Blood Institute (NIH; 5R01HL092141, 5R01HL093579, 1U24HL094373, and 1P20HL113452 to D.J.L. and 5R01HL098481 to J.W.C.). We are also grateful for the generous funding support from the Carlyle Fraser Heart Center of Emory University Hospital Midtown.
Footnotes
Disclosures D.J.L is a cofounder of Sulfagenix, Inc. Sulfagenix is currently developing H2S–based therapeutics for cardiovascular disease.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen-Solal A, Beauvais F, Logeart D. Heart failure and diabetes mellitus: Epidemiology and management of an alarming association. J Card Fail. 2008;14:615–625. doi: 10.1016/j.cardfail.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal. 2009;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2s as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302:H2410–2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 13.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: Mechanisms and applications. Br J Pharmacol. 2011;164:853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc. 2012;1:e004093. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: Evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci U S A. 2008;105:11430–11435. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavu M, Gundewar S, Lefer DJ. Gene therapy for ischemic heart disease. J Mol Cell Cardiol. 2011;50:742–750. doi: 10.1016/j.yjmcc.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via ampk-enos-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 21.Koitabashi N, Kass DA. Reverse remodeling in heart failure--mechanisms and therapeutic opportunities. Nat Rev Cardiol. 2012;9:147–157. doi: 10.1038/nrcardio.2011.172. [DOI] [PubMed] [Google Scholar]
- 22.Solomon SD, Pfeffer MA. The decreasing incidence of left ventricular remodeling following myocardial infarction. Basic Res Cardiol. 1997;92:61–65. doi: 10.1007/BF00805561. [DOI] [PubMed] [Google Scholar]
- 23.Maack C, Kartes T, Kilter H, Schafers HJ, Nickenig G, Bohm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-gtpase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 24.Mishra PK, Tyagi N, Sen U, Givvimani S, Tyagi SC. H2S ameliorates oxidative and proteolytic stresses and protects the heart against adverse remodeling in chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H451–456. doi: 10.1152/ajpheart.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 28.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci. 2012;8:430–441. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villaschi S, Nicosia RF. Angiogenic role of endogenous basic fibroblast growth factor released by rat aorta after injury. Am J Pathol. 1993;143:181–190. [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke JP. NO and angiogenesis. Atheroscler Suppl. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 32.Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osipov RM, Robich MP, Feng J, Liu Y, Clements RT, Glazer HP, Sodha NR, Szabo C, Bianchi C, Sellke FW. Effect of hydrogen sulfide in a porcine model of myocardial ischemia-reperfusion: Comparison of different administration regimens and characterization of the cellular mechanisms of protection. J Cardiovasc Pharmacol. 2009;54:287–297. doi: 10.1097/FJC.0b013e3181b2b72b. [DOI] [PubMed] [Google Scholar]
- 34.Osipov RM, Robich MP, Feng J, Chan V, Clements RT, Deyo RJ, Szabo C, Sellke FW. Effect of hydrogen sulfide on myocardial protection in the setting of cardioplegia and cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2010;10:506–512. doi: 10.1510/icvts.2009.219535. [DOI] [PubMed] [Google Scholar]
- 35.Shen BQ, Lee DY, Zioncheck TF. Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a kdr/flk-1 receptor and a protein kinase c signaling pathway. J Biol Chem. 1999;274:33057–33063. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- 36.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 37.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. Faseb J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 38.Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor e2-related factor 2. Drug Metab Dispos. 2007;35:995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 39.Thu VT, Kim HK, Ha SH, Yoo JY, Park WS, Kim N, Oh GT, Han J. Glutathione peroxidase 1 protects mitochondria against hypoxia/reoxygenation damage in mouse hearts. Pflugers Arch. 2010;460:55–68. doi: 10.1007/s00424-010-0811-7. [DOI] [PubMed] [Google Scholar]
- 40.Ardanaz N, Yang XP, Cifuentes ME, Haurani MJ, Jackson KW, Liao TD, Carretero OA, Pagano PJ. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin ii hypertension. Hypertension. 2010;55:116–123. doi: 10.1161/HYPERTENSIONAHA.109.135715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otterbein LE, Choi AM. Heme oxygenase: Colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 42.Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul BD, Snyder SH. H(2)s signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 44.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein s-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 45.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of nf-kappab mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Hydrogen sulfide protects against cellular senescence via s-sulfhydration of keap1 and activation of nrf2. Antioxid Redox Signal. 2013;18:1906–19. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 48.Um HC, Jang JH, Kim DH, Lee C, Surh YJ. Nitric oxide activates nrf2 through s nitrosylation of keap1 in pc12 cells. Nitric Oxide. 2011;25:161–168. doi: 10.1016/j.niox.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W, Zhu X, Lanceta LB, Sanganalmath SK, Dawn B, Shinmura K, Rokosh GD, Wang S, Bolli R. Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-{kappa}b-dependent pathway. Circulation. 2009;120:1222–1230. doi: 10.1161/CIRCULATIONAHA.108.778688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.