Abstract
Objective
To examine the prescribing patterns of medications quantified by the performance measures for acute myocardial infarction (AMI).
Background
Current performance measures for AMI are designed to improve quality by quantifying the use of evidence-based treatments. However, these measures only assess medication prescription. Whether patients receive optimal dosing of secondary prevention medications at the time of and following discharge after AMI is unknown.
Methods
We assessed treatment doses of beta-blockers, statins, and ACE/ARBs at discharge and 12 months after AMI among 6748 patients from 31 hospitals enrolled in 2 US registries (2003-08). Prescribed doses were categorized as none, low (<50% target [defined from seminal clinical trials]), moderate (50-74% target), or goal (≥75% target). Patients with contraindications were excluded from analyses for that medication.
Results
Most eligible patients (>87%) were prescribed some dose of each medication at discharge, although only 1 in 3 patients were prescribed these medications at goal doses. Of patients not discharged on goal doses, up-titration during follow-up occurred infrequently (~25% of patients for each medication). At 12 months, goal doses of beta-blockers, statins, and ACE/ARBs were achieved in only 12%, 26%, and 32% of eligible patients, respectively. After multivariable adjustment, prescription of goal dose at discharge was strongly associated with being at goal dose at follow-up: beta-blockers, adjusted odds ratio (OR): 6.08 (95% CI: 3.70-10.01); statins, adjusted OR: 8.22 (95% CI: 6.20-10.90); ACE/ARBs, adjusted OR: 5.80 (95% CI: 2.56-13.16); p<0.001 for each.
Conclusions
Although nearly all patients after an AMI are discharged on appropriate secondary prevention medications, dose increases occur infrequently, and most patients are prescribed doses below those with proven efficacy in clinical trials. Integration of dose intensity into performance measures may help improve the use of optimal medical therapy after AMI.
Keywords: myocardial infarction, secondary prevention, performance measures
In an effort to standardize and improve the quality of care provided to patients with acute myocardial infarction (AMI), the American College of Cardiology and American Heart Association developed performance measures to quantify the use of evidence-based treatments.(1) The goal of these measures is to promote the widespread and uniform application of best practices in AMI care and, in turn, improve patients’ survival and quality of life.(2) Current performance measures assess whether patients are prescribed certain medications (i.e. yes/no) but not the potency of treatment (i.e., dose). However, trials comparing low vs. high doses of these medications have demonstrated that optimal dosing is necessary to achieve the full clinical benefit of these therapies.(3-7) Thus, it is possible that a large number of treated patients are receiving relatively ineffective therapy but yet still fulfill the requirements of current performance measures.
Initiating lower doses of secondary prevention medications at hospital discharge may be reasonable, particularly in patients with marginal hemodynamics (e.g., low blood pressure or heart rate) or left ventricular (LV) systolic dysfunction. It is desirable, however, for these therapies to be up-titrated shortly after discharge to the levels with established benefit in clinical trials, but the degree to which providers are intensifying treatment during outpatient follow-up also is not currently known. If patients are being sub-optimally dosed with secondary prevention medications at hospital discharge and if medication up-titration occurs infrequently during subsequent follow-up, this may explain why the findings from clinical trials (where there was clear evidence of benefit for each medicine promoted by performance measures) have been discordant from those in clinical practice (where the impact of performance measures on reducing mortality has been underwhelming(8-10)).
Accordingly, we examined the prescribing patterns of medications quantified by the performance measures (beta-blockers, statins, and angiotensin converting enzyme inhibitor [ACE] or angiotensin II receptor blocker [ARB]) in a large, multi-center cohort of patients hospitalized with AMI. We explored prescribing patterns at the time of hospital discharge and 12 months after discharge, as well as outpatient intensification of therapy among those initially discharged on medication doses that were below goal.
METHODS
Study population and protocol
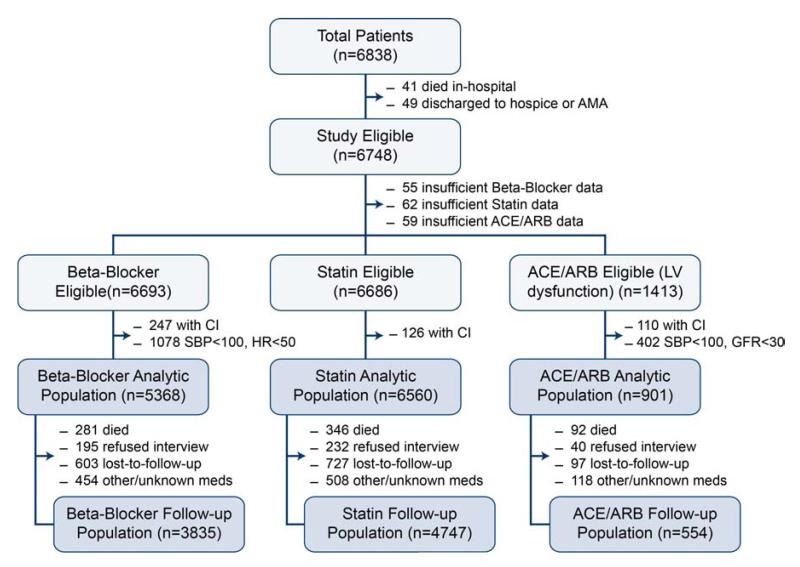
Patients were enrolled in either of two multicenter, prospective cohort studies of unselected patients hospitalized with AMI in the U.S. Between January 2003 and June 2004, 2498 patients with AMI were recruited from 19 US hospitals into the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) study.(11) Similarly, between June 2005 and December 2008, 4340 patients with AMI from 24 US hospitals were enrolled into the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) study (12 hospitals participated in both registries).(12) Both registries employed identical inclusion and exclusion criteria and were coordinated by the Mid America Heart Institute. Patients were required to have biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of an AMI, including prolonged ischemic signs/symptoms (≥20 minutes) or electrocardiographic ST changes during the initial 24 hours of admission. Baseline data, including discharge medications and doses, were obtained through chart abstraction and a structured interview by trained research staff within 24 to 72 hours following admission. To be eligible for the current study, patients had to survive to hospital discharge and not be discharged against medical advice or to hospice (90 patients excluded). Thus, our study sample included 6748 patients from 31 hospitals, 12 of which participated in both PREMIER and TRIUMPH (Figure 1).
Figure 1. Flowchart of Patients.
*indicates patients who reported taking zero medications, patients who were taking medications not FDA-approved for patients after myocardial infarction or with concurrent heart failure, or patients with doses that were unknown. CI, contraindications
Detailed follow-up interviews were attempted on all survivors at 1, 6, and 12 months after AMI. In addition to a report of interval events and an assessment of health status, participants were asked to read the names and doses of their medications from their prescription bottles and to report the number of outpatient visits to cardiologists, cardiac surgeons, and primary care providers (for care related to their “heart condition”). For this study’s follow-up analyses, we excluded 503 patients who participated in the interview but reported taking zero medications of any type (cardiac or otherwise), as we believed that the therapeutic decisions in these cases were not likely under the control of a physician.
For each of the 3 medication classes, we excluded patients with chart-documented contraindications (e.g., heart rate <50 bpm or systolic blood pressure [SBP] <100 mmHg for beta-blockers, SBP <100 mmHg or a glomerular filtration rate <30 mL/min/1.73m2 for ACE/ARBs, patient sensitivities/allergies for each medication class), which were prospectively abstracted from patients’ medical records. Furthermore, for analyses of ACE/ARBs, we included only patients with LV systolic dysfunction at the time of AMI (ejection fraction <40%) (Figure 1). Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent for baseline and follow-up assessments.
Medication dose assessment
Our primary outcome was whether a patient reported taking a goal dose of beta-blocker, statin, and ACE/ARB at 12-months after AMI. In order to standardize comparisons of medications within the same class (e.g., metoprolol and carvedilol for beta-blockers), we classified medication doses into categories, relative to the target dose for that medication. The target dose for each medication was defined by the landmark clinical trials that established clinical benefit for each medication in AMI (see Appendix eTables 1-3 for target doses for each medication, the clinical trials demonstrating clinical efficacy, and the mean achieved doses in the trials). Beta-blockers, statins and ACE/ARBs that were not indicated by FDA labeling for patients after AMI or with concurrent heart failure (and thus did not have a target dose for these conditions) were considered “other” and excluded from the respective analyses. For example, medications such as labetolol and losartan are only indicated for the treatment of hypertension and, thus, do not have established target doses for secondary prevention in AMI. Overall, this exclusion affected 1% of patients taking beta-blockers, 0% of patients taking statins, and 6% of patients taking ACE/ARBs.
A person was considered to be taking a goal dose of a medication if the dose was at least 75% of the target dose. A dose that was 50-74% of target was considered moderate intensity, whereas doses below 50% of target were considered low. Although we excluded patients with SBP <100 mmHg from the beta-blocker and ACE/ARB analyses, as these patients would be difficult to get on any dose of these medications, patients with SBP <110 mmHg may similarly be difficult to up-titrate. As such, a 5th category of patients was created for the beta-blocker and ACE/ARB analyses that included these patients, which was labeled on medication/unable to titrate. The percentages of patients at the various dose categories (goal, moderate, low, none, on medication/unable to titrate) of beta-blockers, statins, and ACE/ARBs were examined at the time of hospital discharge and at 12-month follow-up.
Medication up-titration
We also examined rates of up-titration during the first year of follow-up, which was defined as an increase in the dose category of a medication from discharge to follow-up (e.g., increasing from a low dose to a moderate or goal dose). Although 12 month follow-up was examined, some patients (20% of cohort) only had 6-month follow-up data, in which case the medications reported at 6-months were used for the follow-up analyses. As up-titration of medications requires active decision making on the part of a physician, we examined outpatient follow-up intensity (defined as the patient-reported monthly rate of outpatient visits to cardiologists, cardiac surgeons, or primary care providers) for cardiologists and for all physicians (cardiologists and primary care physicians) to determine its association with achieving a goal dose of each medication at follow-up.
Statistical analysis
Baseline characteristics of patients treated with beta-blockers, statins, and ACE/ARBs were summarized with proportions for categorical variables, means with standard deviations for non-skewed continuous variables, and medians with interquartile ranges for skewed continuous variables. The percentage of patients at the various dose categories (goal, moderate, low, none) of beta-blockers, statins, and ACE/ARBs were summarized at the time of hospital discharge and at 12-month follow-up, and the percentage of patients at goal at each time point was compared using the McNemar’s test. The mean outpatient follow-up rate was compared between those who did vs. did not achieve goal dose of each medication at follow-up using t-tests.
For each of the 3 medication classes, we constructed multivariable logistic regression models to identify factors associated with achieving goal dose at follow-up. Patients on medications/unable to titrate were excluded from these analyses. We used hierarchical random effects models to adjust for patient clustering by site. Variables included in the model were selected a priori based on clinical judgment of factors that might impact medication titration. All 3 models included the following variables: discharge dose, age, sex, race, hypertension, diabetes mellitus, chronic lung disease, depression (as assessed with the 9-item Patient Health Questionnaire(13)), type of AMI (ST- or non-ST-elevation), Global Registry of Acute Coronary Events (GRACE) score(14), and the intensity of outpatient follow-up (monthly rate of physician visits). In addition to these variables, the beta-blocker model included SBP, heart rate, and LV systolic dysfunction at hospital discharge whereas the ACE/ARB model included SBP and estimated glomerular filtration rate at hospital discharge.
We conducted a number of sensitivity analyses. First, we evaluated the distribution of discharge SBPs to determine if the majority of patients who were discharged on low doses of beta-blockers and ACE/ARBs had lower blood pressures. Second, as it is recommended that beta-blockers be more slowly up-titrated in patients with LV systolic dysfunction, we repeated the analyses, restricting the model for only patients with normal or mild LV dysfunction (ejection fraction ≥40%). Third, for the statin model, even though data supports treating all patients after AMI with high statin doses,(6,15) we added low density lipoprotein cholesterol (LDL-C) levels to the multivariable model to assess whether in-hospital LDL-C levels were associated with physicians’ dosing of statins at follow-up. Fourth, we additionally adjusted for follow-up intensity to cardiologists specifically, to evaluate if type of provider visited was associated with a greater likelihood of goal dosing at follow-up. For each of these sensitivity analyses and for the main models, cubic splines were considered to account for non-normality of data on age, heart rate, SBP, and low density lipoprotein.
Missing data analysis
Among patients who survived 12 months, 4% of study participants were contacted but refused to participate in the interview and 11% were lost to follow-up (see Figure 1). To account for potential bias attributable to those with missing follow-up data, we calculated a non-parsimonious propensity score with successful follow-up as the dependent variable. An inversely weighted propensity score was assigned to each responder(16) to provide greater weight to the data of patients who were most like those without follow-up. Results were comparable with and without weighting, so only the unweighted analyses are presented.
All analyses were conducted using SAS v9.2 (SAS Institute, Inc., Cary, NC), and evaluated at a 2-sided significance level of <0.05.
RESULTS
Patient population
Of the 6838 patients enrolled in PREMIER and TRIUMPH, 41 did not survive to hospital discharge and 49 were discharged to hospice or left the hospital against medical advice. Of the remaining 6748 patients, 1413 (20.9%) had left ventricular systolic dysfunction that was at least moderate in severity and were thus eligible for the ACE/ARB analyses (Figure 1). Baseline characteristics of the patients in the study cohort who were eligible for beta-blocker, statin, and ACE/ARB therapy are shown in Table 1. The mean age of patients was ~60 years, and two-thirds were male and of white race. The mean SBP was >120mm Hg in each group, and most patients underwent either percutaneous or surgical coronary revascularization.
Table 1. Baseline characteristics.
| Beta-Blocker n=5368 |
Statin n=6560 |
ACE/ARB n=901 |
|
|---|---|---|---|
| Sociodemographics | |||
| Age (years) | 59.9 ± 12.6 | 59.6 ± 12.6 | 60.5 ± 12.7 |
| Female sex | 32.5% | 32.8% | 28.2% |
| White race | 69.2% | 70.1% | 63.6% |
| High school education | 79.0% | 79.1% | 75.7% |
| Lives alone | 23.5% | 23.5% | 27.1% |
| Comorbidities | |||
| Hypertension | 67.5% | 65.3% | 67.6% |
| Depression | 19.2% | 19.9% | 21.7% |
| Diabetes mellitus | 31.2% | 29.9% | 33.9% |
| Prior myocardial infarction | 20.4% | 21.1% | 29.3% |
| Prior stroke/transient ischemic attack | 7.5% | 7.4% | 8.5% |
| Prior heart failure | 8.9% | 9.3% | 19.8% |
| Current smoking | 35.7% | 37.4% | 36.2% |
| Body mass index (kg/m2) | 29.7 ± 6.5 | 29.4 ± 6.4 | 28.9 ± 6.1 |
| Estimated GFR* (mL/min/1.73m2) | 77 ± 29 | 78 ± 29 | 76 ± 27 |
| LDL-cholesterol (mg/dL) | 101 (77-129) | 101 (77-129) | 97 (71-126) |
| Clinical Presentation | |||
| Systolic blood pressure* (mmHg) | 124 ± 17 | 120 ± 19 | 120 ± 16 |
| Heart rate* (bpm) | 73 ± 12 | 73 ± 12 | 76 ± 13 |
| Peak troponin (ng/dL) | 5.6 (1.3-26.1) | 6.1 (1.4-28.3) | 7.2 (1.7-38.7) |
| LV ejection fraction (%) | 50 (40-57) | 50 (40-55) | 30 (25-35) |
| LV systolic dysfunction (mod/severe) | 19.4% | 21.1% | 100% |
| ST-Elevation myocardial infarction | 42.2% | 43.5% | 45.3% |
| GRACE discharge risk score | 103 ± 31 | 102 ± 31 | 111 ± 32 |
| Treatment Characteristics | |||
| Cardiac catheterization | 90.6% | 90.7% | 90.1% |
| Percutaneous coronary intervention | 62.6% | 63.1% | 55.6% |
| Bypass graft surgery | 10.4% | 10.0% | 10.2% |
| Length of stay (days) | 4 (3-6) | 4 (3-6) | 5 (3-8) |
ACE/ARB, angiotensin converting enzyme inhibitor/angiotensin II receptor blocker; GFR, glomerular filtration rate; LDL, low-density lipoprotein; LV, left ventricle Data are presented as mean ± SD (continuous non-skewed) or median (IQR) (continuous skewed), or percentage (categorical)
Assessed at hospital discharge
Discharge medications
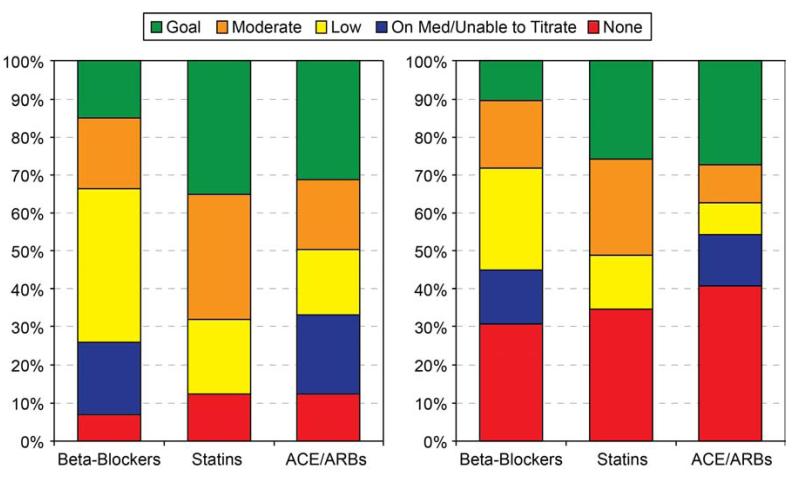
At hospital discharge, most eligible patients were discharged on some dose of the 3 secondary prevention medications: beta-blockers, 93%; statins, 88%, and ACE/ARB, 88% (Figure 2). However, 40% of patients were discharged on low doses of beta-blockers despite SBPs ≥110 mmHg, and only 19% of patients considered eligible for titration were discharged on goal doses. Patients without LV systolic dysfunction were discharged on goal doses of beta-blockers at similar rates as those with LV dysfunction (14.6% vs. 16.3%, p=0.18). One-third of patients were discharged on goal doses of statins, and 19% were on low doses. Among patients with LV dysfunction, 21% of patients were discharged on a low or moderate dose of ACE/ARB but were considered unable to titrate due to marginal blood pressures. Among the remainder, 17% were discharged on low doses despite reasonable SBP and 40% were discharged on goal doses of ACE/ARBs.
Figure 2. Frequency of dose categories for beta blockers, statins, and ACE/ARBs after acute myocardial infarction. Panel A displays dose categories at hospital discharge. Panel B displays dose categories at follow-up.
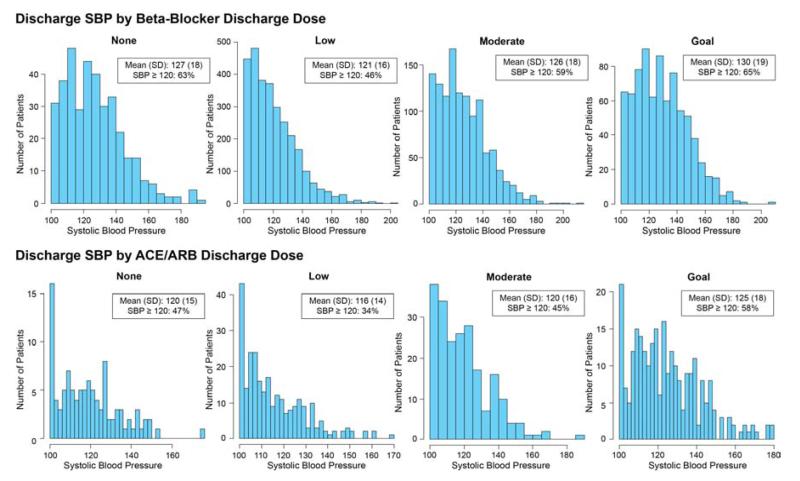
The distribution of SBPs for patients within each discharge dose category for beta-blockers and ACE/ARBs is shown in Figure 3. While patients with higher SBPs were more likely to be discharged on higher doses of these medications, 46% of patients on low doses and 59% of patients on moderate doses of beta-blockers had SBPs of at least 120 mmHg just prior to hospital discharge to allow for a potentially higher dose. Similarly, for those eligible for ACE/ARB therapy, 34% of patients on low doses and 45% on moderate doses had a SBP of at least 120 mmHg just prior to hospital discharge.
Figure 3. Systolic Blood Pressure at Discharge by Dose Category.
ACE/ARB, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers
Up-titration during outpatient follow-up
Twelve months after AMI, only 60-70% of patients reported taking any dose of beta-blockers, statins, or ACE/ARBs compared with ~90% at hospital discharge (Figure 2). Goal doses of medications were achieved less frequently at 12-months than at discharge, with only 12% (p<0.001 for change from discharge to 12 months), 26% (p<0.001), and 32% (p=0.74) of eligible patients on goal doses of beta-blockers, statins, and ACE/ARBs, respectively. Patients with LV systolic dysfunction were more likely to be on goal doses of beta-blockers at 12 months than those without LV systolic dysfunction (16.2% vs. 9.2%, p<0.001). Up-titration of medication dose during outpatient follow-up occurred infrequently, with dose increases in only 20.4% of patients on beta-blockers, 24.4% of patients on statins, and 31.9% of patients on ACE/ARB (Appendix eTable 4).
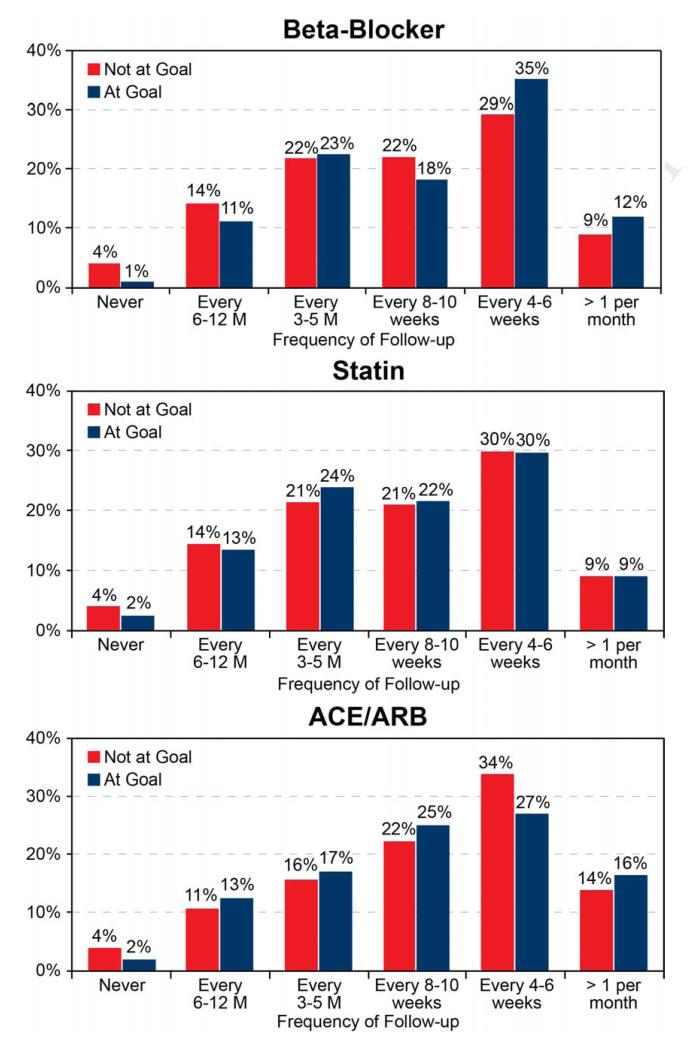
For each of the 3 medications, the frequency of physician follow-up for patients who were and were not at goal doses is shown in Figure 4 (frequency of follow-up to a cardiologist or cardiac surgeon is shown in Appendix eFigure 1). There was a modestly higher follow-up frequency in patients who achieved a goal beta-blocker dose at follow-up compared to those who did not (mean frequency: every 7.5 weeks vs. every 8.2 weeks, respectively; p=0.014). For statins and ACE/ARBs, there were no significant differences in the mean frequency of physician visits during follow-up among patients who did and who did not achieve goal doses of treatment (statins: 8.2 weeks vs. 8.0 weeks, p=0.76; ACE/ARBs: 6.6 weeks vs. 7.1 weeks, p=0.35). Patients who achieved goal doses at follow-up had more frequent visits to cardiologists than those who did not (beta-blockers: 14.0 weeks vs. 16.0 weeks, p<0.001; statins: 14.4 weeks vs. 16.0 weeks, p=0.022; ACE/ARBs: 12.4 weeks vs. 14.0 weeks, p=0.13).
Figure 4. Outpatient Follow-up Intensity.
Frequency of follow-up for patients who did and who did not achieve goal dose at follow-up
In site-level hierarchical logistic regression models adjusting for sociodemographic and clinical factors, including intensity of outpatient follow-up, a patient who was discharged on a goal dose of medication was 6-8 times more likely to be on a goal dose at the 12-month follow-up as compared with those not discharged on goal medication doses: beta-blockers (adjusted odds ratio [OR], 6.08 [95% CI: 3.70-10.01]), statins (adjusted OR, 8.22 [95% CI: 6.20-10.90]), and ACE/ARBs (adjusted OR, 5.80 [95% CI: 2.56-13.16]); all p <0.001 (Table 2).Results were similar when adjusted for cardiac-specific follow-up intensity: beta-blockers (adjusted odds ratio [OR], 6.06 [95% CI: 3.68-9.97]), statins (adjusted OR, 8.21 [95% CI: 6.20-10.90]), and ACE/ARBs (adjusted OR 5.42 [95% CI: 2.40-12.23]); all p <0.001.
Table 2. Patient and treatment factors associated with being at goal medication dose at 12-month follow-up.
| Beta-Blocker | Statin | ACE/ARB | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Medication dose at discharge | ||||||
| Low dose | 0.50 (0.30-0.83) | 0.008 | 0.67 (0.47-0.95) | 0.024 | 0.86 (0.34-2.15) | 0.748 |
| Moderate dose | 0.93 (0.55-1.59) | 0.799 | 1.36 (1.01-1.82) | 0.043 | 1.83 (0.78-4.27) | 0.162 |
| Goal dose | 6.08 (3.70-10.01) | <0.001 | 8.22 (6.20-10.90) | <0.001 | 5.80 (2.56-13.16) | <0.001 |
|
| ||||||
| Age (per 1 y) | 0.98 (0.97-1.00) | 0.047 | 1.00 (0.98-1.02) | 0.880 | 1.00 (0.97-1.03) | 0.934 |
| Female sex | 0.94 (0.72-1.23) | 0.638 | 1.01 (0.86-1.19) | 0.872 | 0.75 (0.43-1.29) | 0.296 |
| White race | 1.25 (0.93-1.68) | 0.145 | 1.02 (0.85-1.24) | 0.809 | 1.04 (0.61-1.79) | 0.880 |
| Hypertension | 1.46 (1.07-2.00) | 0.017 | 1.02 (0.87-1.20) | 0.830 | 1.33 (0.75-2.34) | 0.327 |
| Depression | 0.98 (0.71-1.36) | 0.910 | 1.02 (0.84-1.24) | 0.807 | 0.67 (0.35-1.30) | 0.235 |
| Diabetes mellitus | 1.40 (1.08-1.82) | 0.011 | 0.86 (0.72-1.02) | 0.083 | 0.97 (0.59-1.61) | 0.906 |
| Chronic lung disease | 1.26 (0.83-1.90) | 0.277 | 0.89 (0.66-1.19) | 0.433 | 1.33 (0.64-2.76) | 0.443 |
| ST-elevation MI | 1.04 (0.80-1.36) | 0.752 | 1.06 (0.91-1.24) | 0.429 | 0.83 (0.50-1.38) | 0.465 |
| GRACE score (per 1 pt) | 1.00 (1.00-1.01) | 0.365 | 1.00 (1.00-1.01) | 0.407 | 1.00 (0.98-1.01) | 0.724 |
| Follow-up rate (per 1 visit per month) |
1.09 (0.82-1.45) | 0.560 | 1.01 (0.85-1.21) | 0.875 | 1.33 (0.87-2.03) | 0.184 |
| Left Ventricular Dysfunction | 1.88 (1.40-2.53) | <0.001 | NA | NA | ||
| SBP* (per 1 mmHg) | 1.00 (0.99-1.01) | 0.727 | NA | 1.01 (1.00-1.03) | 0.092 | |
| Heart rate* (per 1 bpm) | 1.00 (0.99-1.01) | 0.426 | NA | NA | ||
| GFR* (per 1 mL/min/1.73m2) | NA | NA | 1.00 (0.99-1.01) | 0.627 | ||
ACE/ARB, angiotensin converting enzyme inhibitor/angiotensin II receptor blocker; MI, myocardial infarction; GRACE, Global Registry of Acute Coronary Events; GFR, glomerular filtration rate
Assessed at hospital discharge
In these models, patients with hypertension and diabetes were more likely to be on goal doses of beta-blockers at follow-up. In addition, patients with LV systolic dysfunction at the time of AMI were nearly 2 times more likely to be on goal doses of beta-blockers at follow-up than those without LV dysfunction (OR 1.88, 95% CI 1.40-2.53, p<0.001). No other patient factors, other than discharge dose, were significantly associated with being on a goal dose of any of the 3 medications at follow-up. Notably, follow-up intensity was not significantly associated with being on goal dose for any medication at follow-up. However, when follow-up was restricted to cardiologists, follow-up intensity was significantly associated with achieving goal dose at follow-up (adjusted OR [95% CI] for cardiology follow-up rate [per 1 visit per month]; beta-blockers: 1.69 [1.05-2.72], p=0.031; statins: 1.37 [1.03-1.83], p=0.033; ACE/ARBs: 2.48 [0.99-6.22], p=0.053). In sensitivity analyses, the strong association between goal dose of medication at discharge and follow-up was not materially altered, including additional adjustment for in-hospital LDL-C levels (statin analysis) or limiting the study sample to only those patients without LV systolic dysfunction (Appendix eTable 5).
DISCUSSION
In a large, prospective cohort of patients hospitalized with AMI, we found that the use of evidence-based medications at hospital discharge was high, with ~90% of all eligible patients treated with beta-blockers, statins, and ACE/ARBs. However, the prescribed doses for these medications were below those examined in clinical trials, with nearly 85% of patients discharged on beta-blocker doses and two-thirds of patients discharged on statin and ACE/ARB doses that were substantially below (<75%) the doses with established efficacy. This is particularly concerning in the case of statins, where there should be few clinical reasons not to start a patient on a goal statin dose early in the AMI hospitalization. While discharging patients on sub-optimal doses of beta-blockers and ACE/ARBs may be appropriate clinical care if these doses are then actively increased to goal doses during outpatient follow-up, up-titration was uncommon during the 12 months following AMI—occurring in only 25% of patients—and was not influenced by blood pressure or heart rate during hospitalization. In fact, the strongest predictor of being on a goal dose for any of the 3 medications during follow-up was being on a goal medication dose at hospital discharge. Collectively, our findings suggest the majority of patients with AMI may be undertreated, despite meeting criteria for performance measures for secondary prevention medications. These findings highlight the limitation of current performance measures that credit providers for using any dose of medication, even if well below doses with established clinical benefit.
Prior Studies
Actively titrating secondary prevention therapy to goal doses is particularly important as the efficacy of these medications has been demonstrated to be dose-related. For statins, two large clinical trials have shown that higher statin doses are superior in reducing the risk of rehospitalization and death after an AMI.(6,7,17) A trial comparing low versus high doses of lisinopril and a second comparative effectiveness study of different doses of losartan and candesartan both demonstrated that higher doses of these classes of medications reduced the risk of heart failure hospitalizations and death.(4,5) Finally, for beta blockers, two trials of heart failure patients showed that target doses of bucindilol and carvedilol were associated with more improvement in ejection fraction,(3,18) fewer hospitalizations,(3) and lower mortality compared with low or moderate doses.(3) While there has been some conflicting evidence published from an observational registry in which patients treated with low doses of beta blockers had lower mortality than those discharged on high doses,(19) most studies—and, in particular, clinical trials that do not have inherent treatment bias found in registries—have demonstrated that the higher treatment doses are most effective in reducing morbidity and mortality after an AMI.
Although several studies have reported rates of medication treatment among patients hospitalized with AMI at discharge and follow-up,(20-23) these studies have not examined treatment doses or intensification of therapy over time. Therefore, they did not establish whether patients were being treated at doses with established efficacy from clinical trials. Studies that have examined medication dosing are limited; however, these have demonstrated similar patterns of medication dosing as what we found. A study of 606 patients admitted with AMI from 4 hospitals in 1995 found that while 58% of patients without contraindications were discharged on some dose of beta-blocker, 76% were discharged on doses ≤25% of the target doses from clinical trials and only 11% were discharged on doses of >50% of the target doses.(24) While these authors did not have information on follow-up, our study highlights that, despite 15 years of quality improvement, little has changed regarding optimal dosing of secondary prevention medications. A second study of 382 patients with AMI from 41 hospitals found that 76% of patients discharged on a statin were on the same dose 1 year later, with intensification of therapy occurring in only 12%.(25) Our data both support and extend the findings of prior studies by examining patterns of medication prescription and dosing at both hospital discharge and outpatient follow-up, evaluating 3 proven therapies in AMI, and including data from over 30 centers for greater generalizability.
Potential Explanations
There are several potential reasons as to why patients might have been treated at doses far lower than those with established efficacy. First, some patients who are on lower doses of medications are truly receiving their maximally tolerated dose (e.g., low blood pressure for beta-blockers and ACE/ARBs). Furthermore, there may be some patients who were not up-titrated due to side effects, such as light-headedness or myalgias, or due to patient preference. However, given our sensitivity analyses, it is unlikely that low blood pressure or other dose-limiting side effects were the primary limiting factors in achieving goal doses in the majority of patients.
Second, as hospitalization stays for AMI have continued to decrease over the past decade,(26) clinicians today have less time to optimize medical therapy during the index hospitalization and thus defer intensification of medication therapy until outpatient follow-up. However, we found that medication up-titration occurred infrequently, suggesting there is significant clinical inertia in intensifying treatment during the outpatient period. Interestingly, we found that outpatient follow-up intensity was not associated with an increased likelihood of achieving goal dose at follow-up. However, when we restricted follow-up to cardiologists, there was a modest association between follow-up intensity and goal dose at follow-up, indicating that specialists may be more aggressive in up-titrating these medications. The general lack of active up-titration in the outpatient setting may be because some clinicians do not view up-titration of these medications as an important therapeutic goal, are unaware of the target medication doses (i.e., the doses with proven clinical efficacy), or have other competing medical issues that they need to address during follow-up visits.(27) As current performance measures evaluate only whether patients are on a medication, clinicians also may mistakenly equate being on a treatment as being on effective treatment. This inertia has been shown to be pervasive in the outpatient management of other chronic medical conditions such as hypertension(28) and diabetes.(29) Thus, strategies such as improved care coordination at discharge(30) or outpatient tools that assist providers with automating medication titrations (e.g., pharmacist-assisted monitoring, clinical reminders, education and feedback(31)) may lead to greater success in treatment intensification during follow-up. Since the protective effects of these medications have been shown to be dose-related,(4-7) the inability to achieve goal doses at discharge or to intensify treatment during follow-up represents an important gap in the current quality of AMI care.
Implications
The findings of our study have important implications for performance measurement in AMI. The current measures do not distinguish between those hospitals that make robust or meager efforts to optimize medical therapy dosing in patients with AMI. Rates of medication use at clinically-proven doses might be improved if performance measures incorporated assessments of medication dosing. Inclusion of optimal drug dosing at discharge has the potential to improve outcomes, although this would understandably be more challenging in terms of data collection. There is also a need to further our understanding of outpatient care, as the current practice of evaluating AMI care only at hospital discharge has substantial limitations in comprehensively capturing the quality of care of AMI patients. Outpatient registries, such as PINNACLE,(32) may provide additional insights into how best to track and optimize outpatient cardiac care.
Limitations
There are several limitations to the current analyses that warrant consideration when interpreting our results. First, our analyses on medication up-titration during follow-up used information from vital signs, creatinine, and adverse side effects at hospital discharge. As a result, some patients may have been misclassified as not being at their maximally tolerated dose of medication at follow-up (either due to new or worsening medical factors [e.g., new heart failure or renal dysfunction], side effects [e.g., light-headedness, myalgias], or patient preferences). However, as we excluded patients with SBP <110 mmHg from these up-titration analyses, we suspect this is relevant to a minority of patients and does not diminish our finding that most patients are not on goal doses of these important medications 12 months after discharge. More in-depth qualitative research, with detailed interviews of patients and physicians, would be particularly useful in deepening our understanding of why up-titration did not occur more frequently in many patients.
Second, medications without a specific indication for treatment of post-AMI or heart failure patients were excluded in the study (e.g., nebivolol, olmesartan) but might be appropriate for particular patients. However, these excluded patients only represented 1% of patients taking beta-blockers and 6% taking ACE/ARBs, and therefore were unlikely to have affected our findings. Third, we did not have follow-up data on ~15% of surviving patients; however, we performed a sensitivity analysis in which we weighted the responses of participants by the inverse of their likelihood to follow-up, to provide greater weight to the data of patients who were most like those without follow-up. As results were comparable with and without weighting, it is unlikely that our results were significantly biased by loss-to-follow-up. Finally, while we identified gaps in achieving optimal dosing of secondary prevention medications in post-MI patients, we did not evaluate the effectiveness of optimal dosing of these medications because of the likelihood of significant bias by indication with such analyses, whereby patients at higher risk of adverse outcomes [e.g., LV dysfunction] are more likely to receive goal doses of medication or undergo more frequent medication up-titration than lower-risk patients.
Conclusion
We found that the majority of patients hospitalized with an AMI were routinely discharged on secondary prevention medications at doses substantially below the levels proven to be efficacious in clinical trials. These doses were infrequently increased as outpatients, even after one year. Since being on a target medication dose at discharge was the strongest predictor of being on a target dose at follow-up, our findings suggest that clinicians should attempt to maximize the doses of secondary prevention therapy during the index hospitalization and to up-titrate these medications during outpatient follow-up. More in-depth qualitative research is necessary to deepen our understanding of why up-titration did not occur more frequently and could provide important insights as to mechanisms by which care can be improved. Performance measures, in turn, may need to incorporate doses of medications to better achieve their goal of truly optimal medical therapy.
Supplementary Material
Acknowledgments
Funding Source: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, Blood Institute): SCCOR Grant #P50HL077113. PREMIER was principally supported by a grant from Cardiovascular Therapeutics, Inc., Palo Alto, CA. The funding organizations did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Maddox is supported by a Career Development Grant Award from the Veterans Affairs Health Services Research and Development Service. Dr. Daugherty is supported by a Career Development Grant Award (K08HL103776) from the National Heart Lung and Blood Institute. Dr. Chan is supported by a Career Development Grant Award (K23HL102224) from the National Heart, Lung, and Blood Institute. Dr. Spertus is supported by a Clinical and Translational Science Award (1UL1RR033179).
Abbreviations
- ACE
angiotensin converting enzyme inhibitor
- AMI
acute myocardial infarction
- ARB
angiotensin II receptor blocker
- GRACE
Global Registry of Acute Coronary Events
- LDL-C
low density lipoprotein cholesterol
- LV
left ventricular
- PREMIER
Prospective Registry Evaluating Myocardial Infarction: Events and Recovery
- SBP
systolic blood pressure
- TRIUMPH
Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: The authors report no potential conflicts of interest.
REFERENCES
- 1.Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation. 2008;118:2596–648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 2.Peterson ED, Roe MT, Mulgund J, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–20. doi: 10.1001/jama.295.16.1912. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Gilbert EM, Abraham WT, et al. MOCHA Investigators Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996;94:2807–16. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 4.Svanström H, Pasternak B, Hviid A. Association of treatment with losartan vs candesartan and mortality among patients with heart failure. JAMA. 2012;307:1506–12. doi: 10.1001/jama.2012.452. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Poole-Wilson PA, Armstrong PW, et al. ATLAS Study Group Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation. 1999;100:2312–8. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 6.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 7.de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–16. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 8.Werner RM, Bradlow ET. Relationship between Medicare’s hospital compare performance measures and mortality rates. JAMA. 2006;296:2694–702. doi: 10.1001/jama.296.22.2694. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 10.Glickman SW, Ou FS, DeLong ER, et al. Pay for performance, quality of care, and outcomes in acute myocardial infarction. JAMA. 2007;297:2373–80. doi: 10.1001/jama.297.21.2373. [DOI] [PubMed] [Google Scholar]
- 11.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–97. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 16.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–60. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 17.Murphy SA, Cannon CP, Wiviott SD, et al. Effect of intensive lipid-lowering therapy on mortality after acute coronary syndrome (a patient-level analysis of the Aggrastat to Zocor and Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 trials) Am J Cardiol. 2007;100:1047–51. doi: 10.1016/j.amjcard.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 18.Bristow MR, O’Connell JB, Gilbert EM, et al. Bucindolol Investigators Dose-response of chronic beta-blocker treatment in heart failure from either idiopathic dilated or ischemic cardiomyopathy. Circulation. 1994;89:1632–42. doi: 10.1161/01.cir.89.4.1632. [DOI] [PubMed] [Google Scholar]
- 19.Barron HV, Viskin S, Lundstrom RJ, et al. Beta-blocker dosages and mortality after myocardial infarction: data from a large health maintenance organization. Arch Intern Med. 1998;158:449–53. doi: 10.1001/archinte.158.5.449. [DOI] [PubMed] [Google Scholar]
- 20.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–12. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 21.Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152:454, e1–8. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, Olomu A, Corser W, Rovner DR, Holmes-Rovner M. Outpatient medication use and health outcomes in post-acute coronary syndrome patients. Am J Manag Care. 2006;12:581–7. [PubMed] [Google Scholar]
- 23.Bi Y, Gao R, Patel A, et al. Evidence-based medication use among Chinese patients with acute coronary syndromes at the time of hospital discharge and 1 year after hospitalization: results from the Clinical Pathways for Acute Coronary Syndromes in China (CPACS) study. Am Heart J. 2009;157:509–516. e1. doi: 10.1016/j.ahj.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Viskin S, Kitzis I, Lev E, et al. Treatment with beta-adrenergic blocking agents after myocardial infarction: from randomized trials to clinical practice. J Am Coll Cardiol. 1995;25:1327–32. doi: 10.1016/0735-1097(94)00552-2. [DOI] [PubMed] [Google Scholar]
- 25.Melloni C, Shah BR, Ou FS, et al. Lipid-lowering intensification and low-density lipoprotein cholesterol achievement from hospital admission to 1-year follow-up after an acute coronary syndrome event: results from the Medications ApplIed aNd SusTAINed Over Time (MAINTAIN) registry. Am Heart J. 2010;160:1121–9. 1129, e1. doi: 10.1016/j.ahj.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Saczynski JS, Lessard D, Spencer FA, et al. Declining length of stay for patients hospitalized with AMI: impact on mortality and readmissions. Am J Med. 2010;123:1007–15. doi: 10.1016/j.amjmed.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825–34. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari P, Hess L, Pechere-Bertschi A, Muggli F, Burnier M. Reasons for not intensifying antihypertensive treatment (RIAT): a primary care antihypertensive intervention study. J Hypertens. 2004;22:1221–9. doi: 10.1097/00004872-200406000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Ziemer DC, Miller CD, Rhee MK, et al. Clinical inertia contributes to poor diabetes control in a primary care setting. Diabetes Educ. 2005;31:564–71. doi: 10.1177/0145721705279050. [DOI] [PubMed] [Google Scholar]
- 30.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301:603–18. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 31.Ziemer DC, Doyle JP, Barnes CS, et al. An intervention to overcome clinical inertia and improve diabetes mellitus control in a primary care setting: Improving Primary Care of African Americans with Diabetes (IPCAAD) 8. Arch Intern Med. 2006;166:507–13. doi: 10.1001/archinte.166.5.507. [DOI] [PubMed] [Google Scholar]
- 32.Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.