Abstract
Ovarian cancer (OVCA) has a high incidence of recurrence and a high rate of mortality. We performed a pilot study to evaluate the usefulness of tumor autoantibodies to tumor associated antigens (TAA) to predict OVCA recurrence. A validation study with 56 antigens, previously identified in the initial phase of the study, along with 13 known tumor antigens on protein arrays was performed on an independent cohort of recurrent and non-recurrent OVCA patients. Statistical analyses revealed that a panel of 3 antigens predicted recurrence at a median time of 9.07 months prior to clinical recurrence in a study population, where majority of patients had CA125 values less than 35 U/ml, with an average sensitivity, specificity and accuracy of 94.7%, 86.7% and 93.3% respectively. One of the top 3 antigens has been associated with the development of polymyositis (PM) which has been shown in some cases to precede the occurrence of ovarian carcinoma. Our results indicate that these 3 antigens have potential for predicting recurrence at an early time and may have better prognostic utility than CA125 alone for early therapeutic intervention. These biomarkers could guide us to identify those patients that could benefit most from maintenance or consolidation therapy.
Keywords: Ovarian cancer, recurrence, humoral immune response, tumor autoantibodies, protein arrays
1. Introduction
The asymptomatic nature of OVCA together with lack of effective diagnostic screening tools results in extreme difficulty for detecting this disease at an early stage. In approximately 70% of cases, OVCA is detected at an advanced stage [1]. Despite an initial response to primary treatment more than 85% of patients with late stage serous adenocarcinoma will experience OVCA recurrence after the completion of front-line treatment even with optimal surgical cytoreduction and platinum-based combination chemotherapy [2,3]. Patients are labeled as having platinum-sensitive tumors when their relapse occurs at least 6 months following their last platinum treatment compared to patients with platinum-resistant tumors who fail to achieve complete response after front-line therapy or relapse in less than 6 months from the completion of therapy [4]. Over the years CA125 has emerged as an useful biomarker for monitoring of OVCA recurrence [5]. The study conducted by Krivak and colleagues [6] indicated that following surgery and 6 cycles of chemotherapy, OVCA patients with persistently abnormal CA125 levels > 35 U/ml were 2.45 times more likely to have a disease progression (95% CI: 1.52-3.95, P < 0.001) and the risk of death for those patients was more than 2.78 times (95% CI: 1.66-4.65, P < 0.001) than those with CA125 less than 35 U/ml. Several studies evaluated the risk of recurrence in epithelial OVCA patients with rising CA125 values below the upper limit of normal (< 35 U/ml). Wilder and colleagues [7] reported that OVCA patients who had three progressively rising CA125 levels within a normal range (< 35 U/ml) at 1-3 months follow-up intervals were associated with a high risk of tumor recurrence.
Tumor autoantibodies develop at very early stage, well before the clinical manifestations of the disease because of the activation of humoral immune responses due to the presence of small amounts of TAAs even at very low tumor burden [8]. Thus, antibodies against tumor specific proteins may provide the earliest candidate biomarkers for detecting OVCA as well as for monitoring OVCA during the first-line chemotherapy that will provide a signal for the risk of developing OVCA recurrence. We applied a robust method, “epitomics” [9], which is a combination of high-throughput cloning of tumor antigens, biopanning of relevant antigens with OVCA-specific IgGs, and protein microarray-based serological screening. We previously identified biomarkers that were diagnostically useful for the early detection of OVCA [9,10]. The objective of the present study was to evaluate the efficacy of those diagnostic biomarkers for predicting recurrence in platinum-sensitive OVCA patients where a majority of the population had CA125 within the normal range (< 35 U/ml). We found that a subset of antigens from our previously identified diagnostic biomarker panel was able to discriminate recurrent from non-recurrent OVCA patients at a median time of 9.07 months prior to clinical recurrence. One of the antigens in the biomarker panel has been linked to the development of PM that precedes the occurrence of OVCA [11,12].
2. Materials and methods
2.1. Study population
Patients diagnosed and treated for late stage serous OVCA at Karmanos Cancer Institute or St. John Hospital & Medical Center (Detroit, MI) or Oakwood Hospital & Medical Center (Dearborn, MI) were entered onto the study at the time of their diagnosis or during a return visit within 5 years of initial diagnosis. Medical records were reviewed to determine CA125 levels, disease status, chemotherapy status, and time to recurrence (TTR) over a multi-year period. Cases were limited to those diagnosed between 1997 and 2007 to ensure sufficient follow up. On the basis of this information patients were divided into two groups: 1) No Recurrence (NR), defined as no clinical evidence of disease for at least 48 months, and 2) Recurrent Disease (R), defined as clinical evidence of disease and/or doubling of CA125 within approximately 3 years of diagnosis (range 11–39 months). Recurrent disease patients selected for the validation set had a disease-free interval of at least six months (range 6.6–34).
2.2. Blood sample collection and processing
Blood was collected and processed as discussed in our previous study [9]. Blood samples were selected for use on the basis of time since diagnosis, CA125 level, disease status, and chemotherapy status at the time of blood collection. For the initial study we used specimens from 3 time points for all cases (R and NR); the specimen obtained at time of enrollment and at two post-diagnosis intervals. (Supplementary Table 1A).
For the validation study, samples were collected from recurrent cases at a median time of 9.07 months (range = 2.1 to 18.9 months) prior to clinical recurrence. Most patients had a normal CA125 and no clinical evidence of disease at that time. For non-recurrent cases, samples were collected at least 11 months after completion of chemotherapy, with no evidence of disease and a normal CA125 level. Patients’ characteristics are summarized in Supplementary Table 1A (Initial Study) and Supplementary Table 1B (Validation Study).
Study procedures were approved by the Wayne State University, St. John Hospital & Medical Center, and Oakwood Hospital & Medical Center Institutional Review Boards. All participants provided written informed consent.
2.3. Serological screening of protein antigen arrays
Robotically printed protein arrays comprising 174 individual T7 phage bearing antigens (initial study), 80 individual T7 phage bearing tumor antigens (validation study) and thirteen known proteins (Supplementary Table S3) were performed as previously described [9]. The known overexpressed proteins except p53 (recombinant histidine-tagged p53 prepared in our lab) were purchased commercially, (Supplementary Table S3).
In both the initial and validation study we used empty phage vectors that served as negative controls. The initial serological screening of the nitrocellulose membranes (arrays) was performed with serum samples that were obtained from recurrent (n = 5) and non-recurrent (n = 5) OVCA patients by following the experimental procedures as described in our previous study [9]. For the validation study, the membranes were processed with samples from the initial study (see above) along with serum samples obtained from independent cohorts comprising recurrent (n = 25) and non-recurrent (n = 5). Immunoscreening was performed by following the same method as discussed earlier [9]. For the initial antigen selection serological study and the validation study 1:100 serum dilution and rabbit-anti human secondary antibody conjugated with IR-Dye800 (Rockland, Gilbertville, PA, USA) at 1:5000 dilution were used. The arrays were scanned using Odyssey at 800 nm wavelength by following manufacturer’s instructions. The fluorescence intensity at each spot was quantified using ImaGene™ software. (Biodiscovery, Inc., El Segundo, CA, USA).
Nitrocellulose membranes were separately treated with mouse anti-T7 antibody directed toward T7 phage coat protein (EMD Bioscience Inc: Novagen, SanDiego, CA, USA). For data normalization Alexa fluor 680 goat anti-mouse IgG secondary antibody (Molecular probes, Invitrogen, Grand Island, NY, USA) at 1:10000 dilution was used and the membranes were scanned using Odyssey at 700 nm wavelength.
2.4. Statistical analyses
2.4.1. Background correction and normalization
The same procedure was followed for both the initial and the validation study. The image quantified files were read into R using the limma package suite of software. Any measurement with a “0” weight (defined as an empty or poor spot) was set to missing. Each of the assays (initial and validation study) was background corrected using the “minimum” method. This created the “red” channel intensity measurement for each assay. We created a pseudo “green” channel array using the point-wise median of the 1536 intensity values from the 3 assays (for initial study) and 12 assays (for validation study) performed only with the T7-antibodies. Then each assay was normalized to this “green” channel assay using the “median” method. Subsequently for each of the assays, we calculated the median corrected intensity measurement for each of the antigens, which were measured in triplicate on each assay. This dataset was used to conduct all of the analyses that are discussed in the following results section.
2.4.2. Statistical analyses for the initial study
As this screening dataset had only 10 patients (5 recurrent, 5 non-recurrent), many different statistical methods were used to derive a list of antigens to analyze in the set of patients in the validation experiment. The statistical methods used included t-tests and the Wilcoxon rank sum test (non-parametric analog to the t-test). Antigens that were significant at 0.05 for any of the 3 tests were retained for the validation study.
2.4.3. Statistical analyses for the validation study
The 10 patients that were measured for the initial study were also measured in the validation study to validate the reproducibility of the antigen measurements in addition to the 30 new patient samples (25 recurrent, 5 non-recurrent). As the recurrent and non-recurrent sample sizes were quite different, weighted analyses were performed for the validation study. We initially determined the nominal level of significance using logistic regression, t-tests and Wilcoxon rank sum tests for each antigen for the 30 new patient samples. Bootstrapping was then used in conjunction with both logistic regression and classification and regression trees (CART) to evaluate if any antigens were predictive of recurrence status. Briefly, we created a bootstrapped sample of 40 patients (ensuring that the 10 patients that were measured in the initial study were always in the training dataset). A prediction model was created (either a single antigen logistic regression or CART model) using the bootstrapped sample. Then we applied the model to the holdout patient samples (not in the bootstrapped sample) and retained the predictive probability of recurrence. Over 10,000 bootstrapped samples, each of the new 30 patient samples was held out approximately 46% of the time. We summarized the predicted values for each antigen for the pooled recurrent cases using the median and inter-quartile range (IQR). A similar summarization was done for the non-recurrent patients. Those antigens for which the IQR was greater than 0.5 for the recurrent cases and less than 0.5 for the non-recurrent cases were deemed significant. Using the IQR instead of a confidence interval is necessary since individual patients may be poorly predicted; the IQR will yield antigens that predict well over all samples. Individual plots of the 95% bootstrapped predicted confidence intervals were produced to determine which patients were poorly predicted by the model.
3. Results
The goal of this study was the evaluation of a classifier using autoantibodies directed against TAAs as a potential prediction tool for detecting OVCA recurrence in platinum-sensitive serous adenocarcinoma at a sufficiently early time that could impact treatment disposition. We performed an initial study based on immunoscreening of our diagnostic biomarkers previously identified for early detection to select a biomarker panel that was able to discriminate recurrent OVCA from non-recurrent OVCA at first and second post diagnosis intervals (Supplementary Table S2). Next, we performed a validation study where we evaluated the utility of this biomarker panel compared to CA125 for predicting OVCA recurrence at a median time of 9.07 months in a population in which the majority of patients showed recurrence prior to a rise in CA125 level.
3.1. Initial biomarker selection for detecting early OVCA recurrence by profiling tumor autoantibody response
In the discovery phase we performed a serological screening of protein arrays comprising 174 previously identified diagnostic biomarkerswith serum obtained at 3 time points from recurrent (n = 5) and non-recurrent (n = 5) OVCA (Supplementary Table S2). We identified antigens that were able to distinguish recurrent OVCA from non-recurrent OVCA at 2 different intervals after diagnosis.
For both the recurrent and non-recurrent OVCA patients, we used the assay from serum taken at first post diagnosis interval. All of these OVCA patients had a CA125 value less than 35 U/ml at that time. A t-test and Wilcoxon rank sum test were performed for each antigen. We identified 17 statistically significant antigens (from either test) that discriminated recurrent from non-recurrent OVCA patients at the first post diagnosis interval (p < 0.05) (Table 1). Four antigens 2002Mec1 BP4 P3 A11, Mec1 1A3, Mec1 4B5, and Mec1 5C7 were identified that discriminated between the recurrent and non-recurrent groups.
Table 1.
Performance of antigens in discriminating recurrent from Non-recurrent ovarian cancer patients at 2 different diagnosis intervals
| First post diagnosis interval (Method A) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Antigen | TP | FN | FP | TN | Sensitivity | Specificity | PPV | NPV |
| 02Mecl_BP4_Pl_Dl 1 < −0.193 | 4 | 1 | 0 | 5 | 80 | 100 | 100.0 | 83.3 |
| 02Mecl_BP4_P3_Al 1 ≥ 0.288 | 5 | 0 | 0 | 5 | 100 | 100 | 100.0 | 100.0 |
| Mecl_lA3 < 0.135 | 5 | 0 | 0 | 5 | 100 | 100 | 100.0 | 100.0 |
| Mecl_lB4 < 0.123 | 4 | 1 | 0 | 5 | 80 | 100 | 100.0 | 83.3 |
| Mecl_2D7 ≥ −0.75 | 5 | 0 | 1 | 4 | 100 | 80 | 83.3 | 100.0 |
| Mecl_2G9 ≥ 0.197 | 5 | 0 | 1 | 4 | 100 | 80 | 83.3 | 100.0 |
| Mecl_3A7 ≥ −0.105 | 4 | 1 | 0 | 5 | 80 | 100 | 100.0 | 83.3 |
| Mecl_3D5 ≥ −0.425 | 5 | 0 | 1 | 4 | 100 | 80 | 83.3 | 100.0 |
| Mecl_3Gl ≥ −0.687 | 5 | 0 | 1 | 4 | 100 | 80 | 83.3 | 100.0 |
| Mecl_4B5 <0.331 | 5 | 0 | 0 | 5 | 100 | 100 | 100.0 | 100.0 |
| Mecl_4B7 < −0.271 | 4 | 1 | 0 | 5 | 80 | 100 | 100.0 | 83.3 |
| Mecl_4F8 < 0.355 | 5 | 0 | 1 | 4 | 100 | 80 | 83.3 | 100.0 |
| Mecl_4G9 < −0.538 | 4 | 1 | 0 | 5 | 80 | 100 | 100.0 | 83.3 |
| Mecl_5A3 < 0.083 | 5 | 0 | 2 | 3 | 100 | 60 | 71.4 | 100.0 |
| Mecl_5A7 < −0.437 | 4 | 1 | 0 | 5 | 80 | 100 | 100.0 | 83.3 |
| Mecl_5C7 < −0.414 | 5 | 0 | 0 | 5 | 100 | 100 | 100.0 | 100.0 |
| Mecl_5H10 < −0.767 | 4 | 1 | 0 | 5 | 80 | 100 | 100.0 | 83.3 |
| CA125 ≥ 35 | 0 | 5 | 0 | 5 | 0 | 100 | NaN* | 50.0 |
|
| ||||||||
| First post diagnosis interval (Method B) | ||||||||
|
| ||||||||
| Antigen | TP | FN | FP | TN | Sensitivity | Specificity | PPV | NPV |
|
| ||||||||
| 02Mec 1 _BP4_P2_A2 ≥ −0.017 | 4 | 0 | 0 | 3 | 100 | 100 | 100 | 100 |
| 02Mecl_BP4_P3_F10 ≥ −0.335 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75 |
| Mecl.lEl ≥ 0.202 | 5 | 0 | 0 | 3 | 100 | 100 | 100 | 100 |
| Mecl_4B5 < −0.039 | 5 | 0 | 0 | 3 | 100 | 100 | 100 | 100 |
| Mecl_4C10 ≥ −0.128 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75 |
| Mecl_4D9 < −0.254 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75 |
| Mecl_4F2 ≥ 0.607 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75 |
| Mecl_5A7 < −0.241 | 5 | 0 | 0 | 3 | 100 | 100 | 100 | 100 |
| Mecl_5C7 < −0.094 | 5 | 0 | 0 | 3 | 100 | 100 | 100 | 100 |
| Mecl_5H6 ≥ 0.28 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75 |
| P111_BP4_P4_F6 ≥ −0.12 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75 |
| P156_BP4_P2_H3 < 0.486 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75 |
| P87_BP3_P2_C10 ≥ 0.083 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75 |
|
| ||||||||
| Second post diagnosis interval (Method C) | ||||||||
|
| ||||||||
| Antigen | TP | FN | FP | TN | Sensitivity | Specificity | PPV | NPV |
|
| ||||||||
| Mecl_2B3 ≥ 0.297 | 4 | 1 | 0 | 4 | 80 | 100 | 100 | 80.0 |
| Mecl_2H5 ≥ 0.084 | 4 | 1 | 0 | 4 | 80 | 100 | 100 | 80.0 |
| Mecl_3A12 < −0.062 | 4 | 1 | 0 | 4 | 80 | 100 | 100 | 80.0 |
| Mecl_3D7 < 0.044 | 5 | 0 | 0 | 3 | 100 | 100 | 100 | 100.0 |
| Mecl_3F9 ≥0.116 | 3 | 2 | 0 | 4 | 60 | 100 | 100 | 66.7 |
| Mecl_3Gl 1 ≥ −0.134 | 4 | 1 | 0 | 4 | 80 | 100 | 100 | 80.0 |
| Mecl_4E8 < −0.389 | 4 | 1 | 0 | 4 | 80 | 100 | 100 | 80.0 |
| Mecl_5C12 ≥ −0.426 | 5 | 0 | 0 | 4 | 100 | 100 | 100 | 100.0 |
| MH2.1 ≥ −0.37 | 5 | 0 | 0 | 3 | 100 | 100 | 100 | 100.0 |
| P156_BP4_P2_C5 < 0.233 | 4 | 1 | 0 | 3 | 80 | 100 | 100 | 75.0 |
| P156_BP4_P2_H3 < 0.151 | 5 | 0 | 0 | 4 | 100 | 100 | 100 | 100.0 |
NOTE: Method A: Normalized values at first post diagnosis interval for each patient were used for the statistical analyses(see Materials and Methods).
Methods B and C: The normalized values at first and second post diagnosis intervals for each patient were subtracted from their values closest to their time of diagnosis and statistical analyses were performed (see Materials and Methods).
TP = True positive. FN = False negative. FP = False positive. TN = True negative. PPV = Positive predictive value. NPV = Negative predictive value.
As a second approach we also evaluated samples at first and second post diagnosis intervals. The normalized values for each patient were subtracted from their corresponding values closest to the time of diagnosis and then both the t-test and Wilcoxon rank sum (Mann-Whitney) tests were applied to antigens in this data set. Analyses at first post diagnosis interval revealed the identification of 13 statistically significant antigens that differentiated recurrent from non-recurrent OVCA patients from either test. As summarized in Table 1,4 antigens obtained by Wilcoxon’s test namely, Mec1 1E1, Mec1 4B5, Mec1 5A7 and Mec1 5C7 completely discriminated the 5 recurrent patients from the 3 non-recurrent patients (p < 0.05). Analyses at second post diagnosis interval identified 11 statistically significant antigens obtained from either a t-test or Wilcoxon rank sum (Mann-Whitney) analyses (p < 0.05), 2 of which namely, Mec1 5C12 and P156 BP4 P2 H3, completely discriminated the 5 recurrent patients from the 4 non-recurrent patients (Table 1).
3.2. Validation of biomarkers for detection of OVCA recurrence at an early time
Combination of all of the panels of antigens obtained from different classification analyses as discussed above resulted in 56 antigens that were used for the validation study along with 13 known tumor antigens (shown to be overexpressed in recurrent OVCA). Serological immunoscreening of protein arrays was performed with serum obtained from an independent cohort of recurrent (n = 25) and non-recurrent (n = 5) OVCA patients as well as with serum samples used for the initial study (see Materials and Methods). All the samples that were used for the initial study of antigen selection were only included in the training model and not used in the testing set during the validation process.
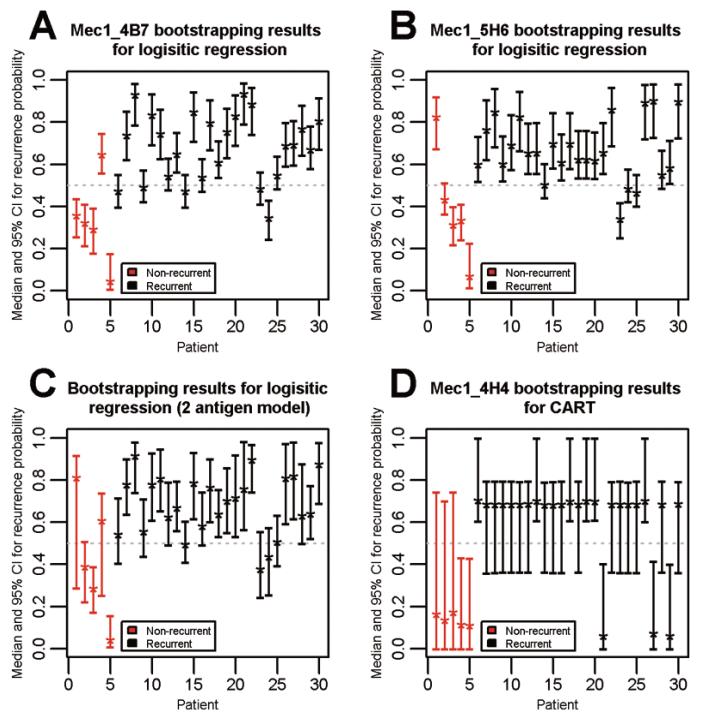
There were 28 antigens statistically significant at 0.05 predicting recurrence status using weighted logistic regression on the 30 newly measured patient samples. Only 2 of those antigens (Mec1 4B7, Mec1 5H6) were statistically significant using our bootstrapped algorithm. The median and pooled IQR values for Mec1 4B7 antigen were 0.695 (0.541, 0.817) and 0.322 (0.237, 0.399), and for Mec1 5H6 antigen were 0.652 (0.568, 0.774) and 0.352 (0.271, 0.456) for recurrent and non-recurrent cases respectively. The median and 95% confidence interval of the predicted probability of recurrence for each patient sample are shown in the Figs 1(A-B). Only a few samples out of 30 for each antigen were poorly predicted. A rule of Mec1 4H4 or Mec1 4B7 would be accurate for all but 1 non-recurrent patient. A rule of Mec1 4H4 or Mec1 5H6 would be accurate for all but 1 non-recurrent patient (a different patient) (Fig. 2). Combining these antigens in a single logistic regression model does not improve prediction (Fig. 1C).
Fig. 1.
Determination of median and 95% confidence interval of the predicted probability of recurrence of each ovarian cancer patient. This measurement was based on the performance of antigen biomarkers namely, Mec1 4B7 (A), Mec1 5H6 (B) individually, in combination (C), using logistic regression bootstrapped algorithm, and Mec1 4H4 (D) using CART bootstrapped algorithm. (Colours are visible in the online version of the article; http://dx.doi.org/10.3233/CBM-2012-0265)
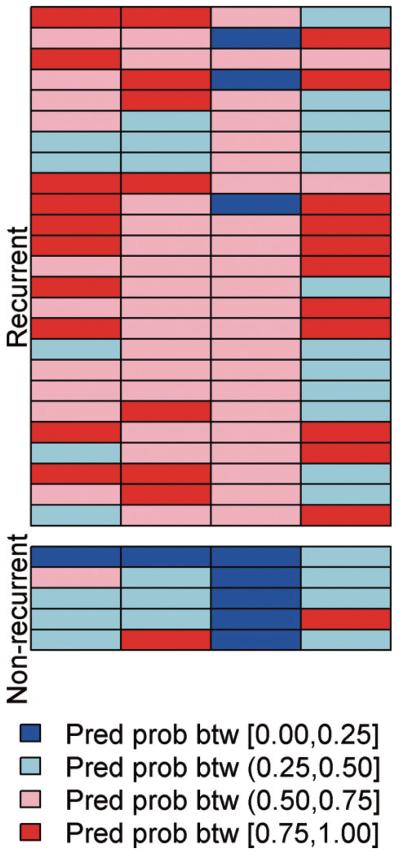
Fig. 2.
Representation of predicted probability of recurrence of ovarian cancer patients based on the performance of each biomarker derived from bootstrapped samples. Representation of predicted probability of recurrence of OVCA patients based on the performance of each biomarker derived from bootstrapped samples. The predicted probabilities for Mec1 4B7 and Mec1 5H6 were computed as the median predicted value from the “testing set” from 10,000 bootstrapped logistic regression analyses (which always included the 10 samples previously used in the training set). The predicted probabilities for Mec1 4H4 and p53 were computed as the median predicted value from the “testing set” from 10,000 bootstrapped CART analyses (which always included the 10 samples previously used in the training set). The predicted recurrence probabilities are classified and color-coded into 4 groups with break points at 0.25, 0.50 and 0.75. The samples with light or dark blue are predicted to be non-recurrent based on the models while those with light or dark red are predicted to be recurrent based on the models. The testing samples are all displayed, stratified by their true recurrence status. (Colours are visible in the online version of the article; http://dx.doi.org/10.3233/CBM-2012-0265)
There were 13 antigens significant at 0.05 for either a t-test or a Wilcoxon rank sum test. We then used CART on these 13 antigens to determine the optimal threshold to allow us to estimate sensitivity and specificity as shown in Table 2. Only 1 antigen, Mec1 4H4, was found to be statistically significant using our CART bootstrapped algorithm. The median and pooled IQR for this antigen were 0.682 (0.620, 0.723) and 0.138 (0.067, 0.389) for recurrent and non-recurrent cases respectively. The median and 95% confidence interval of the predicted probability of recurrence for each patient sample are shown in Fig. 1D. Note that the confidence intervals are much wider for the CART analysis than the logistic regression analysis due to the categorization involved with CART. Only 3 recurrent patient samples had incorrect median recurrence probabilities.
Table 2.
The performance of 13 antigens obtained by CART analysis
| Antigen/threshold | TP | FN | FP | TN | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Mecl_lB4 < 0.111 | 15 | 10 | 0 | 5 | 60 | 100 | 100 | 33.3 |
| Mecl_2B3 ≥ −0.274 | 17 | 8 | 0 | 5 | 68 | 100 | 100 | 38.5 |
| Mecl_2Hl < −0.122 | 16 | 9 | 0 | 5 | 64 | 100 | 100 | 35.7 |
| Mecl_3D5 < 0.406 | 15 | 10 | 0 | 5 | 60 | 100 | 100 | 33.3 |
| Mecl_3D7 ≥ −0.154 | 17 | 8 | 0 | 5 | 68 | 100 | 100 | 38.5 |
| MeclJGl ≥ −0.333 | 13 | 12 | 0 | 5 | 52 | 100 | 100 | 29.4 |
| Mecl_4B5 ≥ −0.473 | 24 | 1 | 2 | 3 | 96 | 60 | 92.3 | 75.0 |
| Mecl_4B7 ≥ −0.704 | 25 | 0 | 1 | 4 | 100 | 80 | 96.2 | 100.0 |
| Mecl_4E8 < 0.112 | 15 | 10 | 0 | 5 | 60 | 100 | 100 | 33.3 |
| Mecl_4H4 < 0.212 | 22 | 3 | 0 | 5 | 88 | 100 | 100 | 62.5 |
| Mecl_5A3 ≥ −0.021 | 21 | 4 | 0 | 5 | 84 | 100 | 100 | 55.6 |
| Mecl_5H6 ≥ −0.78 | 24 | 1 | 1 | 4 | 96 | 80 | 96.0 | 80.0 |
| MH2.2 < 0.29 | 11 | 14 | 0 | 5 | 44 | 100 | 100 | 26.3 |
| CA125 | 2 | 23 | 0 | 5 | 8 | 100 | 100 | 17.9 |
TP = True positive. FN = False negative. FP = False positive. TN = True negative. PPV = Positive predictive value. NPV = Negative predictive value.
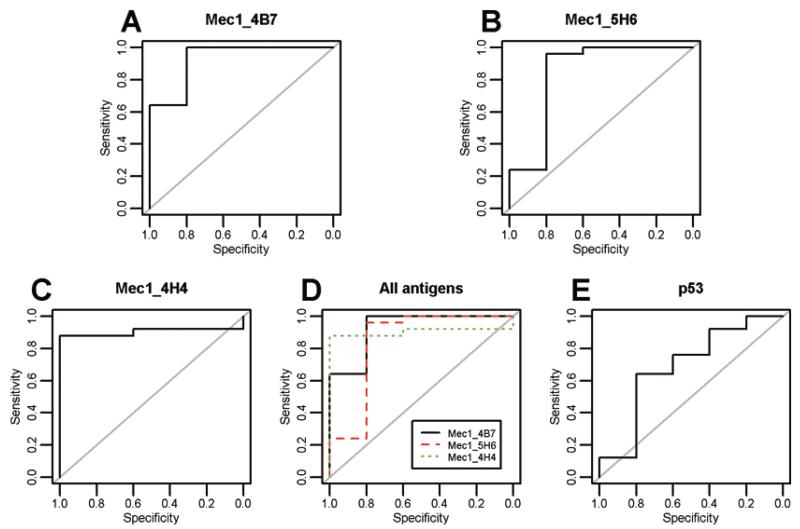
We further examined these 3 antigens, Mec1 4B7, Mec1 4H4, and Mec1 5H6 that were significant through either analysis. Supplementary Fig. S1 shows the relationship between the 2 remaining antigens (after backwards step-wise selection on the 3 in a logistic regression model) and recurrence status of the validation samples. There is perfect non-linear discrimination between the recurrent and non-recurrent samples. The receiver operating characteristic (ROC) curves for each of the 3 antigens are shown in Figs 3(A-C). The three lines are plotted on the same axis in Fig. 3D. The area under the curves (AUC) for Mec1 4B7, Mec1 4H4, and Mec1 5H6 were 0.928, 0.904 and 0.840 respectively.
Fig. 3.
Comparison of receiver operating characteristic (ROC) curves of the 3 antigens to p53. ROC curves of the 3 antigens Mec1 4B7 (A), Mec1 4H4 (B), Mec1 5H6 (C) individually and in combination (D) and p53 (E). (Colours are visible in the online version of the article; http://dx.doi.org/10.3233/CBM-2012-0265)
We also measured 13 proteins commonly expressed in OVCA on the same assay and applied the same statistical methods for the data analyses as the other antigen biomarkers (discussed above). We analyzed the unnormalized log transformed values for these protein antigens because they lacked the first 11 N-terminal amino acids of 10B T7 phage coat protein that was necessary for their reactivity with the T7 antibody (please see background correction and normalization in Materials and Methods). However upon evaluation using the bootstrap method described above, none of the 13 protein biomarkers had non-overlapping IQR values between recurrent and non-recurrent samples. A backwards stepwise logistic regression involving the nominally significant markers resulted in only E-cadherin being significant. Combination of protein biomarkers with the other top 3 significant biomarkers, only MEC1 4B7 and MEC1 4H4 antigens still retained their significance. The sensitivity and specificity values for the optimally determined thresholds are listed in Supplementary Table S3. The bootstrap evaluation for a CART analysis also yielded no markers with non-overlapping IQR values between recurrent and non-recurrent samples. A CART analysis with only the protein predictors yielded p53 as the significant predictor; although it is not as strong a predictor relative to the significance of our top 3 antigens, see the ROC curve (Figs 3(A-E)).
4. Discussion
The management of recurrent OVCA is a major clinical challenge because relapse after platinum based front-line chemotherapy represents an aggressive disease state which currently has no clinical biomarkers that can indicate when to reinitiate therapy [13]. We are targeting this test as an earlier indication of recurrence in platinum-sensitive serous adenocarcinoma of the ovary so that the second-line chemotherapy treatment can be implemented sooner than CA-125 could detect disease for a better therapeutic outcome.
Our results indicated that top 3 biomarkers were able to predict recurrence at a median time of 9.07 months prior to clinical recurrence of the disease with an average sensitivity, specificity, and accuracy of 94.7%, 86.7% and 93.3% (Fig. 3D), in a population where 92% (23/25) recurrent OVCA patients had CA125 less than 35 U/ml at that time. Our results also indicated that proteins known to be overexpressed in OVCA were not useful autoantigen recurrence biomarkers. In the same patient population CA125 alone detected recurrence with a low sensitivity of 8%, although all the non-recurrent OVCA patients were correctly categorized by CA125 as indicated by the high assay specificity (Table 2). The low sensitivity of CA125 was due to the enrollment of a particular group of recurrent OVCA patients for this study where the majority of patients had CA125 values below 35 U/ml at first post diagnosis interval before recurrence. It is noteworthy that of the 12 recurrent patients on whom we obtained complete longitudinal CA125 data, 11 had normal CA125 levels for an extended period prior to their recurrence (average interval 9 months, range 5.5-11.7 months). Clinical documentation of recurrence was not noted for a median of 9.07 months (range 2.1-18.9) after the appearance of biomarkers detected by our method. A limitation of this study is that few non-recurrentpatients (OVCA patients who remained disease free after primary chemotherapy for greater than 3 years) were available for the validation study. Generally, monitoring of disease during or after front-line chemotherapy in OVCA patients with low CA125 levels is dependent on imaging studies that sometimes fails to detect the metastases that fall below the resolution limits of this technology. Therefore, the biomarker panel that we identified may be useful for predicting recurrence at an early time in OVCA patient population whose CA125 values are within the normal range.
Among the top peptide antigen biomarkers, one of the antigens (Mec1 4B7) represented a peptide epitope of a known gene product, histidyl t-RNA synthetase (Table 3). Histidyl-tRNA synthetase (HARS) also known as histidine-tRNA ligase, is an enzyme which in humans is encoded by the HARS gene. The protein encoded by this gene is a cytoplasmic enzyme which belongs to the class II family of aminoacyl tR-NA synthetases [14]. Autoantibodies to histidyl t-RNA synthetase, termed as anti-Jo-1, or to other amino acyl t-RNA synthetase occur in 25% of patients with PM and dermatomyositis [11]. Iavazzo and colleagues presented a case report for a patient who developed PM after she was treated for ovarian carcinoma recurrence [12]. In general, PM appears to arise in cancer patients prior to diagnosis [15,16].
Table 3.
Description of top 3 antigen biomarkers
| Antigen Biomarkers |
Description of the genes that are in- frame with T7 10B gene |
Peptide sequences of Epitope/Mimotopes, in-frame with T7 10 B gene |
Size of the peptide |
Description of the sequences that Mi- motopes mimic |
Unigene # | Region of similarity of AA | Antigen expression in any type of cancer |
|---|---|---|---|---|---|---|---|
| Mecl_4B7 | NM.002109.3, Homo sapiens histidyl-tRNA synthetase (HARS), mRNA |
Epitope EVDVRREDLVEE IKRRTGQPLCIC |
24 AA | N/A | Hs.528050 | 486–509 Score = 82.5 bits (187), Expect = 2e–18 Identities = 24/24 (100%), Positives = 24/24 (100%), Gaps = 0/24 (0%) Query 1 EVDVRREDLVEEIKRRTGQPLCIC 24 EVDVRREDLVEEIKRRTGQPLCIC Sbjct 486 EVDVRREDLVEEIKRRTGQPLCIC 509 |
Autoantibodies to histidyl t_RNA synthetase were shown to be present in patients diagnosed with polymyositis or dermatomyositis [11] |
| Mecl_5H6 | ref|NT.034772.6|, Homo sapiens chr- omosome 5 genomic contig, GRCh37.p5 |
Mimotope NSFLMTSSKPR |
11 AA | sp|095944.2| NCTR2.HUMAN, Natural cytotoxicity triggering receptor 2 |
Hs. 194721 | 66–73 Score = 23.5 bits (48), Expect = 0.035 Identities = 7/8 (88%), Positives = 7/8 (88%), Gaps = 0/8 (0%) Query 4 LMTSSKPR 11 LT SSKPR Sbjct 66 LVTSSKPR 73 |
The cytolytic effect of natural killer cells in killing the neurob- lastoma and glioblas- toma target cells is mediated by natural cytotoxicity triggering receptor 2 [26] |
| Mecl_4H4 | NM.014671.2, Homo sapiens ubiquitin protein ligase E3C (UBE3C), mRNA |
Mimotope PGCSTTLS |
8 AA | sp|094966.2| UBP19.HUMAN, Ubiquitin carboxyl- terminal hydrolase 19 |
Hs.721972 | 887–894 Score = 18.9 bits (37), Expect = 0.88 Identities = 6/8 (75%), Positives = 6/8 (75%), Gaps = 0/8 (0%) Query 1 PGCSTTLS 8 PGC T LS Sbjct 887 PGCTTLLS 894 |
Ubiquitin carboxyl-terminal hydrolase 1 was reported as tumor suppressor and biomarker for hepatocellular carcinoma [27] |
The remaining 2 peptide antigens contained an open reading frame with the T7 10B gene with a frameshift within the natural reading frame of the gene (Table 3). These peptides are termed as mimotopes because they mimic linear or conformationalepitopes of an immunogen [17,18].
A variety of other tumor biomarkers have been reported to be useful for monitoring response to therapy or indicating relapse during follow-up visits. Anastasi and colleagues conducted a follow-up retrospective study for survival analysis of 8/32 patients with advanced OVCA by evaluating the levels of human epididymis protein 4 (HE4) and CA125 in the serum samples that were collected at the time of diagnosis and at intervals during a 16–20 month period after surgery. Their study showed that 5/8 patients with recurrent disease had an increase in HE4 level above the cut-off value that preceded the rise of CA125 by 5-8 months [19]. Another study showed that the level of Osteopontin (OPN), a putative plasma biomarker, increased earlier than CA125 in 90% of the patients developing progressive or recurrent epithelial OVCA(median lead time, 3 months) although its role in predicting clinical response to therapy was considered inferior to CA125 [20]. Tassi and colleagues [21] reported significant elevation in the expression of Mammaglobin B (MGB-2), a secretoglobin family member, in epithelial OVCA. Univariate survival analysis on 106 OVCA patients enrolled in their study revealed significant correlation of MGB-2 expression with reduced risks of cancer-related death, recurrence and disease progression (p < 0.05). In another study, the utility of a biomarker panel comprised of HE4, MMP7 and Glycodelin was evaluated to predict recurrence in a longitudinal monitoring cohort of 30 patients with advanced OVCA. The results indicated that in 27/30 patients who experienced recurrence following initial response to chemotherapy, this biomarker panel predicted recurrence with a sensitivity of 100% compared to 96% for CA125 alone. In 56% patients, the level of one or more panel biomarkers was elevated 6–69 weeks before the rise in CA125 and prior to other clinical evidence of recurrence [22].
Although autoantibodies to TAAs develop at the early onset of the disease, only a few have been evaluated as prognostic biomarkers because very little data on the evidence of tumor autoantibodies in monitoring disease or predicting recurrence in OVCA patients are available. Reports from Vogl and colleagues [23] revealed 46% prevalence of circulating p53 autoantibodies in a study population comprising 83 OVCA patients. Their study also indicated that in a bivariate analysis, patients with anti-p53 autoantibodies had a 1.96-fold risk for relapse (95% confidence interval 1.02–3.78).
Peptide biomarkers that are indicative of a poor response to therapy during disease monitoring as well as in predicting recurrence at an early time could provide the clinician information to modify patient treatment. Such modifications could include prolonging frontline therapy, initiating maintenance therapy, or early treatment of recurrent disease. These treatment modifications could potentially result in more durable response and greater survival among OVCA patients with the potential for novel therapeutics such as vaccines or other biologics. To date, CA125 is most extensively used in monitoring OVCA during routine follow-up visits because in about 80% of patients an increase in the level of CA125 may be the first indication of relapse that precedes recurrence by 3–5 months [13]. There has been considerable debate on the beneficial outcome of OVCA patients from the recommencement of early chemotherapy treatment due to a rise in CA125 values during their disease monitoring phase after the completion of therapy. The recent report from MRC/EORTC trial demonstrated that OVCA patients with a rising CA125 who received chemotherapy treatments prior to the appearance of clinical symptoms of recurrence had no survival benefit [24]. The limitation of their study was that it took a long time for them to enroll patients and as a result clinician bias may have been introduced for not registering patients who were considered likely to benefit from early chemotherapy. Furthermore, the lack of a benefit in early treatment has been argued to be a result of enrolling patients with poor prognoses [25]. Our goal for future studies is to evaluate the potential utility of the top 3 biomarkers for predicting recurrence in a larger OVCA patient population for early therapeutic intervention to improve mortality rates.
Supplementary Material
Acknowledgments
The authors would like to thank Sruthi Renati, Nadine Dawood, Chandani Patel and Sabreena Kammo for technical assistance. This work was supported by funds from the Gail Purtan OVCA Research Fund, The Rais Foundation, The Janis Warren Ovarian Cancer Fund, The Barbara and Fred Erb Endowed Chair in Cancer Genetics, and by grants from National Institutes of Health (NIH), R21/R33-CA100740, and U01-CA117748.
References
- [1].Schink JC. Current initial therapy of stage III and IV ovarian cancer: challenges for managed care. Semin Oncol. 1999;26:2–7. [PubMed] [Google Scholar]
- [2].Mutch DG. Surgical management of ovarian cancer. Semin Oncol. 2002;29:3–8. doi: 10.1053/sonc.2002.31589. [DOI] [PubMed] [Google Scholar]
- [3].Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. doi: 10.1007/978-1-4757-3587-1_4. [DOI] [PubMed] [Google Scholar]
- [4].Chua TC, Liauw W, Robertson G, Morris DL. Second-line treatment of first relapse recurrent ovarian cancer. Aust N Z J Obstet Gynaecol. 2010;50:465–471. doi: 10.1111/j.1479-828X.2010.01209.x. [DOI] [PubMed] [Google Scholar]
- [5].Mann WJ, Patsner B, Cohen H, Loesch M. Preoperative serum CA-125 levels in patients with surgical stage I invasive ovarian adenocarcinoma. J Natl Cancer Inst. 1988;80:208–209. doi: 10.1093/jnci/80.3.208. [DOI] [PubMed] [Google Scholar]
- [6].Krivak TC, Tian C, Rose GS, Armstrong DK, Maxwell GL. A Gynecologic Oncology Group Study of serum CA-125 levels in patients with stage III optimally debulked ovarian cancer treated with intraperitoneal compared to in-travenous chemotherapy: an analysis of patients enrolled in GOG 172. Gynecol Oncol. 2009;115:81–85. doi: 10.1016/j.ygyno.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wilder JL, Pavlik E, Straughn JM, Kirby T, Higgins RV, DePriest PD, Ueland FR, Kryscio RJ, Whitley RJ, Nagell J. Clinical implications of a rising serum CA-125 within the normal range in patients with epithelial ovarian cancer: a preliminary investigation. Gynecol Oncol. 2003;89:233–235. doi: 10.1016/s0090-8258(03)00051-9. [DOI] [PubMed] [Google Scholar]
- [8].Li Y, Karjalainen A, Koskinen H, Hemminki K, Vainio H, Shnaidman M, Ying Z, Pukkala E, Brandt-Rauf PW. p53 autoantibodies predict subsequent development of cancer. Int J Cancer. 2005;114:157–160. doi: 10.1002/ijc.20715. [DOI] [PubMed] [Google Scholar]
- [9].Chatterjee M, Mohapatra S, Ionan A, Bawa G, Ali-Fehmi R, Wang X, Nowak J, Ye B, Nahhas FA, Lu K, Witkin SS, Fishman D, Munkarah A, Morris R, Levin NK, Shirley NN, Tromp G, Abrams J, Draghici S, Tain-sky MA. Diagnostic markers of ovarian cancer by high-throughput antigen cloning and detection on arrays. Cancer Res. 2006;66:1181–1190. doi: 10.1158/0008-5472.CAN-04-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Draghici S, Chatterjee M, Tainsky MA. Epitomics: serum screening for the early detection of cancer on microar-rays using complex panels of tumor antigens. Expert Rev Mol Diagn. 2005;5:735–743. doi: 10.1586/14737159.5.5.735. [DOI] [PubMed] [Google Scholar]
- [11].Howard OM, Dong HF, Yang D, Raben N, Nagara-ju K, Rosen A, Casciola-Rosen L, Hartlein M, Kron M, Yang D, Yiadom K, Dwivedi S, Plotz PH, Oppenheim JJ. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med. 2002;196:781–791. doi: 10.1084/jem.20020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iavazzo C, Vorgias G, Papadakis M, Manikis P, Mavro-matis I, Akrivos T. Polymyositis in a patient with recurring ovarian cancer and history of unrelated breast cancer. Arch Gynecol Obstet. 2007;276:81–84. doi: 10.1007/s00404-006-0307-z. [DOI] [PubMed] [Google Scholar]
- [13].Rustin GJ, Nelstrop AE, Tuxen MK, Lambert HE. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Ann Oncol. 1996;7:361–364. doi: 10.1093/oxfordjournals.annonc.a010602. [DOI] [PubMed] [Google Scholar]
- [14].Wasmuth JJ, Carlock LR. Chromosomal localization of human gene for histidyl-tRNA synthetase: clustering of genes encoding aminoacyl-tRNA synthetases on human chromosome 5. Somat Cell Mol Genet. 1986;12:513–517. doi: 10.1007/BF01539922. [DOI] [PubMed] [Google Scholar]
- [15].Ghosh A, Malak TM, Pool AJ. Polymyositis and ovarian carcinoma: a case report. Arch Gynecol Obstet. 2007;275:195–197. doi: 10.1007/s00404-006-0198-z. [DOI] [PubMed] [Google Scholar]
- [16].Sigurgeirsson B, Lindelof B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363–367. doi: 10.1056/NEJM199202063260602. [DOI] [PubMed] [Google Scholar]
- [17].Regenmortel MHV. Molecular dissection of protein antigens and the prediction of epitopes. In: Van Regenmortel MHV, Muller S, editors. Synthetic Peptides as Antigens. Elsevier; Amsterdam: 1999. pp. 1–78. [Google Scholar]
- [18].Meloen RH, Puijk WC, Slootstra JW. Mimotopes: re-alization of an unlikely concept. J Mol Recognit. 2000;13:352–359. doi: 10.1002/1099-1352(200011/12)13:6<352::AID-JMR509>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [19].Anastasi E, Marchei GG, Viggiani V, Gennarini G, Frati L, Reale MG. HE4: a new potential early biomarker for the recurrence of ovarian cancer. Tumour Biol. 2010;31:113–119. doi: 10.1007/s13277-009-0015-y. [DOI] [PubMed] [Google Scholar]
- [20].Schorge JO, Drake RD, Lee H, Skates SJ, Rajanbabu R, Miller DS, Kim JH, Cramer DW, Berkowitz RS, Mok SC. Osteopontin as an adjunct to CA125 in detecting recurrent ovarian cancer. Clin Cancer Res. 2004;10:3474–3478. doi: 10.1158/1078-0432.CCR-03-0365. [DOI] [PubMed] [Google Scholar]
- [21].Tassi RA, Calza S, Ravaggi A, Bignotti E, Odicino FE, Tognon G, Donzelli C, Falchetti M, Rossi E, Todeschini P, Romani C, Bandiera E, Zanotti L, Pecorelli S, Santin AD. Mammaglobin B is an independent prognostic marker in epithelial ovarian cancer and its expression is associated with reduced risk of disease recurrence. BMC Cancer. 2009;9:253. doi: 10.1186/1471-2407-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, Malinowski DP, Fischer TJ, Berchuck A. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008;110:374–382. doi: 10.1016/j.ygyno.2008.04.041. [DOI] [PubMed] [Google Scholar]
- [23].Vogl FD, Stickeler E, Weyermann M, Kohler T, Grill HJ, Negri G, Kreienberg R, Runnebaum IB. p53 autoantibodies in patients with primary ovarian cancer are associated with higher age, advanced stage and a higher proportion of p53-positive tumor cells. Oncology. 1999;57:324–329. doi: 10.1159/000012069. [DOI] [PubMed] [Google Scholar]
- [24].Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C, Qian W, Parmar MK, Swart AM. Early versus delayedtreatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376:1155–1163. doi: 10.1016/S0140-6736(10)61268-8. [DOI] [PubMed] [Google Scholar]
- [25].Morris RT, Monk BJ. Ovarian cancer: relevant therapy, not timing, is paramount. Lancet. 2010;376:1120–1122. doi: 10.1016/S0140-6736(10)61515-2. [DOI] [PubMed] [Google Scholar]
- [26].Sivori S, Parolini S, Marcenaro E, Castriconi R, Pende D, Millo R, Moretta A. Involvement of natural cytotoxicity receptors in human natural killer cell-mediated lysis of neuroblastoma and glioblastoma cell lines. J Neuroimmunol. 2000;107:220–225. doi: 10.1016/s0165-5728(00)00221-6. [DOI] [PubMed] [Google Scholar]
- [27].Yu J, Tao Q, Cheung KF, Jin H, Poon FF, Wang X, Li H, Cheng YY, Rocken C, Ebert MP, Chan AT, Sung JJ. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology. 2008;48:508–518. doi: 10.1002/hep.22343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.