Abstract
Objective
To explore the prevention of recurrent candiduria using natural based approaches and to study the antimicrobial effect of Hibiscus sabdariffa (H. sabdariffa) extract and the biofilm forming capacity of Candida albicans strains in the present of the H. sabdariffa extract.
Methods
In this particular study, six strains of fluconazole resistant Candida albicans isolated from recurrent candiduria were used. The susceptibility of fungal isolates, time-kill curves and biofilm forming capacity in the present of the H. sabdariffa extract were determined.
Results
Various levels minimum inhibitory concentration of the extract were observed against all the isolates. Minimum inhibitory concentration values ranged from 0.5 to 2.0 mg/mL. Time-kill experiment demonstrated that the effect was fungistatic. The biofilm inhibition assay results showed that H. sabdariffa extract inhibited biofilm production of all the isolates.
Conclusions
The results of the study support the potential effect of H. sabdariffa extract for preventing recurrent candiduria and emphasize the significance of the plant extract approach as a potential antifungal agent.
Keywords: Hibiscus sabdariffa, Biofilm, Candiduria, Candida albicans, UTIs
1. Introduction
Candida albicans (C. albicans) is commensal yeast normally present in small numbers as a skin, oral, gut or vaginal flora and is found in 15%-60% of the population. C. albicans is traditionally regarded as a low grade pathogen. A common form of candidiasis that is restricted to the mucosal membranes in the mouth or vagina known as thrush, which is usually easily treated[1]. Systemic candidal infections have emerged as important causes of morbidity and mortality in immunocompromised patients[2]. The emergence of fungal urinary tract infections (UTIs) poses an interesting diagnostic and therapeutic challenge. During the past decade, there has been a worldwide trend in increasing occurrences of fungal UTIs. C. albicans was reported as the most common cause of fungal UTIs (candiduria)[3]. Candiduria may be resolved spontaneously, but may also result in serious complications if treated inadequately, and recurrent or persistent infections are frequent. Many risk factors for candiduria found including urinary system intervention, catheter use and immunosuppression history. There are other risk factors for candiduria including older age, sex, antibiotic exposure and diabetes[4].
Candiduria has raised alarms with the global infectious diseases community because of the limited therapeutic options. The limited success over the past decade of prevention and control candiduria highlights the difficulty of limiting the problem once it has already been established[5]. In contrast to antibacterial agents, antifungal agents have less of a clinical benefit. This is due to the higher level of toxicity to human cells because both fungi and the host cells are eukaryotic and share similar cellular structures. In recurrent candiduria, long-term antifungals are administered to the patients and this is generally unfavourable from the points of medical cost and the potential development of fungal resistance[6].
Current pharmacologic treatment options for symptomatic candiduria include amphotericin B, flucytosine, or azole. Due to its ease of administration and pharmacokinetic profile, fluconazole has emerged as the most commonly used drug to treat candiduria. In addition, fluconazole's adverse effect profile may be more tolerable than the complications associated with other antifungal agents. However, the failure of fluconazole treatments may be associated with either some Candida species innate resistance or due to an acquired resistance to the drug. The emergence of fluconazole resistance in C. albicans was observed after reports showed that the long-term use of fluconazole for prophylaxis lead to the emergence of resistance[7].
Therefore, promoting the application of natural plant-derived antimicrobials as a possible approach to prevent recurrent candiduria is very desirable and the potential effect of natural options for long-term prevention of infections should be explored. Hence, in this study we examined the in vitro antimicrobial activities of Hibiscus sabdariffa (H. sabdariffa) extract against fluconazole resistant C. albicans isolated from recurrent candiduria. H. sabdariffa is a common herbal drink consumed both hot and cold by people around the world and used in traditional medicine in the treatment of hypertension. The infusion is made from the calyces of the H. sabdariffa[8]. Relatively few studies have been carried out to evaluate the antimicrobial activities of this plant against common human pathogens[9],[10]. However, no study has been reported concerning the effect of the plant in biofilm forming uropathogenic C. albicans and the combination effect of H. sabdariffa with other antifungal drugs. The aim of this study was to assess the antifungal activity of H. sabdariffa extract against C. albicans from recurrent candiduria in order to better understand their therapeutic properties.
2. Materials and methods
2.1. Preparation of plant extracts
Air dried Hibiscus calyces were purchased from the local market. The identity of the plant was confirmed by comparing collected voucher specimen with those of a known identity located in the Herbarium of the College of Science, Taibah University. A voucher specimen was deposited at the Herbarium under the reference number 1433/14. The extracts were prepared according to a previously described protocol[11]. Using 1 L of 80% aqueous methanol (BDH, UK), the phenolics were extracted from 100 g of the grinded calyces. The mixture was then sonicated for 20 min and then filtered through Whatman No. 2 filter paper. The solvents were then evaporated using a rotary evaporator at 40 °C under reduced pressure and the extracts were stored in tightly, sealed glass vials and considered to be H. sabdariffa extract, and was used for the antibacterial assays. Dried plant extracts were then dissolved in methanol to give a stock solution of 500 mg/mL and then filter sterilized before use.
2.2. Fungal strains and growth conditions
In this particular study, six strains of fluconazole resistant C. albicans, CA1, CA2, CA3, CA4, CA5 and CA6 were used. All were obtained from patients with recurrent candiduria which had been isolated from Ohad Hospital, Almadinah, Saudi Arabia. Fungal identities were confirmed using standard microbiologic procedures and the use of API ID 32C strips (Biomerieux, France).
2.3. Determination of antimicrobial activity
The fluconazole and H. sabdariffa extracts minimum inhibitory concentrations (MICs) and time-kill curves were determined according to the approved CLSI standard reference method for broth dilution and antifungal susceptibility testing of yeasts M27-A3[12]. MIC plates were incubated at 35 °C for 48 h. MICs were defined as the lowest concentration of the drug eliminating cell growth using MIC-0. MIC-0 was defined as the lowest drug combination resulting in optically clear wells.
2.4. Effect on C. albicans biofilm formation
The ability to form a Candida biofilm was tested on 96-well tissue culture plates and biofilm was quantified by measuring the optical density after dyeing it with 0.1% crystal violet in accordance to a previously described protocol[13]. This method measures the cells adhering to the bottom of microtiter plate wells after repetitive cycles of washing. Briefly, strains from an overnight culture were inoculated with the tested organism in the presence and absence of sub-MIC concentrations of the extract at 37 °C without agitation for 48 h in yeast nitrogen base broth +0.5% glucose. The attached cells were washed, fixed and stained with crystal violet and air dried. About 0.15 mL of 95% ethanol was added in each well and the plates were incubated for 10 min. Biofilm formation was quantified by measuring the absorbance at 550 nm. Each strain and each concentration were assayed in three wells on each plate, and all experiments were replicated at least three times.
2.5. Statistical analysis
Data was analysed in SPSS statistical software. P<0.05 was considered to be statistically significant when compared with control.
3. Results
3.1. Antifungal activities
Various levels MIC of H. sabdariffa extract were observed against all the isolates. MICs values ranged from 0.5 to 2.0 mg/mL. All the isolates showed resistance to fluconazole with MICs values of >16 µg/mL (P<0.05). The results are summarized in Table 1.
Table 1. Antifungal activities of H. sabdariffa extract and fluconazole against C. albicans.
| Strain | Antifungal agents (µg/mL) | Minimum inhibitory concentration |
| CA1 | Fluconazole | 64.0 |
| H. sabdariffa extract | 2.0 | |
| CA2 | Fluconazole | 32.0 |
| H. sabdariffa extract | 1.0 | |
| CA3 | Fluconazole | 32.0 |
| H. sabdariffa extract | 2.0 | |
| CA4 | Fluconazole | 128.0 |
| H. sabdariffa extract | 2.0 | |
| CA5 | Fluconazole | 64.0 |
| H. sabdariffa extract | 0.5 | |
| CA6 | Fluconazole | 64.0 |
| H. sabdariffa extract | 1.0 |
CA: C. albicans
3.2. Mode of action
Time-kill curves were performed for the isolates using different concentrations of the H. sabdariffa extract ranging from 0.5-2× the MIC value. The results obtained for the time-kill curves are summarized in Figure 1. For the six strains, the effects were fungistatic (P<0.05). Fungistatic activity has been defined as <3 log reduction in CFU/mL using time-kill[14]. The present study allows us to establish that H. sabdariffa has a fungistatic effect against the C. albicans isolates.
Figure 1. Time-kill experiments evaluating the activity of H. sabdariffa extract against C. albicans isolates.
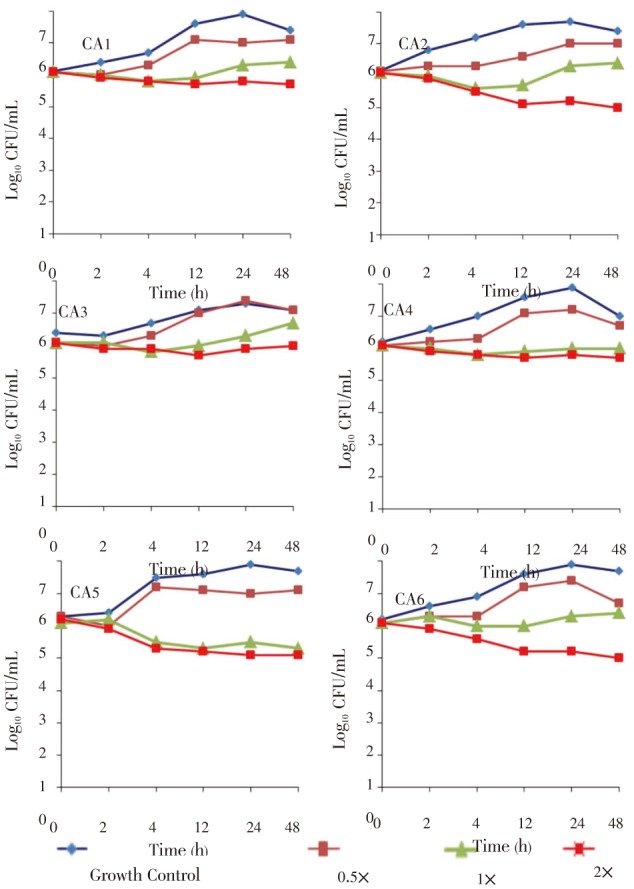
Fungistatic activity was defined as a reduction in the numbers of viable bacteria of <3 log10 CFU/mL at any of the incubation times tested. P<0.05, between growth control and different concentrations of the extracts; ×: Minimum inhibitory concentration of the extract; CA: C. albicans.
3.3. Inhibition of biofilm formation by H. sabdariffa
There is no previous data that suggests H. sabdariffa may inhibit biofilm formation against candiduria isolates. Thus, the current study was initiated to evaluate H. sabdariffa extracts on those pathogens that are clinically relevant. The results showed that the H. sabdariffa extract inhibited biofilm production on all of the isolates. The biofilm inhibition varies between the different isolates and ranged from 33%-55% (P<0.05). The results obtained for the biofilm formation assays are summarized in Figure 2.
Figure 2. Inhibition of biofilm formation in microtiter plates of C. albicans isolates by H. sabdariffa extract.
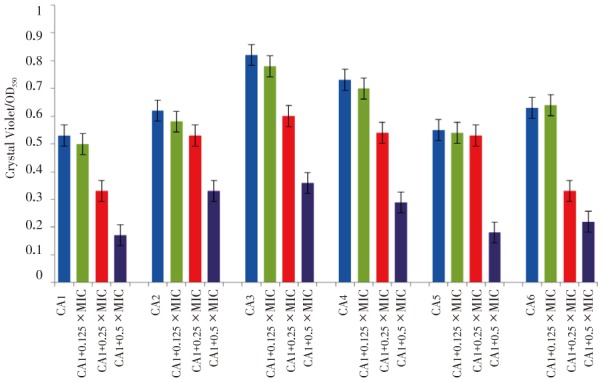
All strains showed reduced biofilm formation in the presence of different concentrations of H. sabdariffa extract. Biofilm formation was determined by crystal violet staining. Values are means of three independent experiments. P<0.05, between growth control and different concentrations of the extracts. X: Minimum inhibitory concentration of the extract, OD: Optical density, CA: C. albicans.
4. Discussion
Few studies have been carried out to evaluate the antimicrobial activities of H. sabdariffa extract against common bacterial pathogens. Extracts of the plant significantly enhanced the inhibitory effects of antibacterial agents against different bacterial species[15]. However, there has been only one study reported the effect of the plant extract against fungal pathogens. H. sabdariffa was found to be effective at all levels in inhibiting C. albicans (non candiduria isolates) but no other Candida species[9]. Previous studies have shown this effect in terms of inhibition zones or agar dilution methods. In our study, we chose the most appropriate and reliable method for antimicrobial susceptibility testing (i.e., the MIC using microdilution method). The advantage of this technique was that it generated a quantitative result in which with further analysis provided a clearer interpretation of the results in terms of time-kill. The present study allows us to establish, for the first time, the effect of H. sabdariffa extract against C. albicans isolated from recurrent UTIs.
The high potency of H. sabdariffa against these strains gives scientific basis for its use in folk medicine for treating and preventing of UTI. The mechanism of the action is not fully understood but it has been suggested that H. sabdariffa products contain phenolic compounds including flavonoids and cyanidin[16],[17]. Phenolic compounds have been reported to exhibit antimicrobial activities[11],[18],[19]. The antimicrobial effect of the phenolic compounds may be due to iron deprivation or hydrogen bonding with vital proteins such as microbial enzymes[20].
Nearly all Candida species strain possess different ranges of virulence factors, including the use of several adherence mechanisms to colonize uroepithelium of both the upper and lower urinary tract. Biofilm forming uropathogens are often associated with long term persistence and display a dramatically increased resistance to antimicrobial agents. Agents interfering with biofilm formation represent a novel approach to control C. albicans infections. By affecting C. albicans virulence properties, this may minimize the appearance of resistant strains[21],[22].
H. sabdariffa contains a unique polymeric compound known as proanthocyanidin. Research shows that proanthocyanidin from cranberry extracts inhibit the biofilm formation of C. albicans isolated from oral infection[13]. The present study suggests that proanthocyanidin from H. sabdariffa has the same anti-biofilm inhibition effect against C. albicans strains isolated from UTIs.
The use of plant extracts and phytochemicals with known antimicrobial properties is becoming a very common practice worldwide and is of great significance in therapeutic use. The wide extensive use of antifungal agents leads to the selection of clinical isolates that show multidrug resistance. On the other hand, few synthetic antifungals agents are being developed. The identification of plant extracts with antimicrobial properties would be of an immense practical use as a safe and effective means of controlling clinical infections[23],[24].
In conclusion, the current study shows that H. sabdariffa extract significantly inhibits the C. albicans growth and prevents the formation of in vitro biofilm. The antifungal activities of the extract that were observed provide basic evidence for the potential effects of this plant for preventing recurrent candiduria caused by C. albicans. It also emphasizes a significant approach of the plants extract as an antimicrobial agent. Although a preventive effect of H. sabdariffa has been shown in vitro, further studies with a more robust methodology would be needed in order to determine the in vivo clinical efficiency of the extract to prevent recurrent UTIs.
Acknowledgments
The authors are grateful to Mr. Mohamed Abdulsamad and all the staff in the Department of Medical Microbiology, Ohad Hospital who made a great effort to collect and identify the fungal isolates. We acknowledge the Deanship of Scientific Research of Taibah University for providing funding for this research (Grant No.432/3088).
Comments
Background
H. sabdariffa, commonly known as karkade, grows and is cultivated in different parts of the world such as Africa and Asia. The calyces parts of plant is used in traditional medicine in the treatment of hypertension. Many research studies have been carried out to validate its traditional uses.
Research frontiers
Current article aimed to evaluate and compare the antifungal potential and activity of the methanolic extract against C. albicans isolated from candiduria. The authors also examined the biofilm inhibition activity of the extract. This paper describes for the first time the extract effect against fungal isolates from candiduria.
Related reports
Relatively, few studies have been carried out to evaluate the antimicrobial activities of this plant against common human pathogens (Darwish et al., 2010). This study focuses on the antifungal effect of H. sabdariffa extract against C. albicans isolated from recurrent UTIs.
Innovations and breakthroughs
This study has shown that H. sabdariffa extract can be further developed for use in the treatment of infectious diseases.
Applications
This report finds wide applications in medicine. H. sabdariffa extract may be used as a local source of antifungal agents to treat and prevent UTIs.
Peer review
Generally the presentation of the paper is good. Methodology is appropriate and the results are presented properly. Based on the results, the authors reported a new application of this valuable medicinal plant. The results of biofilm inhibition indicate that this plant can be used to prevent recurrent UTIs.
Footnotes
Foundation Project: Supported by the Deanship of Scientific Research of Taibah University (Grant No.432/3088).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. J Microbiol. 2011;49:171–177. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- 2.Barnes RA. Early diagnosis of fungal infection in immunocompromised patients. J Antimicrob Chemother. 2008;61(Suppl 1):3–6. doi: 10.1093/jac/dkm424. [DOI] [PubMed] [Google Scholar]
- 3.Fisher JF. Candida urinary tract infections-epidemiology, pathogenesis, diagnosis, and treatment: executive summary. Clin Infect Dis. 2011;52(Suppl 6):S429–432. doi: 10.1093/cid/cir108. [DOI] [PubMed] [Google Scholar]
- 4.Guler S, Ural O, Findik D, Arslan U. Risk factors for nosocomial candiduria. Saudi Med J. 2006;27:1706–1710. [PubMed] [Google Scholar]
- 5.Bukhary ZA. Candiduria: a review of clinical significance and management. Saudi J Kidney Dis Transpl. 2008;19(3):350–360. [PubMed] [Google Scholar]
- 6.Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125(Suppl 1):S3–13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Sobel JD, Kauffman CA, McKinsey D, Zervos M, Vazquez JA, Karchmer AW, et al. et al. Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. The National Institute of Allergy and Infectious Diseases (NIAID) Mycoses Study Group. Clin Infect Dis. 2000;30:19–24. doi: 10.1086/313580. [DOI] [PubMed] [Google Scholar]
- 8.McKay DL, Chen CY, Saltzman E, Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr. 2010;140:298–303. doi: 10.3945/jn.109.115097. [DOI] [PubMed] [Google Scholar]
- 9.Rukayadi Y, Shim JS, Hwang JK. Screening of Thai medicinal plants for anticandidal activity. Mycoses. 2008;51:308–312. doi: 10.1111/j.1439-0507.2008.01497.x. [DOI] [PubMed] [Google Scholar]
- 10.Fullerton M, Khatiwada J, Johnson JU, Davis S, Williams LL. Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Escherichia coli O157:H7 isolated from food, veterinary, and clinical samples. J Med Food. 2011;14:950–956. doi: 10.1089/jmf.2010.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foo LY, Lu Y, Howell AB, Vorsa N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 12.Clinical Laboratory Standards Institute-CLSI . Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, M27-A3. 3rd ed. Wayne: CLSI; 2008. [Google Scholar]
- 13.Feldman M, Tanabe S, Howell A, Grenier D. Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement Altern Med. 2012;12:6. doi: 10.1186/1472-6882-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst EJ, Roling EE, Petzold CR, Keele DJ, Klepser ME. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob Agents Chemother. 2002;46:3846–3853. doi: 10.1128/AAC.46.12.3846-3853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darwish RM, Aburjai TA. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement Altern Med. 2010;10:9. doi: 10.1186/1472-6882-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng CH, Chyau CC, Chan KC, Chan TH, Wang CJ, Huang CN. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress while improving insulin resistance. J Agric Food Chem. 2011;59:9901–9909. doi: 10.1021/jf2022379. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Arroyo S, Herranz-López M, Beltrán-Debón R, Borrás-Linares I, Barrajón-Catalán E, Joven J, et al. et al. Bioavailability study of a polyphenol-enriched extract from Hibiscus sabdariffa in rats and associated antioxidant status. Mol Nutr Food Res. 2012;56:1590–1595. doi: 10.1002/mnfr.201200091. [DOI] [PubMed] [Google Scholar]
- 18.Viskelis P, Rubinskiene M, Jasutiene I, Sarkinas A, Daubaras R, Cesoniene L. Anthocyanins, antioxidative, and antimicrobial properties of American cranberry (Vaccinium macrocarpon Ait.) and their press cakes. J Food Sci. 2009;74:C157–161. doi: 10.1111/j.1750-3841.2009.01066.x. [DOI] [PubMed] [Google Scholar]
- 19.Diarra MS, Block G, Rempel H, Oomah BD, Harrison J, McCallum J, et al. et al. In vitro and in vivo antibacterial activities of cranberry press cake extracts alone or in combination with β-lactams against Staphylococcus aureus. BMC Complement Altern Med. 2013;13:90. doi: 10.1186/1472-6882-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo M, Perez C, Wei Y, Rapoza E, Su G, Bou-Abdallah F, et al. et al. Iron-binding properties of plant phenolics and cranberry's bio-effects. Dalton Trans. 2007;43:4951–4961. doi: 10.1039/b705136k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher JF, Kavanagh K, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infection: pathogenesis. Clin Infect Dis. 2011;52(Suppl 6):S437–451. doi: 10.1093/cid/cir110. [DOI] [PubMed] [Google Scholar]
- 23.Savoia D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol. 2012;7:979–990. doi: 10.2217/fmb.12.68. [DOI] [PubMed] [Google Scholar]
- 24.Högberg LD, Heddini A, Cars O. The global need for effective antibiotics: challenges and recent advances. Trends Pharmacol Sci. 2010;31:509–515. doi: 10.1016/j.tips.2010.08.002. [DOI] [PubMed] [Google Scholar]


