Abstract
Objective
To assess the antioxidant activity of Ficus pseudopalma Blanco (Moraceae) (F. pseudopalma) and characterize the active components present in it.
Methods
Column chromatography of crude ethanol leaf extract of F. pseudopalma was performed and seven fractions were obtained, labeled as F1, F2, F3, F4, F5, F6, F7. DPPH, FRAP, Griess, Fenton and superoxide radical scavenging assays were performed to assess the antioxidant ability of the fractions. Thin layer chromatography (TLC), high performance liquid chromatography and Fourier transfer infrared spectroscopy (FTIR) were performed to identify and characterize the bioactive component present in each fractions of F. pseudopalma.
Results
DPPH and FRAP assay showed that F5, F6 and F7 exhibited the good proton accepting ability and reducing power as compared to the other fractions. All fractions exhibited a good nitric oxide radical scavenging activity wherein F1, F2 and F3 showed the highest inhibition. However, all of the fractions exhibited a stimulatory activity on hydroxyl and superoxide radicals. Lupeol matched one of the spots on the thin layer chromatography chromatogram of the fractions. Linear gradient high performance liquid chromatography and spiking of lupeol with the fraction revealed the presence of 5.84 mg/L lupeol in F6. Infrared spectra of the fractions revealed the presence of C-C, OH, aromatic C=C and C=O groups.
Conclusions
The identified lupeol in F. pseudopalma may be responsible for the exhibited antioxidant property of the plant. Furthermore, knowing the antioxidant capability of the plant, F. pseudopalma can be developed into products which can help prevent the occurrence of oxidative stress related diseases.
Keywords: Antioxidant, Oxidative stress, Free radical scavenging, Reducing power, Phenolic acids, Ficus pseudopalma Blanco
1. Introduction
Physiologically, normal metabolic processes of the body produce significant amounts of reactive oxygen species (ROS). The damaging effects brought by these ROS are being counteracted by the cellular antioxidant defense system of the body which consist of enzymatic and non-enzymatic components[1]. However, at certain conditions, oxidative stress is triggered due to the imbalance between the production of ROS and the antioxidant systems of the body[2],[3]. Oxidative stress are usually related to high risk health conditions such as cardiovascular disease, neurodegenerative diseases, diabetes, cancer and inflammation[4]–[6].
Henceforth, potential antioxidative agents are being utilized and tested in order to alleviate the occurrence of such health conditions. In this regard, phytochemicals from various natural products had become the subject of most drug development researches.
Plants are the most commonly known sources of natural antioxidants which includes ascorbate, tocopherols, polyphenolic compounds and terpenoids[7],[8]. The numerous health benefits that people obtained from natural products is not only related to their antioxidative components but also to some of their phytochemical contents which work hand in hand in order to elicit functions that may specifically be beneficial in the treatment of certain diseases[8].
Ficus pseudopalma Blanco (F. pseudopalma), an endemic medicinal plant in Philippines, was reported to have an antioxidant activity[9],[10]. As presented in a study, F. pseudopalma contains terpenoids and sterols, which includes, α-amyrin acetate, β-amyrin acetate, ursenone and lupeol acetate[11], and they were reported to have antioxidant properties[12]. Terpenoids are among the commonly useful phytochemicals isolated from plants. They have various biological functions that help in maintaining good health. A study conducted by Goto et al. (2010) relating to peroxisome proliferator-activated receptors revealed that terpenoids can regulate the activity of peroxisome proliferator-activated receptors so as to maintain the energy homeostasis in the body and manage obesity induced metabolic disorders including type 2 diabetes, hyperlipidemia, insulin resistance and cardiovascular disease[13].
Lupeol is one of the identified compounds in F. pseudopalma which has several biological activities[11]. These activities were described in some review articles wherein according to them lupeol has anti-inflammatory, antimicrobial, antiprotozoal and anti-tumor activities[14],[15]. In this regard, lupeol can also be used as a nutraceutical and chemopreventive agent[15].
According to Stuart, F. pseudopalma is used as herbal medicine for the treatment of kidney stones and diabetes[16]. The plant also exhibits no toxicity to tested female Sprague Dawley rats at dose of 2 000 mg/kg body weight[17], which is why the young shoots and leaves can be eaten as salad and mixed with other vegetables. The anti-urolithiatic property of the plant was verified with the study conducted by Acosta et al. (2013) which reported that the crude dichloromethane extract of F. pseudopalma significantly decreased the creatinine and urine oxalates levels of ethylene-glycol induced male Sprague Dawley rats[17], which supports the ethnobonatical use of F. pseudopalma as an agent that cure kidney stones.
Considering the health benefits one can derive from F. pseudopalma, its biological, biochemical and pharmacological studies were scarce. Moreover, chemical profiling of the antioxidative components present in the plant is not yet been fully elucidated. Hence, the plant remains underutilized and insignificant. Therefore this study was undertaken to back up its ethnobotanical uses while intensively evaluating the antioxidative components of F. pseudopalma as well as assessing its potential role as a powerful antioxidant and a scavenger of free radicals.
2. Materials and methods
Fresh leaves of F. pseudopalma Blanco were collected from Brgy. San Jose, Pili, Camarines Sur, Philippines. All reagents and solvents used were either analytical or high performance liquid chromatography (HPLC) grade and were obtained from Sigma-Aldrich Co., Singapore.
2.1. Plant preparation and extraction
Plant preparation and extraction was done accordingly as what were described[9]. In brief, air-dried leaves were ground to fine powder using Wiley mill and sieved in 20 mm mesh size. The powder sample was kept in a clean, dried, well-sealed amber glass container to protect it from sunlight and contamination.
About 1 kg powdered leaves were soaked with 2.5 L 95% ethanol in a percolator for 4 d while changing the extracting solvent every after 24 h. The collected ethanol extract was then concentrated using the rotary evaporator (Eyela, USA) at 40 °C until syrupy consistency was obtained. The syrupy extract was further evaporated until it became dry. The air-dried crude extract was weighed, obtaining 4.572% yield and then kept in amber-colored container under 0 °C until use.
2.2. Column chromatography
Different factions from the crude ethanolic leaf extract was obtained through sequential elution column chromatography. Silica gel G-50, 100-200 mesh (Hi-media, India) was used as the stationary phase. Five grams of silica gel was added with 10 mL of hexane. The mixture was then transferred to a column and was allowed to stabilize for 20 min. The crude ethanolic leaf extract of F. pseudoplama (1.5 g) was dissolved in 2 mL 95% ethyl alcohol to form a slurry. After the stabilization of the column, the crude ethanol leaf extract slurry was loaded to the column and was continuously eluted with the mobile phase (hexane, ethyl acetate, acetone and methanol). Approximately 10 mL of eluents were collected in pre-weighed vials. A total of seven fractions were collected and were labeled accordingly: hexane (F1), hexane: ethyl acetate (F2), ethyl acetate (F3), ethyl acetate: acetone (F4), acetone (F5), acetone: methanol (F6) and methanol (F7) fractions. These fractions were concentrated by removing the solvent through rotary evaporator (Eyela, USA). The fractionated extracts that were obtained were kept in a refrigerator at -20 °C until use.
2.3. Micro-scale antioxidant tests
Procedures for the antioxidant tests were performed according to the discussed procedures[9].
2.3.1. DPPH radical scavenging
The hydrogen donating ability of each fractions was evaluated using the stable DPPH radical. Each fractions were prepared in ethanol at different concentrations. Ten microliter of each fractions at various concentrations were loaded in a 96-well microplate (triplicate). Then 140 µL of 6.58×10−5 mol/L DPPH solution was added to each well. The microplate was incubated for 30 min at 25 °C in the dark. The absorbance of the fractions were measured at 517 nm. The free radical scavenging activity was expressed as the percentage inhibition of free radical by each fraction.
2.3.2. FRAP assay
The reducing power can be measured through the intensity of the Prussian blue color that results from the direct reduction of potassium ferricyanide [K3Fe3(CN)6] to potassium ferrocyanide [K3Fe2(CN)6][18].
Different concentrations of each fraction (40 µL) were mixed with 1.0 mol/L hydrochloric acid (100 µL), 1% sodium dodecylsulphate (20 µL) and 1% potassium ferricyanide (30 µL) in an Eppendorf tube. The mixtures were incubated at 50 °C for 20 min. Then, 30 µL of 0.1% ferric chloride was added to each tubes. Aliquots of the mixtures were transferred to a 96-well microplate and absorbance was read at 750 nm.
2.3.3. Nitric oxide radical scavenging
The ability of the fractions to scavenge nitric oxide radical was assessed through the Griess reaction[19]. One hundred microliters of each fractions of the ethanolic leaf extract of F. pseudopalma, at different concentrations, were mixed with 10 mmol/L sodium nitroprusside (400 µL) and phosphate buffered saline, pH 7.4 (100 µL). The mixture was incubated for 150 min at 25 °C. After incubation, 100 µL of each mixture was transferred to a new tube and was added with 0.33% sulfanilamide (200 µL). The resulting mixture is incubated for 5 min at 25 °C. Then 0.1% napthylethylenediamine (200 µL) was added to each tubes. The tubes were incubated for another 30 min 25°C. An aliquot of 250 µL of the resulting mixture was transferred to a 96-well microplate in triplicate and was read at 540 nm.
2.3.4. Hydroxyl radical scavenging
The method used for the scavenging of the highly reactive hydroxyl radical was determined by Valko M, et al. with slight modification[20]. Before starting, Fenton reagent-a mixture of 2.5 mL 0.1 mmol/L FeCl3, 0.625 mL 1.5% H2O2 and 1.88 mL 0.002 9% EDTA-was prepared. Briefly, 50 µL of varied concentration of sample was loaded in each designated plates followed by the addition of 200 µL Fenton reagent. The absorbance of each wells were read a 500 nm as an optimized wavelength of the blank away from the original 288 nm.
2.3.5. Superoxide radical scavenging
Five microliter of each sample was loaded in designated wells. After, 50 µL 73 µmol/L NADH, 50 µL 156 µmol/L nitrobluetetrazolium and 50 µL 60 µmol/L phenazine methosulfate was introduced to the sample loaded wells. To complete the reaction, the mixture was incubated for 5 min at 25 °C and was immediately read at 560 nm[18].
2.4. Structural evaluation of the active antioxidant components
2.4.1. Thin layer chromatography (TLC)
Thin layer chromatography was performed to identify the components present in each fractions of F. pseudopalma. In a TLC plate pre-coated with silica gel (6 cm×8 cm), each fractions were applied in small spots. The TLC chamber was developed using ethyl acetate: toluene: methanol (6:3:1) and was used as the solvent system.
2.4.2. Infrared spectroscopy
Attenuated total reflectance-Fourier transform infrared spectroscopy was performed using Shimadzu IR-Prestige 21 in order to analyze the functional group present on the seven fractions obtained from the crude ethanolic leaf extract of F. pseudopalma.
2.4.3. HPLC analysis
2.4.3.1. Standard preparation
Standard quercetin (24.0 mg), rutin (40.0 mg), gallic acid (22.0 mg) and lupeol (2.7 mg) were accurately weighed and dissolved in vacuum-filtered methanol (HPLC grade) to obtain a stock solution. These solutions were further diluted to 6.67 mg/mL, 2.4 mg/mL, 4.4 mg/mL and 2.7 mg/mL, respectively before the chromatographic analysis.
2.4.3.2. Sample preparation
Seven fractions from the crude ethanolic leaf extract of F. pseudopalma, labeled as F1, F2, F3, F4, F5, F6, and F7, were prepared and dissolved in vacuum-filtered methanol (HPLC grade). Each solutions were filtered using 0.22 µm membrane filter before injecting to the sample port.
2.4.3.3. Apparatus and chromatographic condition
The components present in each fractions were separated using reverse-phase C18 column. The mobile phase was comprised of 70% methanol (A), phosphate buffer, pH 7.2 (B) and ultrapure water (C). Linear elution of the solvent system was programmed at 4% B and 96% C for the first 2 min; 50% A, 1% B and 49% C for 2 to 6 min; 80% A, 2% B and 18% C for 6 to 26 min; 50% A, 1% B and 49% C for 26-30 min; and the last 5 min for 4% B and 96% C. The mobile phase was delivered at 1 mL/min with detection wavelength at 280 nm.
Sixty microliters of each fractions and standard solutions were introduced to the column. The chromatographic peaks obtained of each fractions were compared to that of the peaks obtained by the standard solutions. Spiking was performed in order to confirm the presence of each standard used on the fractions of F. pseudopalma.
2.5. Statistical analysis
Mean±SEM were used to summarize the data gathered from the experiment. Single-factor analysis of variance (ANOVA) was used to determine if there is a significant difference in the mean percentage inhibition on antioxidant assays (DDPH, nitric oxide, hydroxide and superoxide), mean percentage reducing power using FRAP assay. Tukey's HSD was used for post-hoc analyses.
All the statistical tests were performed using Graphpad's Prism 5.0, and SAS 9.0. P-values less than 0.05 indicate significant difference.
3. Results
3.1. Free radical scavenging activity of the fractions of F. pseudopalma
3.1.1. DPPH radical scavenging activity
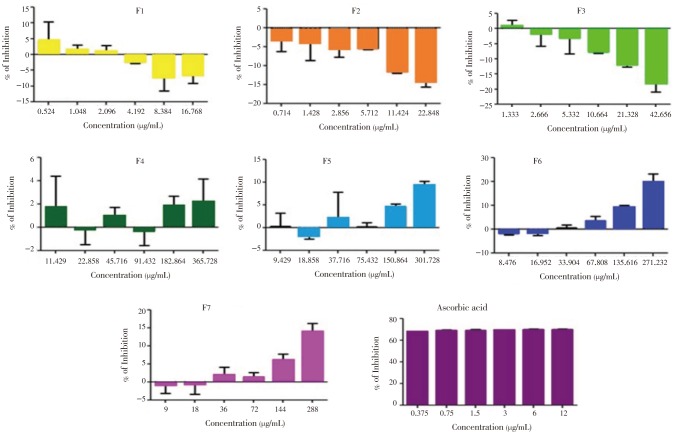
DPPH assay of the fractions from the ethanolic leaf extract of F. pseudopalma showed that F1 (P=0.006), F2 (P=0.008) and F3 (P<0.001) has a significantly least mean percentage of inhibition of DPPH radical compared to the other fractions (Figure 1). The scavenging activity of the F4 is not dependent on the concentration of the fraction (P=0.324) as indicated by its mean percentage inhibition. Moreover, the increase in DPPH scavenging activity is observed in the polar fractions of F. pseudopalma, F5 (P=0.003), F6 (P<0.001), F7 (P<0.001) as shown by their mean percentage inhibition. In connection to this, the highest concentration of the indicated fractions has demonstrated the best DPPH scavenging activity (P<0.05). The activity of each fractions was compared to the standard ascorbic acid, which exhibits a good scavenging activity (P=0.004).
Figure 1. Proton-donating ability of the fractions obtained from the ethanolic leaf extract of F. pseudopalma. Analysis was performed in triplicates (n=3, P>0.05).
3.1.2. Ferric reducing power
The ferric reducing power of the fractions of F. pseudopalma were evaluated using FRAP assay which utilizes the reduction of Fe2+ ions to Fe3+ to form a Prussian blue complex.
As illustrated in Figure 2, the ferric reducing power of almost all the fractions increases with concentrations (P>0.05). Significant differences in the mean percentage reducing power were observed between the concentrations of each fractions. As shown, F1 (P=0.003) and F2 (P<0.001) has exhibited the least ferric reducing power. Whereas, F3 (P<0.001) and F4 (P<0.001) has almost the same activity.
Figure 2. Ferric reducing activity of the fractions obtained from the ethanolic leaf extract of F. pseudopalma. Analysis was performed in triplicates (n=3, P>0.05).
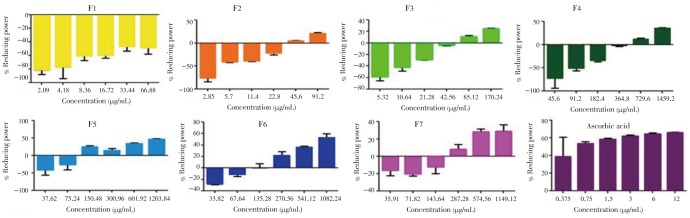
Polar fractions of F. pseudopalma, F5 (P<0.001), F6 (P<0.001) and F7 (P<0.001), had demonstrate the highest mean percentage of reducing power. All fractions were compared to ascorbic acid (P=0.029), which exhibited a good reducing power.
3.1.3. Nitric oxide scavenging activity
Nitric oxide scavenging ability of the fractions of F. pseudopalma was determined using Griess' reaction. As shown in Figure 3, significant difference (P<0.05) was observed between the activity of each fractions. The activity of F1 (P=0.043), F2 (P<0.001) and F3 (P<0.001) showed the highest scavenging ability compared to the other fractions. This is followed by F5 (P<0.001), F6 (P=0.216) and F7 (P<0.001). Among the fractions, F4 (P<0.001) has the lowest scavenging activity. The scavenging ability of all the fractions was compared to ascorbic acid (P<0.001) which elicit a concentration-dependent inhibition of nitric oxide radical production.
Figure 3. Concentration-dependent inhibition of NO• by the fractions obtained from the ethanolic leaf extract of F. pseudopalma.
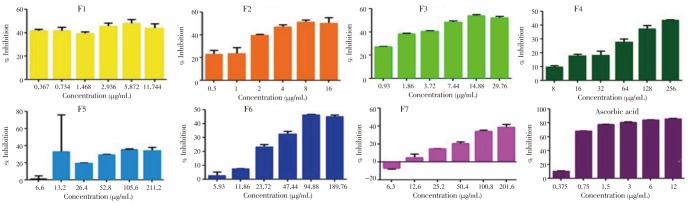
Analysis was performed in triplicates (n=3, P>0.05).
3.1.4. Hydroxyl radical scavenging activity
Hydroxyl radicals (•OH) is among the free radicals that are physiologically produced by the body through its metabolic processes[21]. In the assay, hydroxyl radical was produced by the reaction of H2O2 with EDTA-bound Fe2+.
Almost all the fractions of F. pseudopalma were able to stimulate the production of hydroxyl radicals (Figure 4). Significant differences were observed on scavenging activity of each fractions (P<0.05). Hexane (P<0.001), hexane: ethyl acetate (P<0.001) and ethyl acetate (P<0.001) fractions showed the highest stimulation of hydroxyl radicals. Semi-polar and polar fractions, F4 (P<0.001), F5 (P<0.001), F6 (P<0.001) and F7 (P=0.496), has low stimulatory activity towards hydroxyl radicals as compared to the first three fractions mentioned as indicated by their mean percentage inhibition. In addition to that, difference in the concentration of F7 does not affect its activity towards scavenging of hydroxyl radicals.
Figure 4. Concentration-dependent stimulation of •OH by the fractions obtained from the ethanolic leaf extract of F. pseudopalma. Analysis was performed in triplicates (n=3, P>0.05).
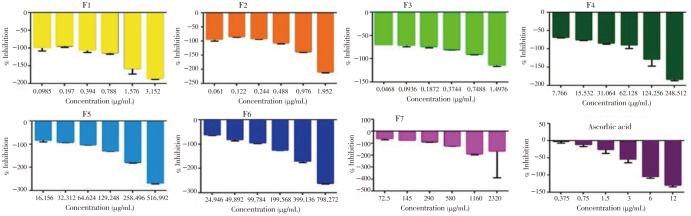
3.1.5. Superoxide radical scavenging activity
Another common oxygen radical in the body is superoxide radicals (•O−2)[21]. It is produced with the •OH and continue to a cascade of free radical formation in the body. In this experiment, •O−2 are produced from phenazine methosulfate-NADH and direct reduction of nitrobluetetrazolium.
As demonstrated in Figure 5, there is a significant difference in the superoxide radical scavenging ability of each fractions (P>0.05). The nonpolar fractions such as hexane (F5,12=3.648, P=0.031), hexane: ethyl acetate (P=0.069) and ethyl acetate (P=0.001) exhibited the highest stimulatory effect towards superoxide radicals. They were followed by the semi-polar and polar fractions: F4 (P<0.001), F5 (P=0.040), F6 (P=0.019) and F7 (P<0.001), which have lower stimulatory effect as compared to the first three fractions that were mentioned.
Figure 5. Concentration-dependent stimulation of •O−2 by the fractions obtained from the ethanolic leaf extract of F. pseudopalma. Analysis was performed in triplicates (n=3, P>0.05).
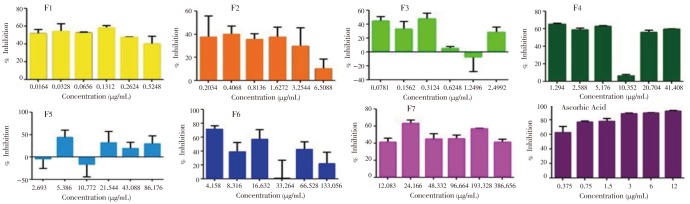
On the other hand, ascorbic acid (P<0.001) was able to inhibit the production of superoxide radical as opposed to the exhibited activities of the fractions of F. pseudopalma.
3.2. Structural evaluation of the active antioxidant components
3.2.1. Thin layer chromatography (TLC)
The TLC chromatogram of the fractions revealed that fractions 1, 2 and 3 have the most number of components and one of which corresponds to lupeol (Rf=0.831). Other fractions showed lesser number of components. In order to further verify the results and confirm the compounds present in the fractions of F. pseudopalma, infrared spectroscopy and HPLC analysis was performed.
3.2.2. Infrared spectroscopy
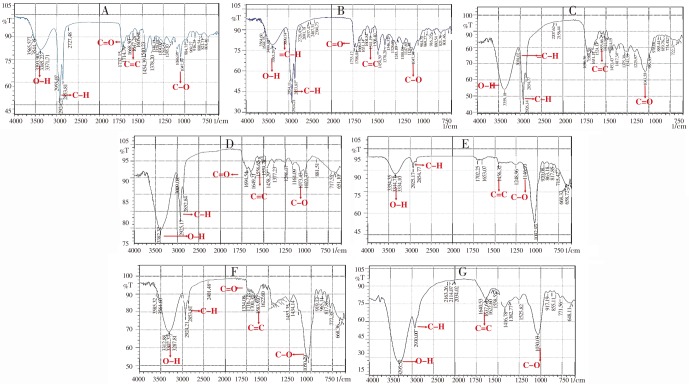
Infrared spectra of the fractions obtained from F. pseudopalma revealed the presence of alkane and alcohol functional group (Figures 6 and 7). The sharp peak for the IR spectra of each fraction with values ranging from 2 800-3 000 cm− corresponds to a C-H stretch functional group of alkane. Terminal alkene group (=C-H) was observed at hexane: ethyl acetate (50: 50) and ethyl acetate fractions. Of the seven spectra, methanol fraction showed a more prominent broad peak that corresponds to an O-H stretch (3 300-3 400 cm−) of phenol or alcohol functional group. Aromatic C=C was also shown in the spectra of all the fractions. Other functional groups that were assumed to be present in each fraction are summarized in Table 1.
Figure 6. Infrared spectra of the 7 fractions from the crude ethanolic leaf extract of F. pseudopalma.
A: Hexane fraction; B: Hexane: ethyl acetate fraction; C: Ethyl acetate fraction; D: Ethyl acetate: acetone fraction; E: Acetone fraction; F: Acetone: methanol fraction; G: Methanol fraction.
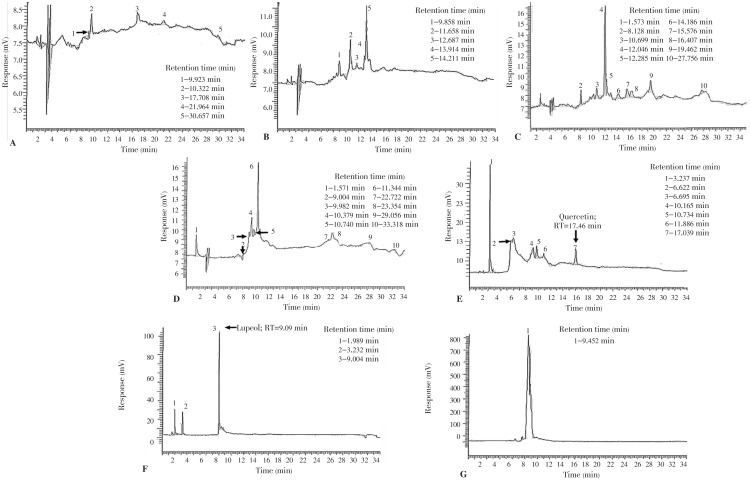
Figure 7. HPLC chromatogram of the crude ethanolic leaf extract of F. pseudopalma.
A: hexane fraction; B: hexane:ethyl acetate fraction; C: ethyl acetate fraction; D: ethyl acetate: acetone fraction; E: acetone fraction; F: acetone:methanol fraction; G: methanol fraction. The numbers correspond to the major peaks observed with their corresponding retention time.
Table 1. Summary of the infrared spectra of each fractions of F. pseudopalma.
| Chemicals | Infrared spectra |
||||||
| Hexane | Hexane: ethyl acetate | Ethyl acetate | Ethyl acetate:acetone | Acetone | Acetone: methanol | Methanol | |
| Alkane C-H | 2853.81 | 2853.81 | 2854.77 | 2852.84 | 2854.77 | 2853.81 | 2930.00 |
| 2924.21 | 2924.21 | 2926.14 | 2925.17 | 2925.17 | 2924.21 | ||
| 2955.07 | 2954.11 | ||||||
| Alkene =C-H | - | 3099.74 | 3038.02 | - | - | - | - |
| C=C | 1647.28 | 1651.14 | 1651.14 | 1641.49 | 1653.07 | 1651.14 | 1640.53 |
| 1668.50 | 1666.57 | 1668.50 | 1649.21 | 1665.60 | |||
| Alcohol C-O | 1046.43 | 1047.39 | 1043.53 | 1641.49 | 1032.93 | 1050.29 | 1030.03 |
| O-H | 3365.93 | 3371.71 | 3359.18 | 3382.32 | 3334.10 | 3302.27 | 3295.52 |
| 3369.79 | 3385.22 | 3344.71 | 3312.88 | ||||
| 3383.29 | 3393.90 | 3564.60 | |||||
| 3499.02 | 3544.35 | 3354.35 | 3585.82 | ||||
| 3544.35 | 3565.57 | ||||||
| 3564.60 | |||||||
| Ether C-O | 1168.91 | 1068.61 | - | 1073.43 | 1146.73 | 1050.29 | - |
| 1144.80 | |||||||
| Aldehyde Aromatic/Conjugated C=O Saturated C=O | - | 1708.04 | - | - | - | - | - |
| 1732.15 | 1733.12 | - | - | - | 1734.08 | - | |
| C-H | 2727.46 | 2728.43 | |||||
| Ketone Aromatic/Conjugated C=O Cyclic C=O Saturated C=O | - | - | - | - | - | 1683.93 | - |
| - | - | - | - | - | 1716.72 | - | |
| 1714.79 | - | - | - | - | - | - | |
| Arene C-H | 821.71 | 819.78 | 819.78 | 3009.08 | 817.85 | 817.85 | 771.56 |
| 836.18 | 817.85 | ||||||
| Aromatic Ring | 1454.39 | 1454.39 | 1453.43 | 1458.25 | 1456.32 | 1455.35 | 1507.43 |
| 1540.23 | 1508.40 | 1514.19 | 1507.43 | 1653.07 | 1622.20 | 1520.94 | |
| 1605.81 | 1541.19 | 1519.01 | 1521.90 | 1702.25 | 1636.67 | 1543.12 | |
| 1647.28 | 1604.84 | 1544.08 | 1539.26 | 1651.14 | 1558.55 | ||
| 1668.50 | 1651.14 | 1555.66 | 1556.62 | 1665.60 | 1622.20 | ||
| 1714.79 | 1666.57 | 1610.63 | 1641.49 | 1683.93 | 1637.64 | ||
| 1732.15 | 1708.04 | 1624.13 | 1649.21 | 1700.32 | 1640.53 | ||
| 17333.12 | 1651.14 | 1684.89 | 1716.72 | ||||
| 1668.50 | 1694.54 | 1734.08 | |||||
| 1699.36 | |||||||
Spectral analysis of the fractions help in the determination of the active compounds present in each fraction that contribute to their antioxidant activity.
3.2.3. HPLC analysis
High pressure liquid chromatography was performed in order to further confirm the active antioxidative components of each fractions from the crude ethanolic leaf extract of F. pseudopalma.
Chromatograms of all the fractions were compared to different standards which includes quercetin, rutin, gallic acid and lupeol. However, only lupeol was observed on the chromatogram of F6. This finding was further confirmed through spiking wherein the standard lupeol was mixed with the F6 solution and was injected to the HPLC unit. The chromatogram of the spiked F6 (Figure 8) showed that one of the peaks had increased in height and area, which correspond mainly to the presence of lupeol. Quantitative analysis revealed that 5.84 mg/L lupeol was contained in F6 using a standard curve.
Figure 8. HPLC chromatogram of F6.
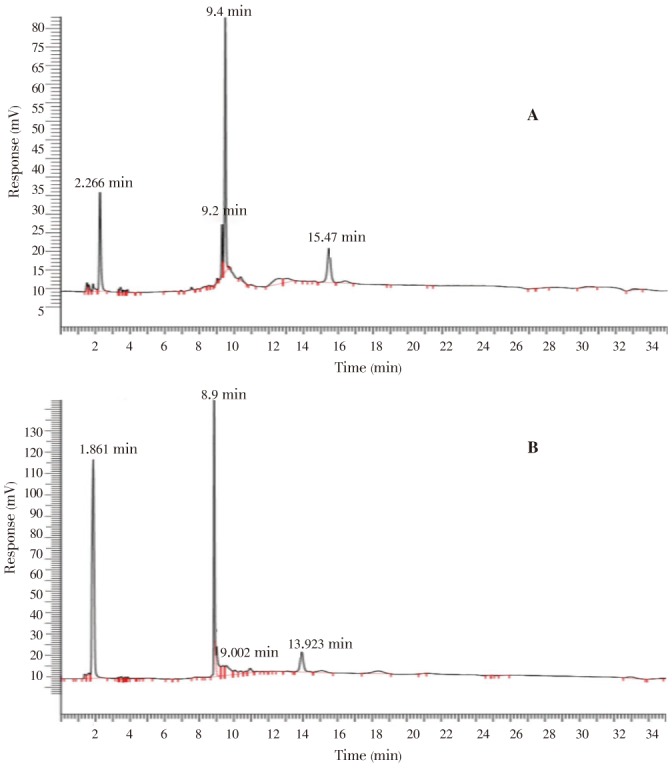
(A) is compared to the chromatogram of F6 spiked with lupeol (B).
4. Discussion
The DPPH and FRAP assay are general tests used to evaluate the antioxidant capacity of substance. The DPPH radical is commonly used for fast evaluation of the antioxidant property of a given compound[22]. The dark purple solution of DPPH is turned to yellow when the DPPH radical subsequently receive an electron or a hydrogen radical from an antioxidant[23]. This change in color can be measured spectrophotometrically at 517 nm. Moreover, the antioxidant activity can be also linked to the reducing power of a bioactive compound[24]. In this assay, the ability of each fractions from the ethanolic leaf extract of F. pseudopalma to directly reduce of Fe3+ to Fe2+ was monitored by measuring the increase in absorbance through the formation of Pearl's Prussian blue complex at 750 nm[18].
As indicated in the results, most of the polar fractions of F. pseudopalma were scavengers of DPPH radicals and has a better reducing power compared to that of the nonpolar and semi-polar fractions. The electron donating property of each of the fractions can potentially neutralize the action of free radicals. In relation to this, the reducing power exhibited by each of the fractions can serve as an indicator of their antioxidant potential.
Specific antioxidant tests were also performed in order to evaluate the influence of the fractions from F. pseudopalma on the activity of free radicals that are physiologically produced by the body. Nitric oxide free radical (NO•) is a biologically active specie that can readily react with other free radicals inside the body which in turn mediate specific biological effects depending on the local chemical environment[25]. NO• is usually linked in inflammation[21]and carcinogenesis[26]. Overproduction of NO• by nitric oxide synthase is commonly observed in patients with rheumatoid arthritis and osteoarthritis[21].
Hydroxyl radicals and superoxide radicals are among the free radicals that are physiologically produced by the body through its metabolic processes[27]. The immune system utilize these reactive species in order to fight the invading pathogens so as to maintain the homeostasis inside the body.
Generally, free radicals do not only cause harmful effects in the body. In fact, free radicals have been also linked to the killing of cancer cells. Cancer cells are normally anaerobic cells which survive in a less oxygenated environment. Exposure of cancer cells to oxidative stress would lead to growth inhibition or even death.
The antioxidant activity of several plant extracts are usually related to the phytochemicals that were isolated from them. Some of the known antioxidant agents are the phenolic acids, flavonoids and terpenoids. In order to determine the active antioxidative component of F. pseudopalma, the fractions of the ethanolic leaf extract were subjected to TLC, high pressure liquid chromatography and infrared spectroscopy to verify the structure of the compound present.
The TLC chromatogram showed that lupeol was present in F1, F2, F3 and F4. However, HPLC analysis revealed that 5.84 mg/L lupeol was present in the acetone: methanol (50: 50) (retention time=9.00 min). Lupeol is a pentacyclic triterpenes that exhibit antioxidative activity through direct scavenging of free radicals and protects membrane permeability[14]. This property can be related to its hepatoprotective and anticancer properties. In one particular study, lupeol compound isolated from Verpis punctata exhibited cytotoxicity (IC50=26.4µg/mL) on A2780 human overian cancer cell line[28].
Additionally, lupeol was also reported to have cardioprotective activity due to its ability to ameliorate lipidemic-oxidative abnormalities in the early stage of hypocholesterolemic atherosclerosis in rats[29]. Other findings showed that lupeol provided 34.4% protection against in vitro low density lipoprotein oxidation and hypotensive activity that makes it a preventive agent against cardiac disorder[30],[31].
Another reported biological activity of lupeol is hepatoprotections. In one related study, lupeol showed some effectiveness in lessening the action of aflatoxin B1, which is a fungal derived toxin[32] that is considered to be the most potent of the mycotoxins[33] which can cause acute hepatotoxicity and liver carcinoma in exposed animal. In relation to that, it was observed that lupeol can substantially normalized degenerative alterations in the hepatocytes with granular cytoplasm[34]. Additionally, lupeol treatment induced growth inhibition and apoptosis in hepatocellular carcinoma SMMC7721 cells by down-regulation of the death receptor 3 expression[35].
Detection of this compound in F. pseudopalma supports the antioxidant activity exhibited by the plant. In addition to that, lupeol may also be the one that contribute to the anticancer property of the plant. A study showed that the ethanolic extract of F. pseudopalma demonstrated a cytotoxic effect against HepG2 cells that was comparable to the activity of curcumin[10].
Based on the aforementioned information, lupeol is indeed one of the most valuable phytochemical constituents of the plant, where several benefits can be derived that include cardioprotection, hepatoprotection and can help in the prevention of cancer progression.
Being one of the local medicinal plant in Philippines which has only meager studies, it is noteworthy to know other uses of F. pseudopalma Blanco (Moraceae). Identification of lupeol in F. pseudopalma supports the exhibited antioxidant activity of the plant which can be used to avoid the prevalence of several diseases related to oxidative stress.
Since little is known about the specific chemical constituents of the plant, further analysis such as liquid chromatography coupled with mass spectroscopy (LC-MS) can be performed in order to elucidate and further confirm the existence of other phytochemicals that may contribute to its pharmaceutical function.
Acknowledgments
The authors would like to extend their gratitude to the Grants-in-Aid of the Philippine Council for Health Research and Development, Department of Science and Technology for providing the financial support for the study with Grant no. FP 120023. The authors are also in gratitude for the Research Center for the Natural and Applied Sciences (RCNAS) of the University of Santo Tomas for the assistance in conducting the analytical experiments included in the study.
Comments
Background
The damaging effects of imbalances in the oxidative state of the cell could trigger development and exacerbation of cardiovascular diseases, hepatocellular maladies and cancer. The biochemical and instrumental identification of lupeol, a biologically active triterpene, in the Philippine-endemic F. pseudopalma Blanco (Moraceae) has provided insights into the potential use of phytochemicals as antioxidants.
Research frontiers
The current research work presents biochemical characterization of antioxidant properties and structural evaluation of phytochemicals, lupeol in particular, present in leaf extracts of F. pseudopalma Blanco (Moraceae).
Related reports
The present work complements the NMR instrumentation done by Ragasa CY et al. in 2009 on the leaf extracts of F. pseudopalma Blanco (Moraceae). Furthermore, the paper supports previous reports on the potential biological and pharmacological property of lupeol and the plant as a whole.
Innovations and breakthroughs
F. pseudopalma Blanco (Moraceae) commonly known as the Philippine fig or niog-niogan in some parts of the country, is being used as an ornamental or medicinal plant. In the present study, the authors showed the antioxidant properties of different organic solvent fractions of the leaf of the plant eventually identifying lupeol as one of the active compents.
Applications
The demonstrated free-radical scavenging activity of the different leaf extracts of F. pseudopalma Blanco (Moraceae) as well as the identification of lupeol as an active component, support the prospective application as an anti-cancer and cardio/hepatoprotective agent.
Peer review
This is an interesting research work in which authors have demonstrated the free-radical scavenging activity of the leaf extracts of F. pseudopalma Blanco (Moraceae) using biochemical tests. Lupeol was identified as one of the possible active components based on chromatographic analysis and infrared spectroscopy. These significant results have shown the importance of phytochemical screening of endemic flora that potentially harbors pharmacologically promising drug candidates.
Footnotes
Foundation Project: Supported by Grants-in-aid of the Philippine Council for Health Research and Development, Department of Science and Technology (Grant no. FP 120023).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Hopps E, Noto D, Caimi G, Averna MR. A novel component of metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis. 2010;20(1):72–77. doi: 10.1016/j.numecd.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Wiernsperger NF. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes Metab. 2008;29(6):579–585. doi: 10.1016/s1262-3636(07)70072-1. [DOI] [PubMed] [Google Scholar]
- 3.Pepe H, Balci SS, Revan S, Akalin PP, Kurtoğlu F. Comparison of oxidative stress and antioxidant capacity before and after running exercises in both sexes. Gend Med. 2009;6(4):587–595. doi: 10.1016/j.genm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Fearon IM, Faux SP. Oxidative stress and cardiovascular disease: Novel tools give free radical insight. J Mol Cell Cardiol. 2009;47(3):372–381. doi: 10.1016/j.yjmcc.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Pashkow FJ. Oxidative stress and inflammation in heart disease: do antioxidants have a role in treatment and/or prevention? Int J Inflamm. 2011 doi: 10.4061/2011/514623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang YZ, Yang S, Wu G. Free radicals, antioxidants and nutrition. Nutrition. 2002;18(10):872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 7.Grassmann J. Terpenoids as plant antioxidants. Vitam Horm. 2005;72:505–535. doi: 10.1016/S0083-6729(05)72015-X. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrios B. Sources of natural phenolics and antioxidants. Trends Food Sci Technol. 2006;17:506–512. [Google Scholar]
- 9.Santiago LA, Valerio VLM. Assessment of antioxidant activities of crude ethanolic leaf extarct of Ficus pseudopalma Blanco (Moraceae) Int J Pharm Front Res. 2013;3(1):15–25. [Google Scholar]
- 10.Bueno PRP, Buno CBM, Santos DLM, Santiago LA. Antioxidant activity of Ficus pseudopalma Blanco and its cytotoxic effect on hepatocellular carcinoma and peripheral blood mononuclear cells. Curr Res Bio Pharm Sci. 2013;2(2):14–21. [Google Scholar]
- 11.Ragasa CY, Tsai P, Shen CC. Terpenoids and sterols from the endemic and endangered Philippine trees, Ficus pseudopalma and Ficus ulmifolia. Philipp J Sci. 2009;138(2):205–209. [Google Scholar]
- 12.Topcu G, Ertas A, Kolak U, Ozturk M, Ulubelen A. Antioxidant activity tests on novel triterpenoids from Salvia macrochlamys. ARKIVOC. 2007;7:195–208. [Google Scholar]
- 13.Goto T, Takahashi N, Hirai S, Kawada T. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res. 2010 doi: 10.1155/2010/483958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wal P, Wal A, Sharma G, Rai AK. Biological activities of lupeol. Syst Rev Pharm. 2011;2(2):96–103. [Google Scholar]
- 15.Gallo MBC, Sarachine MJ. Biological activities of lupeol. Int J Biomed Pharm Sci. 2009;3(1):46–66. [Google Scholar]
- 16.Stuart G. Philippines: StuartXchange; 2011. Niyog-niyogan, Quisqualis indica Linn. Yesterday, today and tomorrow. [Online] Available from: http://www.stuartxchange.org/ niyog-niyogan.html. [Accessed on September 23, 2012] [Google Scholar]
- 17.Acosta CJH, Macabeo APH, Santiago LA. Assessment of the antiurolithiatic and antioxidant properties of Ficus pseudopalma Blanco leaves (Moraceae) Curr Res Bio Pharm Sci. 2013;2(2):22–30. [Google Scholar]
- 18.Ahmed AS, Elgorashi EE, Moodley N, McGaw LJ, Naido V, Eloff JN. The antimicrobial, antioxidative, anti-inflammatory activity and cytotoxicity of different fractions of four South African Bauhinia species used traditionally to treat diarrhea. J Ethnopharmacol. 2012;143(3):826–839. doi: 10.1016/j.jep.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 2008;8:63. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 21.Murrell GA, Dolan MM, Jang D, Szabo C, Warren RF, Hannafin JA. Nitric oxide: an important articular free radical. J Bone Joint Surg Am. 1996;78:265–274. [PubMed] [Google Scholar]
- 22.Bozin B, Mimica-Dukic N, Samojlik I, Goran A, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae) Food Chem. 2008;111:925–929. [Google Scholar]
- 23.Shukla S, Mehta A, John J, Singh S, Mehta P, Vyas SP. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol. 2009;47:1848–1851. doi: 10.1016/j.fct.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 24.Siddhuraju P, Mohan PS, Becker K. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude xtracts from stem bark, leaves, flowers and fruit pulp. Food Chem. 2002;79:61–67. [Google Scholar]
- 25.Mayer O, Jr, Simon J, Holubec L, Pikner R, Trefil L. Folate co-administration improves the effectiveness of fenofibrate to decrease the lipoprotein oxidation and endothelial dysfunction surrogates. Physiol Res. 2006;55:475–481. doi: 10.33549/physiolres.930856. [DOI] [PubMed] [Google Scholar]
- 26.Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, et al. et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A. 2003;100(1):143–148. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong CC, Li HB, Cheng KW, Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. [Google Scholar]
- 28.Chaturvedula VS, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DG. New cytotoxic terpenoids from the wood of Vepris punctata from the Madagascar rainforest. J Nat Prod. 2004;67(5):895–898. doi: 10.1021/np0303512. [DOI] [PubMed] [Google Scholar]
- 29.Sudhahar V, Ashokkumar S, Varalakshmi P. Effect of lupeol and lupeol linolate on lipemic-haptocellular aberrations in rats fed a high cholesterol diet. Mol Nutr Food Res. 2006;50(12):1212–1219. doi: 10.1002/mnfr.200600134. [DOI] [PubMed] [Google Scholar]
- 30.Andrikopoulos NK, Kaliora AC, Assimopolou AN, Papapeorgiou VP. Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytother Res. 2003;7:501–507. doi: 10.1002/ptr.1185. [DOI] [PubMed] [Google Scholar]
- 31.Saleem M, Adhami VM, Siddiqui IA, Mukhtar H. Tea beverage in chemoprention of prostate cancer: a mini-review. Nutr Cancer. 2003;47:13–23. doi: 10.1207/s15327914nc4701_2. [DOI] [PubMed] [Google Scholar]
- 32.Preetha SP, Kanniappan M, Selvakumar E, Nagaraj M, Varalakshmi P. Lupeol ameliorates aflatoxin B1-induced peroxidative hepatic damage in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2006;143:333–339. doi: 10.1016/j.cbpc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Luyendyk JP, Copple B, Barton CC, Ganey PE, Roth RA. Augmentation of aflatoxin B1 hepatotoxicity by endotoxin: involvement of endothelium and coagulation system. Toxicol Sci. 2003;72(1):171–181. doi: 10.1093/toxsci/kfg007. [DOI] [PubMed] [Google Scholar]
- 34.Akçam M, Artan R, Yilmaz A, Ozdem S, Gelen T, Nazıroğlu M. Caffeic acid phenethyl ester modulates aflatoxin B1-induced hepatotoxicity in rats. Cell Biochem Funct. 2013 doi: 10.1002/cbf.2957. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Zhang Y, Zhang L, Yang X, Lv Z. Lupeol, a dietary triterpene, inhibited growth and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest. 2009;27:163–170. doi: 10.1080/07357900802210745. [DOI] [PubMed] [Google Scholar]