Abstract
Objective
To evaluate the hepatoprotective and antioxidant properties of alkaloid extract of Cyclea peltata (C. peltata) against paracetamol/carbon tetra chloride induced liver damage in Wistar rats.
Methods
In vivo paracetamol/carbon tetrachloride induced liver damage in Wistar rats, in vitro free radical scavenging studies, HPTLC estimation of tetrandrine and direct analysis in real time- mass spectrometry of alkaloid extract of C. peltata were used for the validation.
Results
The results showed that pretreatment with alkaloid extract of C. peltata caused significant reduction of serum glutamate pyruvate transaminase, serum glutamate oxaloacetate transaminase, serum alkaline phosphatase, serum cholesterol, liver malondialdehyde levels. The reduced glutathione, catalase, superoxide dismutase levels in liver were increased with alkaloid extract of C. peltata treatment. These results were almost comparable to silymarin and normal control. Histopathological studies also substantiated the biochemical findings. The in vitro hydroxyl, superoxide and DPPH scavenging study of alkaloid extract of C. peltata showed significant free radical scavenging property.
Conclusions
The hepatoprotective property of alkaloid extract of C. peltata against paracetamol/carbon tetrachloride may be due the synergistic action of alkaloids especially tetrandrine, fangchinoline through free radical scavenging and thus preventing oxidative stress.
Keywords: Tetrandrine, Glutathione, Catalase, Superoxide dismutase, Hydroxyl, Malondialdehyde
1. Introduction
India has several traditional medical systems, such as Ayurveda and Unani, which have survived more than 3 000 years, mainly using plant-based drugs. There are many hepatoprotective, antiulcer, antioxidants, gastro protective plants known from the wealth of traditional Ayurveda and folklore medicines but their introduction into modern therapy awaits scientific validation. Scientific research in herbal medicine with hepatoprotective and antioxidant activity may be of great benefit as an alternative therapy for different liver diseases. The exposure to drugs, food additives, dangerous chemicals etc. and their metabolic products makes the liver unstable such as acute or chronic inflammations like hepatitis, cirrhosis etc.
Free radicals are highly reactive molecules or chemical species capable of independent existence which plays an important role in a number of biological processes such as intracellular killing bacteria by phagocytic cells (granulocytes and macrophages), and also implicated in certain cell signalling processes[1]. Their production however, multiplies several folds during pathological conditions, which results in many diseases such as atherogenesis, parkinson's disease, liver diseases, diabetes etc. Antioxidants play a key role in these defense mechanisms by removing free radical intermediates, and inhibiting other oxidation reactions, thus preventing oxidative stress[2]. Hence the rationale for the use of antioxidants is well established in prevention and treatment of diseases where oxidative stress plays a major aetiopathological role[3]. In this aspect, natural antioxidant products got attention for their scientific validation and clinical use.
Cyclea peltata (Poir.) Hook. f. & Thoms. (C. peltata) (Menispermaceae) is locally called Padathaali or Padakkizhangu. It is a much branched climbing shrub found throughout South and East India with tuberous roots, peltate leaves, greenish yellow flowers and drupaceous fruits. In traditional medicine of Kerala, the roots of C. peltata are used against jaundice[4]. The Kurichiya tribe of Kerala use the tuberous roots of this plant along with a little salt to treat stomach pain[5]. The Garo tribe of Balphakram sanctuary in Meghalaya use the crushed root extract as a remedy against small pox[6]. We have already reported the antiulcer property of C. peltata[7]. However this therapeutic claim against liver disease has not been scientifically validated yet. Herein, we report the antioxidant and hepatoprotective properties of the roots of C. peltata against paracetamol (APAP)/carbon tetrachloride (CCl4)-induced liver damage in Wistar rats.
Pharmacological studies using C. peltata showed potent diuretic activity and in vitro anticancer activity, and also inhibits the stone formation induced by ethylene glycol treatment[8]–[10]. The post treatment of C. peltata extract might effectively ameliorate oxidative stress parameters observed in cisplatin-induced renal toxicity and could be used as natural antioxidant against cisplatin-induced oxidative stress[11].
C. peltata roots are reported to contain alkaloids like fangchinoline, d-tetrandrine, dl-tetrandrine, d-isochondrodendrine, cycleapeltine, cycleadrine, cycleacurine, cycleanorine, etc[12],[13]. Tetrandrine is well known to possess activities including antioxidant, plasma glucose lowering[14], anti-inflammatory, immunosuppressive, free radical scavenging[15], anti-fibrotic and anticancer properties. It is used clinically to treat hypertension and silicosis[16],[17]. Fangchinoline is known to inhibit Ca2+ transmembrane movement and histamine release[18]. The Indian sample of root of C. peltata yielded tumor-inhibitory bisbenzylisoquinoline alkaloid tetrandrine as the major alkaloid[4],[19].
2. Materials and methods
2.1. Plant material and preparation of the extract
C. peltata roots were collected from Trivandrum district of Kerala, during August 2011 by Varghese Jancy Shine, Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI) and authenticated by Dr. Mathew Dan, plant taxonomist of the Institute. A voucher specimen has been deposited at the JNTBGRI Herbarium (TBGT 13814 dated September 10, 2011). The roots were washed thoroughly in tap water, shade-dried and powdered. A total of 500 g plant material was extracted with methanol for 48 h using Soxhlet apparatus and dried under reduced pressure using rotoevaporator to yield 100 g methanol crude extract (MCP). Total alkaloid extract was isolated from MCP[20]. About 100 g of MCP was dissolved in dilute H2SO4, filtered and pH was adjusted to 9.5. Free alkaloid was extracted with chloroform. The chloroform layer was filtered and concentrated under reduced pressure using rotoevaporator to yield 9 g alkaloid extract of C. peltata (ACP). It was suspended in 0.5% Tween-80 to required concentrations and used for the experiments.
2.2. Animals
Wistar albino rats, males (200-250 g) and Swiss albino mice, males (25-30 g), obtained from the JNTBGRI Animal House was used for the present study. They were housed under standard conditions and fed commercial rat feed (Lipton India Ltd, Mumbai, India) and boiled water ad libitum. All experiments involving animals were done according to NIH guidelines, after getting the approval of the Institute's Animal Ethics Committee.
2.3. HPTLC estimation of tetrandrine content in ACP
TLC of the ACP was carried out using the solvent system toluene: ethyl acetate: diethylamine (7.2:2:0.8) modified method[7]. The chromatogram was visualized by spraying with Dragendroff's reagent. Co-HPTLC of ACP and authentic tetrandrine (Sigma-Aldrich, USA) was also performed.
2.4. Direct analysis in real time-mass spectrometry (DART-MS) of ACP
The mass spectrometer used was a JMS-T100 LC (Accu ToF) atmospheric pressure ionization time-of-flight mass spectrometer (Jeol, Tokyo, Japan) fitted with a DART ion source. The mass spectrometer was operated in positive-ion mode. The DART ion source was operated with helium gas flowing at 4 L/min. The gas heater was set to 300 °C. The potential on the discharge needle electrode of the DART source was set to 3 000 V. Orifice 1 potential was set at 28 V. The ACP was positioned in the gap between the DART source and mass spectrometer for measurements. Data acquisition was from m/z 10 to 1 050[21].
2.5. APAP-induced liver damage
APAP (Sigma Chemical Company, USA) was suspended in 0.5% gum acacia and administered p.o., at a dose of 2.5 g/kg. This dose is known to cause liver damage in rats[22]. Rats were divided into 6 groups (6 per group). Group I, the normal control group was given a single daily dose of 0.5% Tween-80, p.o., for 4 d. Group II, the APAP control group received a daily dose of 0.5% Tween-80 for 4 d and 2 mL of APAP suspension (2.5 g/kg, per os) on Day 3, 30 min after Tween-80 administration. Groups III, IV and V received a daily dose of ACP 50, 100 and 150 mg/kg respectively p.o., for 4 d and 2 mL of APAP suspension (2.5 g/kg, p.o.) on Day 3, 30 min after ACP administration. Group VI animals received silymarin at a dose of 100 mg/kg p.o., on all the 4 d and 2 mL of APAP suspension (2.5 g/kg) p.o., 30 min after silymarin administration.
2.6. CCl4-induced hepatotoxicity in rats
Rats were divided into 6 groups (n=6). Group I (normal control) animals were administered a single daily dose of 0.5% Tween-80, p.o., for 7 d and received olive oil (8 mL/kg, i.p.) on Day 7. Group II (CCl4 control) received 0.5% Tween-80, p.o. once daily, for 7 d and received 0.2% CCl4 in olive oil (8 mL/kg, i.p.) on Day 7. Group III, IV and V were administered orally ACP 50, 100 and 150 mg/kg once daily for 7 d respectively. Group VI received standard drug silymarin (100 mg/kg, p.o.) once daily for 7 d. Group III-VI animals were administered simultaneously 0.25 mL CCl4 in olive oil (8 mL/kg) on Day 7 after 1 h of administration of the ACP/silymarin[23].
2.7. Assessment of liver function
On the 5th day of the APAP hepatotoxicity study and on the 8th day of the CCl4 hepatotoxicity study, all the animals were sacrificed as per CPCSEA guidelines. Blood samples were collected for evaluating the serum biochemical parameters [glutamate pyruvate transaminase (SGPT), glutamate oxaloacetate transaminase (SGOT), alkaline phosphatase (SAKP), bilirubin and cholesterol] according to standard methods[24]–[27].
2.8. Histopathological studies
The liver samples from the above experiments were preserved in 10% buffered formalin and processed for routine paraffin block preparation. Using a rotary microtome (American Optical Co., USA), sections of thickness of about 5 µm were cut and stained with heamatoxylin and eosin. These were examined under the microscope for histopathological changes, such as necrosis, fatty changes, ballooning degeneration and infiltration of Kupffer cells and lymphocytes.
2.9. Liver tissue homogenate preparation
The liver was washed thoroughly in ice-cold saline to remove the blood. It was then gently blotted between the folds of a filter paper and weighed in an analytical balance. Ten percentage of homogenate was prepared in 0.05 mol/L phosphate buffer (pH 7) using a polytron homogenizer (Remi Motors, India, Pvt Ltd) at 20 °C. The homogenate was centrifuged at 6 000 r/min for 20 min to remove cell debris, unbroken cells, nuclei, erythrocytes and mitochondria. The supernatant was used for further hepatic biochemical assays.
2.10. Estimation of reduced glutathione (GSH)
To estimate GSH, 0.2 mL of tissue homogenate was mixed with 1.8 mL of ethylene diamine tetraacetic acid (EDTA) solution. To this 3 mL precipitating reagent (1.67 g of metaphosphoric acid, 0.2 g of EDTA disodium salt, 30 g sodium chloride in 1L of distilled water) was added and mixed thoroughly and kept for 5 min before centrifugation. To 2 mL of the supernatant, 4 mL of 0.3 mol/L disodium hydrogen phosphate solution and 1 mL of DTNB (5,5-Dithio bis 2-nitro benzoic acid) reagent were added and absorbance was read at 412 nm. Absorbance values were compared with a standard curve generated from known GSH[28].
2.11. Estimation of superoxide oismutase (SOD)
Superoxide radicals react with nitroblue tetrazolium in the presence of NADH and produce formazan blue. SOD removes the superoxide radicals and inhibits the formation of formazan blue. The intensity of colour is inversely proportional to the activity of the enzyme. The reaction mixture contained 1.2 mL sodium pyrophosphate buffer (pH 8.3, 0.025 mol/L), 0.1 mL phenazine methosulphate (186 mmol/L), 0.3 mL nitroblue tetrazolium (NBT) (300 mmol/L), 0.2 mL NADH (780 mmol/L) and approximately diluted enzyme preparations and water in a total volume of 3 mL. After incubation at 30 °C for 90 seconds, the reaction was terminated by the addition of 1 mL glacial acetic acid. The reaction mixture was stirred vigorously and shaken with 4 mL n-butanol. The color intensity of the chromogen in the butanol layer was measured at 560 nm against n-butanol and concentration of SOD was expressed as units/mg protein. Absorbance values were compared with a standard curve generated from known SOD[29].
2.12. Estimation of catalase (CAT) activity
About 500 mg of the liver/stomach samples of the normal and drug treated groups were homogenized (Remi Motors, India, Pvt Ltd) with 10 mL 0.9% saline. The homogenate was used for determination of CAT activity. Decomposition of H2O2 in presence of CAT was followed at 240 nm. One unit of CAT was defined as the amount of enzyme required to decompose 1 mmol of H2O2 per min, at 25 °C and pH 7. Results were expressed as IU of CAT activity/g wet tissue[30].
2.13. Malondialdehyde (MDA) estimation
The liver homogenate was suspended in thiobarbituric acid, and the optical density of the clear pink supernatant was read at 532 nm, after centrifugation. Malondialdehyde bis (dimethyl acetal) was used as standard[31].
2.14. Assesment of in vitro free radical scavenging property of ACP
2.14.1. Hydroxyl radical scavenging activity of ACP
Hydroxyl radicals generated from Fe3+-ascorbate-EDTA- H2O2 were estimated by the degradation of deoxyribose that resulted in thiobarbituric acid reacting substances formation. The reaction mixture contained deoxyribose (2.8 mmol/L), FeCl3 (0.1 mmol/L), KH2PO4-KOH buffer (20 mmol/L, pH 7.4), EDTA (0.1 mmol/L), H2O2 (1 mmol/L), ascorbic acid (0.1 mmol/L) and various concentrations of ACP, in a final volume of 1 mL. The reaction mixture was incubated at 37 °C for 1 h. Degradation of deoxyribose was measured by thiobarbituric acid method and percentage inhibition was calculated. Curcumin was used as reference compound[32].
2.14.2. Superoxide radical scavenging activity of ACP
Superoxide radical scavenging activity of ACP was determined by NBT reduction method[33]. The reaction mixture contained EDTA (0.1 mol/L), 0.0015% NaCN, riboflavin (0.12 mmol/L), NBT (1.5 mmol/L), and various concentrations of ACP and phosphate buffer (67 mmol/L, pH 7.8) in a total volume of 3 mL. The tubes were illuminated under an incandescent lamp for 15 min and thereafter the optical density was measured at 530 nm. The percentage inhibition of superoxide production was evaluated by comparing the absorbance of control and experimental tubes. Curcumin was used as reference compound.
2.14.3. DPPH radical scavenging activity of ACP
DPPH radical scavenging activity was measured by the spectrophotometric method[34]. To a methanolic solution of DPPH (200 µmol/L), 0.05 mL of different concentrations of ACP was dissolved in ethanol and added at different concentrations (10-500 µg/mL). An equal amount of ethanol was added to the control. After 20 min, the decrease in the absorbance of test mixture (due to quenching of DPPH free radicals) was read at 517 nm, and the percentage inhibition was calculated by using the formula given below.
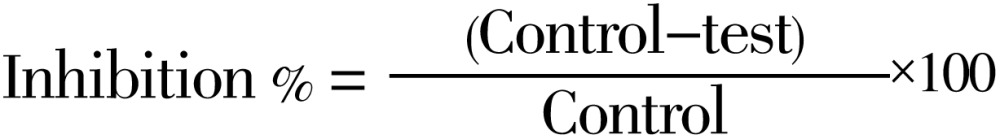 |
2.14.4. Anti-lipid peroxidation effects of ACP
The anti-lipid peroxidant effect of ACP was studied as per standard method[23]. Protein content was determined by the method of Lowry et al[35]. Briefly, 2 g of rat liver tissue was sliced and homogenized with 150 mmol/L KCl-Tris-HCl buffer (pH 7.2). The reaction mixture was composed of 0.25 mL liver homogenate, Tris-HCl buffer (pH 7.2), 0.1 mmol/L ascorbic acid, 4 mmol/L FeCl2 and 0.005 mL of various concentrations of ACP. The mixture was incubated at 37 °C for 1 h in capped tubes. Then, 0.1 mol/L HCl, sodium dodecyl sulphate (9.8%), 0.9 mL distilled water and 2 mL of thiobarbituric acid (0.6%) were added to each tube and vigorously shaken. The tubes were placed in a boiling water bath at 100 °C for 30 min. After cooling, 5 mL of butanol was added and centrifuged at 3 000 r/min for 25 min. The absorbance of the supernatant was measured at 532 nm. The experiment was repeated twice.
2.15. Behavioural and toxic effects
Five groups of 10 mice were administered p.o., 250, 500, 750, 1 000 and 2 000 mg/kg of the ACP. They were observed continuously for 1 h for any gross behavioural changes, like drowsiness, restlessness, writhing, convulsion, piloerection, symptoms of toxicity and mortality if any, and intermittently for the next 6 h and then again, 24 h after dosing with ACP.
2.16. Statistical analysis
The results were expressed as mean±SD. Analysis of variance (ANOVA) was done to compare and analyse the data followed by Duncan's multiple range test. Effects were considered significant at P≤0.01[36].
3. Results
3.1. HPTLC estimation of tetrandrine content in ACP
HPTLC estimation of tetrandrine using different concentration of authentic tetrandrine and ACP has shown 228.4 µg/mg ACP (Figure 1).
Figure 1. HPTLC estimation of tetrandrine.
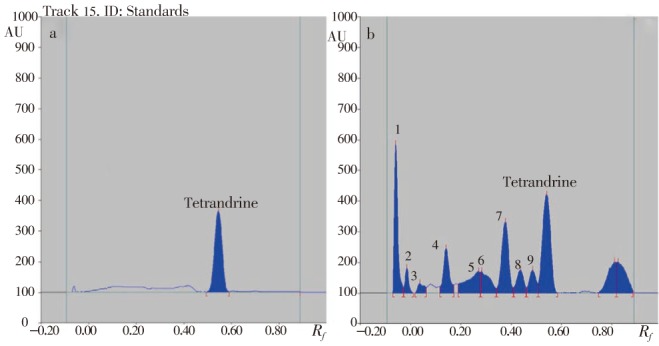
(a): Peak display of standard tetrandrine at 280 nm; (b): Peak display of at 280 nm.
3.2. DART-MS of alkaloid extract of ACP
DART analysis detected the presence of bisbenzyl isoquinoline alkaloids, tetrandrine (C38H42O8N2; MW 622.3), fangchinoline (C37H40N2O6; MW 608.288) and coclaurine (C17H19NO3; MW 285.33), which were detected as peaks of M+1 values 623.365 72 (tetrandrine), 609.350 64 (fangchinoline) and 286.148 81 (coclaurine) respectively (Figure 2).
Figure 2. Representative DART-MS spectra of ACP.
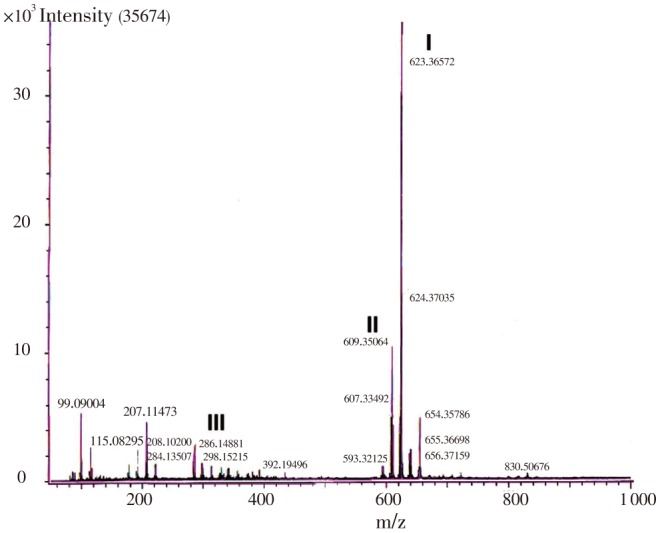
I: tetrandrine (C38H42O8N2; MW 622.30); II: fangchinoline (C37H40N2O6; MW 608.288); III: coclaurine (C17H19NO3; MW 285.33).
3.3. APAP/CCl4-induced hepatotoxicity in rats
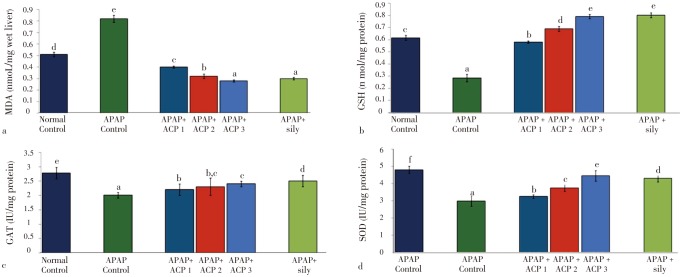
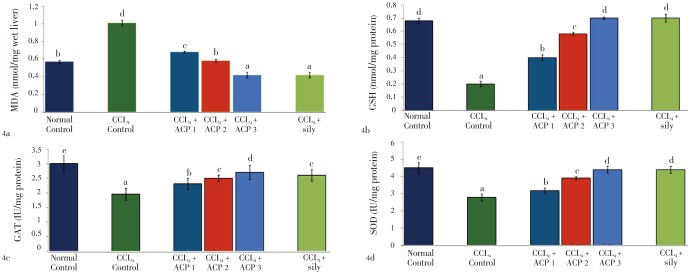
Both APAP and CCl4 produced severe liver damage as indicated by a marked increase in SGOT, SGPT, SAKP, bilirubin and cholesterol values of the toxin groups, in comparison with normal controls. Pretreatment with ACP (50, 100 and 150 mg/kg) caused significant reduction of these values in both the hepatotoxin treated cases, almost comparable to silymarin (100 mg/kg) treated groups (Tables 1 and 2). Liver content of MDA, a marker of lipid peroxidation, increased and GSH, CAT, SOD were lowered after APAP/CCl4 administration. ACP significantly decreased the liver MDA levels and increased the liver GSH, CAT, SOD levels in APAP/CCl4 treated rats in a dose dependent manner. Silymarin (100 mg/kg) pretreatment also showed significant antioxidant property via reduction in MDA and increase in GSH, CAT and SOD levels (Figures 3 and 4).
Table 1. Effect of ACP on serum parameters in APAP-induced hepatic damage in Wistar rats.
| Groups | SGOT (IU/I) | SGPT (IU/I) | SAKP (KA Units) | Cholesterol (mg/dL) | bilirubin (mg/dL) |
| Normal Control | 36.50±4.25a | 47.00±1.80a | 70.40±2.16a | 40.00±1.21b | 0.24±0.02a |
| APAP Control | 113.66±2.40e | 258.20±1.80e | 120.00±1.17e | 62.00±1.31d | 0.97±0.03d |
| APAP+ACP (50 mg/kg) | 67.33±2.56d | 70.92±1.30d | 85.00±2.20d | 48.00±1.26c | 0.37±0.02c |
| APAP+ACP (100 mg/kg) | 51.93±2.15c | 60.92±2.10c | 78.00±2.14c | 40.00±1.50b | 0.27±0.01b |
| APAP+ACP (150 mg/kg) | 42.84±1.55b | 49.88±2.20b | 71.21±2.10b | 37.01±1.12a | 0.24±0.01a |
| APAP+silymarin (100 mg/kg ) | 42.57±2.46b | 50.22±2.10b | 71.15±1.60b | 36.00±2.12a | 0.25±0.01a |
Values are the mean±SD, n=6, ANOVA was followed by Duncan's multiple range test. Means bearing same superscripts do not differ significantly (P≤=0.01).
Table 2. Effect of ACP on serum parameters in CCl4-induced hepatic damage in Wistar rats.
| Groups (units/mL) | SGOT (IU/I) | SGPT (IU/I) | SAKP ((KA Units) | Cholesterol (mg/dL) | bilirubin (mg/dL) |
| Normal Control | 38.90±2.32a | 48.90±2.11a | 51.00±2.10a | 43.00±3.00b | 0.28±0.02a |
| APAP Control | 133.26±2.10e | 278.20±1.89e | 99.10±2.10d | 70.00±1.65e | 1.05±0.02d |
| APAP+ACP (50 mg/kg) | 77.13±1.81d | 120.92±2.10d | 64.00±2.80c | 53.00±1.90d | 0.46±0.01c |
| APAP+ACP (100 mg/kg) | 58.160±2.310c | 64.92±2.15c | 58.00±2.91b | 48.00±1.89c | 0.40±0.02b |
| APAP+ACP (150 mg/kg) | 42.28±1.62b | 51.28±2.20b | 50.00±2.12a | 40.00±1.22a | 0.28±0.02a |
| APAP+silymarin (100 mg/kg ) | 42.57±1.83b | 51.82±2.10b | 51.00±2.21a | 40.00±2.32a | 0.28±0.02a |
Values are the mean±SD, n=6, ANOVA was followed by Duncan's multiple range test. Means bearing same superscripts do not differ significantly (P≤=0.01).
Figure 3. Effect of ACP on liver MDA, GSH, CAT and SOD levels in APAP treated rats.
ACP 1=50 mg/kg, ACP 2=100 mg/kg and ACP 3=150 mg/kg. Values are the mean±SD, n=6, ANOVA was followed by Duncan's multiple range test, means bearing different superscripts differ significantly (P≤0.01).
Figure 4. Effect of ACP on liver MDA, GSH, CAT and SOD levels in carbon tetrachloride (CCl4) treated rats.
ACP 1=50 mg/kg, ACP 2=100 mg/kg and ACP 3=150 mg/kg. Values are the mean±SD, n = 6, analysis of variance (ANOVA) followed by Duncan's multiple range test, means bearing different superscripts differ significantly (P≤=0.01).
3.4. Histopathological studies
Histopathological studies also supported the biochemical studies. Control groups showed normal liver architecture with distinct hepatic cells, sinusoidal space and central vein. In the liver sections of rats intoxicated with APAP, degeneration of normal hepatic cells with intense centrilobular necrosis, broad infiltration of lymphocytes and Kupffer cells and loss of cell boundaries were observed. The liver sections of rats challenged with CCl4 showed necrosis, inflammation and Kupffer cell infiltration. However, administration of ACP/silymarin to the groups intoxicated with APAP/CCl4 produced a marked degree of protection against the toxin-induced liver histological alterations (Figures 5 and 6).
Figure 5. Administration of ACP/silymarin to the groups intoxicated with APAP.
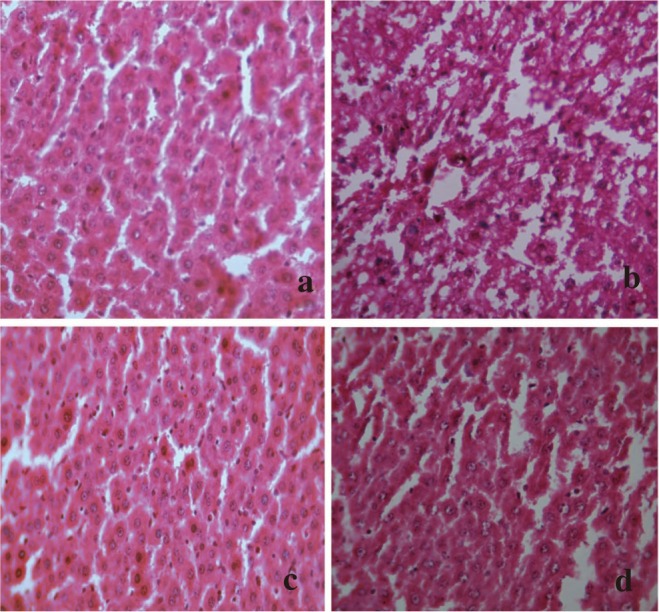
(a): Section of liver of normal control rats showing hepatic cells with well defined nuclei and cytoplasm (350×). (b): Section of APAP treated rat liver showing marked necrosis, extensive vacuolation, broad infiltration of lymphocytes and Kupffer cells, loss of cell boundaries and disappearance of nuclei (350×). (c): Section of APAP+ACP (150 mg/kg) treated rat liver showing marked improvement over APAP control group (350×). (d): Section of APAP+silymarin (100 mg/kg) treated rat liver showing normalcy of hepatic cells (350×).
Figure 6. Administration of ACP/silymarin to the groups intoxicated with CCl4.
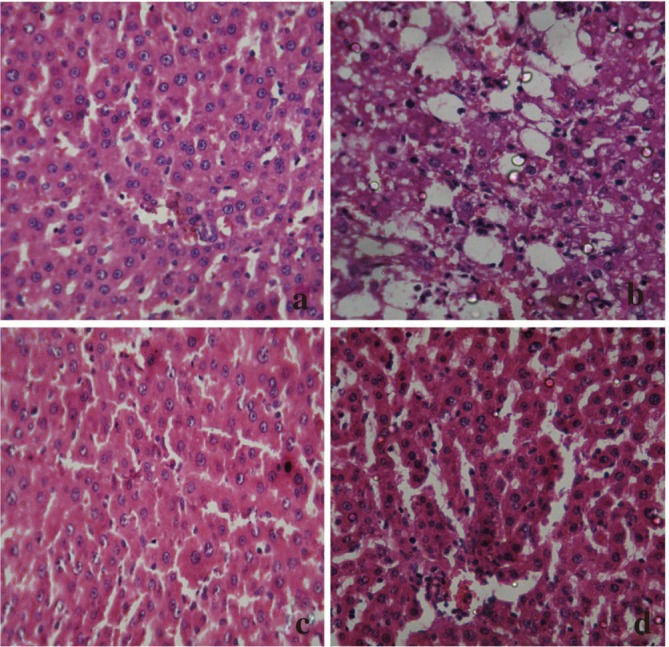
(a): Section of liver of normal control rat showing hepatic cell with well defined nuclei and cytoplasm (100×). (b): Section of CCl4 treated rat liver showing moderate to marked necrosis, inflammatory changes (350×). (c): Section of CCl4+ACP (150 mg/kg) treated rat liver showing improvement over CCl4 control group (350×). (d): Section of CCl4+Silymarin (100 mg/kg) treated rat liver showing normalcy of hepatic cells (350×).
3.5. Assesment of hydroxyl radical scavenging activity
Hydroxyl radical generated by Fe3+/ascorbate/EDTA/H2O2 system initiated the degradation of deoxyribose which was inhibited significantly by ACP and its IC50 value is (38.00±2.68) µg/mL (Table 3).
Table 3. Effect of ACP on in vitro free radical (hydroxyl, superoxide and DPPH) scavenging effects.
| Treatments | Concentration of 50% Inhibition of free radical (µg) |
||
| Hydroxyl | Superoxide | DPPH | |
| ACP | 38.00±2.68b | 41.00±1.23b | 31.00±1.53b |
| Curcumin | 2.89±0.28a | 5.89±0.45a | 2.26±0.51a |
Values are the mean±SD, n=6, analysis of variance (ANOVA) followed by Duncan's multiple range test, means bearing different superscripts differ significantly (P≤=0.01).
3.6. Assesment of superoxide radical scavenging activity
Percentage inhibition of superoxide radical generation by ACP was found to be increasing in a dose dependent manner, showing IC50 value of (41.00±1.23) µg/mL (Table 3).
3.7. DPPH radical scavenging activity
The DPPH scavenging assay showed IC50 value for ACP as (31.00±1.53) µg/mL, standard curcumin having IC50 value of 2.26 µg/mL (Table 3).
3.8. Assesment of anti-lipid peroxidation effects
The ACP treatment showed significant reduction in the MDA levels in vitro dose dependently. The concentration needed for the 50% reduction of lipid peroxidation for ACP was (53.5±1.21) µg/mL (Table 4).
Table 4. Inhibitory effect of ACP on FeCl2-ascorbic acid-induced lipid peroxidation in rat liver homogenate in vitro.
| Treatments | ACP (µg/mL) | MDA (nmol/g wet liver) | MDA inhibition (%) |
| Normal control | - | 1.21±0.40c | - |
| FeCl2-AA | - | 2.56±0.15e | - |
| FeCl2-AA+ACP | 25 | 2.12±0.06d | 17.19a |
| FeCl2-AA+ACP | 50 | 1.99±0.05b | 22.27b |
| FeCl2-AA+ACP | 75 | 0.88±0.02a | 65.63c |
| FeCl2-AA+ACP | 100 | 0.81±0.04a | 68.36c |
Values are the mean±SD, n=3, analysis of variance (ANOVA) followed by Duncan's multiple range test, means bearing different superscripts differ significantly (P≤0.01). AA: Ascorbic acid.
3.9. Behavioural and toxicity studies
In the toxicity study, no mortality occurred within 24 h with the 5 doses of ACP tested. The LD50 was therefore greater than 2 500 mg/kg p.o., in mice (Data not shown).
4. Discussion
Living in a world of inadequately controlled environmental pollution and expanding therapy with potent drugs, the liver, which is the key organ of metabolism and excretion is continually exposed to a variety of xenobiotics and therapeutic agents. Drug detoxification is a complex process that occurs in the endoplasmic reticulum of the hepatocytes which may cause oxidative damage through the production of reactive oxygen species. Therefore, it is valuable to identify natural drugs or compounds that can antagonize the deleterious action of free radicals and act as an antioxidant to protect hepatocytes from such damage.
APAP is a potent analgesic, and antipyretic agent is metabolized in the liver. Toxic doses cause fatal liver damage. It is reported that one percentage of the casualty department attendance is due to APAP[37]. It is established that APAP is bioactivated to a toxic elecrophile, N-acetyl-p-benzoquinoneimine, which binds covalently to tissue macromolecules and probably also oxidizes lipids or critical sulphydryl groups (protein thiols) and alters the homeostasis of calcium[38]–[40]. Carbon tetrachloride has long been used to produce experimental liver necrosis as a model hepatotoxicant and carcinogen. The mitochondria were found to be the component attacked by the poison, and the tricarboxylic acid cycle was disorganized. Mechanistic studies provided evidence that metabolism of carbon tetrachloride via CYP2E1 to highly reactive free radicals (trichloromethyl and trichloromethyl peroxy metabolite) plays a critical role in the postulated mode of action. The free radicals initiate lipid peroxidation by attacking polyunsaturated fatty acids in membranes, setting off a free radical chain reaction sequence. Lipid peroxidation is known to cause membrane disruption, resulting in the loss of membrane integrity and leakage of microsomal enzymes. By-products of lipid peroxidation include reactive aldehydes that can form protein and DNA adducts and may contribute to hepatotoxicity and carcinogenicity, respectively[41].
Due to liver injury the transport function of the hepatocytes gets disturbed, resulting in the leakage of plasma membrane[42], thereby causing an increased enzyme level in the serum. In the present study, the hepatic damage produced by APAP/CCl4 is evident by increased marker enzymes (SGOT, SGPT, SAKP), bilirubin and cholesterol levels in the serum. In both toxicity, if the free radical generated is not inactivated by conjugation with GSH, it reacts with cell proteins and kills the cell[43]. Thus activation of antioxidant defense system by the ACP is the key step in hepatoprotection against APAP/CCl4 hepatotoxicity.
GSH plays a pivotal role in mitochondrial antioxidant defense; its depletion increases the sensitivity of the hepatic tissue to free radical-mediated damage caused by xenobiotic metabolism[44],[45]. In the present study, it was observed that ACP significantly inhibited the liver MDA levels and increases the liver GSH, CAT and SOD levels of APAP/CCl4 treated rats. This may be due to the inhibition of the deleterious effects of the free radicals formed during APAP/CCl4 metabolism by ACP. These findings support the significant antioxidant property of ACP and its free radical scavenging role in vivo. The in vitro free radical scavenging property of ACP also significantly supported the findings. Thus the observed hepatoprotective property of ACP was due to its free radical scavenging property via increasing endogenous antioxidant defense system.
A single dose of CCl4 led to a five-fold increase in liver calcium content. The calcium channel blockers showed a significant reduction in liver calcium content, decrease in AST and ALT levels, and a significant increase in protein synthesis and also a partial inhibition of lipid peroxidation[46]. Weakened cellular membranes allow sufficient leakage of calcium into the cytosol to disrupt intracellular calcium homeostasis, and high calcium levels in the cytosol activate calcium-dependent proteases and phospholipases that further increase the breakdown of the membranes. Similarly, the increase in intracellular calcium can activate endonucleases that can cause chromosomal damage and also contribute to cell death[41]. Lowering the calcium metabolism can reduce the pathological consequences of the attack by toxic metabolites[47].
Tetrandrine and fangchinoline the major alkaloids present in C. peltata are reported to inhibit Ca2+ transmembrane movement[7],[12],[13],[48]. Tetrandrine is well known to possess activities including antioxidant, anti-inflammatory, immunosuppressive, free radical scavenging, anti-fibrotic and anticancer properties. Coclaurine has a potent antispasmodic activity related to an inhibiting effect of extracellular calcium. Thus the synergistic action of alkaloids especially tetrandrine, fangchinoline and coclaurine may contribute to the hepatoprotective property of ACP.
The observed hepatoprotective property of ACP may be due to its antioxidant mechanism via scavenging the free radicals generated efficiently, anti-inflammatory through inhibition of reactive oxygen species and further inhibition of inflammatory cytokines and hepatocyte membrane stabilizing via inhibiting Ca2+ transmembrane movement. Therefore, it may be a promising agent in protecting hepatic tissue from liver injury and further studies are warranted to elucidate the mechanism of action.
Acknowledgments
The authors thank Mr. K.P Pradeep Kumar for photographic assistance and Mr. S Radhakrishna Pillai for technical assistance. The funding from Indian Council of Medical Research (ICMR), New Delhi, in the form of a Senior Research Fellowship (SRF) to the first author is gratefully acknowledged.
Comments
Background
There are many hepatoprotective, antiulcer, antioxidants, gastro protective plants known from the wealth of traditional Ayurveda and folklore medicines, but their introduction into modern therapy awaits scientific validation. Scientific research in herbal medicine with hepatoprotective and antioxidant activity may be of great benefit as an alternative therapy for different liver diseases.
Research frontiers
This paper describes the hepatoprotective property of alkaloid extract of C. peltata against CCl4 and APAP induced hepatic injury and assessed by estimating different biochemical paradigms and in vitro antioxidant parameters.
Related reports
Both CCl4 and APAP are reported to cause hepatic necrosis due to formation of free radicals. The folklore medicine has evidence of effectiveness of herbs in treating various liver disorders.
Innovations and breakthroughs
In traditional medicine of Kerala, the roots of C. peltata are used against jaundice. The Kurichiya tribe of Kerala uses the tuberous roots of this plant along with a little salt to treat stomach pain. The Garo tribe of Balphakram sanctuary in Meghalaya use the crushed root extract as a remedy against small pox. However this therapeutic claim against liver disease has not been scientifically validated yet. Herein, the authors report the antioxidant and hepatoprotective properties of the roots of C. peltata against APAP/CCl4-induced liver damage in Wistar rats.
Applications
C. peltata roots are reported to contain alkaloids like fangchinoline, d-tetrandrine, dl-tetrandrine,d-isochondrodendrine, cycleapeltine, cycleadrine, cycleacurine, cycleanorine, etc. Tetrandrine is well known to possess activities including antioxidant, plasma glucose lowering, anti-inflammatory, immunosuppressive, free radical scavenging, anti-fibrotic and anticancer properties. It is used clinically to treat hypertension and silicosis. Fangchinoline is known to inhibit Ca2+ transmembrane movement and histamine release. This scientific study support and suggest the use of this plant as an adjuvant along with commonly used hepatoprotective agent.
Peer review
The study carried out and presented in this paper is of high quality. The authors have demonstrated the hepatoprotective property of C. peltata in CCl4 and APAP induced liver toxicity in Wistar rats. The property was assessed based on biochemical parameters, antioxidant enzyme levels in liver homogenate and histopathological observations. C. peltata was found to be a promising hepatoprotective agent in CCl4 and APAP rat models.
Footnotes
Foundation Project: Supported by Indian Council of Medical Research (ICMR), New Delhi (Grant No.45/9/2007/BMS/TRM).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 3.Saumya SM, Mahaboob BP. In vitro evaluation of free radical scavenging activities of Panax ginseng and Lagerstroemia speciosa: a comparitive analysis. Int J Pharm Pharm Sci. 2011;3(1):165–169. [Google Scholar]
- 4.NISCAIR . The wealth of India: first supplement series (raw materials), Vol. 2. New Delhi, India: NISCAIR; 2004. pp. 319–321. [Google Scholar]
- 5.Ramachandran VS, Nair VJ. Ethnobotanical studies in Cannanore district, Kerala state (India) J Econ Taxon Bot. 1981;2:65–72. [Google Scholar]
- 6.Kumar Y, Haridasan K, Rao RR. Ethnobotanical notes on certain medicinal plants among some Garo people around Balphakram Sanctuary in Meghalaya. Bull Bot Surv India. 1980;22:161–165. [Google Scholar]
- 7.Shine VJ, Latha PG, Shyamal S, Suja SR, Anuja GI, Sini S, et al. et al. Gastric antisecretory and antiulcer activities of Cyclea peltata (Lam.) Hook. f. & Thoms. in rats. J Ethnopharmacol. 2009;125(2):350–355. doi: 10.1016/j.jep.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 8.Hullatti KK, Gopikrishna UV, Kuppast IJ. Phytochemical investigation and diuretic activity of Cyclea peltata leaf extracts. J Adv Pharm Technol Res. 2011;2(4):241–244. doi: 10.4103/2231-4040.90880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunita P, Rakhi Y. Plants with anticancer activity: need for in vitro study. Vaniki Sandesh. 2005;29(1):32–34. [Google Scholar]
- 10.Christina AJ, Packia Lakshmi M, Nagarajan M, Kurian S. Modulatory effect of Cyclea peltata Lam. on stone formation induced by ethylene glycol treatment in rats. Methods Find Exp Clin Pharmacol. 2002;24(2):77–79. doi: 10.1358/mf.2002.24.2.677130. [DOI] [PubMed] [Google Scholar]
- 11.Vijayan FP, Rani VK, Vineesh VR, Sudha KS, Michael MM, Padikkala J. Protective effect of Cyclea peltata Lam on cisplatin-induced nephrotoxicity and oxidative damage. J Basic Clin Physiol Pharmacol. 2007;18(2):101–114. doi: 10.1515/jbcpp.2007.18.2.101. [DOI] [PubMed] [Google Scholar]
- 12.Rastogi RP, Mehrotra BN. Cyclea peltata (Menispermaceae) In: Rastogi RP, editor. Compendium of Indian medicinal plants, Vol. 2: (1970-1979) New Delhi: Central Drug Research Institute, Lucknow and Publications and Information Directorate; 1999. pp. 237–240. [Google Scholar]
- 13.Reddi TV, Prasanthi S, Ramarao BV. Medicinal and Aromatic Plants of India. In: Khan IA, Khanum A, editors. Role of Biotechnology in Medicinal and Aromatic Plants. Vol. XII. Hyderabad: Ukaaz Publications; 2005. pp. 63–70. [Google Scholar]
- 14.Chen WC, Hayakawa S, Yamamoto T, Huang LW, Liu IM, Cheng JT. The plasma glucose lowering action of tetrandrine in strptozotocin-induced diabetic rats. J Pharm Pharmacol. 2004;56(5):643–648. doi: 10.1211/0022357023330. [DOI] [PubMed] [Google Scholar]
- 15.Cao ZF. Scavenging effect of tetrandrine of active oxygen radicals. Planta Med. 1996;62(5):413–414. doi: 10.1055/s-2006-957928. [DOI] [PubMed] [Google Scholar]
- 16.Qian JQ. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol Sin. 2002;23(12):1086–1092. [PubMed] [Google Scholar]
- 17.Xie QM, Tang HF, Chen JQ, Bian RL. Pharmacological actions of tetrandrine in inflammatory pulmonary diseases. Acta Pharmacol Sin. 2002;23(12):1107–1113. [PubMed] [Google Scholar]
- 18.Nakamura K, Tsuchiya S, Sugimoto Y, Sugimura Y, Yamada Y. Histamine release inhibition activity of bisbenzylisoquinoline alkaloids. Planta Med. 1992;58(6):505–508. doi: 10.1055/s-2006-961536. [DOI] [PubMed] [Google Scholar]
- 19.Gilmore CJ, Bryan RF, Kupchan SM. Conformation and reactivity of the macrocyclic tumor-inhibitory alkaloid tetrandrine. J Am Chem Soc. 1976;98(7):1947–1952. doi: 10.1021/ja00423a049. [DOI] [PubMed] [Google Scholar]
- 20.Hullatti KK, Sharada MS. Comparetive phytochemical investigation of the source of Ayurvedic drug Patha: a chromatographic fingerprinting analysis. Indian J Pharm Sci. 2010;72(1):39–45. doi: 10.4103/0250-474X.62235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajslova J, Cajka T, Vaclavik L. Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis. Trends Analyt Chem. 2011;30(2):204–218. [Google Scholar]
- 22.Mitra SK, Venkataraganna MV, Sundaram R, Gopumadhavan S. Protective effect of HD-03, a herbal formulation against various hepatotoxic agents in rats. J Ethnopharmacol. 1998;63(3):181–186. doi: 10.1016/s0378-8741(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 23.Suja SR, Latha PG, Pushpangadan P, Rajasekharan S. Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L.) Hook against carbon tetrachloride-induced liver damage in Wistar rats. J Ethnopharmacol. 2004;92(1):61–66. doi: 10.1016/j.jep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Reitman S, Frankel SA. Colorimetric method for the determination of serum oxaloacetate and glutamic pyruvate transaminases. Am J Clin Pathol. 1957;28:56–66. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 25.King EJ, Armsrong AR. Calcium, magnesium, phosphorous and phosphatase. In: Varley B, Gowenlock AH, Bell M, editors. Practical clinical biochemistry, Vol. 1. London: Heinemann; 1980. pp. 850–853. [Google Scholar]
- 26.Malloy HT, Evelyn KA. The determination of bilirubin with the photometric calorimeter. J Biol Chem. 1931;119:481–490. [Google Scholar]
- 27.Zlatkis A, Zaik B, Boyle AJ. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953;41(3):486–492. [PubMed] [Google Scholar]
- 28.Ellman GL. Tissue sulphydral groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 29.Kakkar P, Das B, Viswananthan PN. A modified sphectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132. [PubMed] [Google Scholar]
- 30.Hans L. Methods in enzymatic analysis. New York: Academic Press; 1963. [Google Scholar]
- 31.Fong KL, McGay PB, Poyer JL. Evidence that peroxidation of lysosomal membrane is initiated by hydroxyl free radicals produced during flavine enzyme activity. J Biol Chem. 1973;248(22):7792–7797. [PubMed] [Google Scholar]
- 32.Ohkawa H, Onishi N, Yagi K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 33.McCord JM, Fridovich I. Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 34.Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittelforschung. 1996;46(2):169–171. [PubMed] [Google Scholar]
- 35.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 36.Raghava RD. Statistical techniques in agricultural and biological research. New Delhi: Oxford & IBH Publishing Co; 1987. p. 200. [Google Scholar]
- 37.Collins N. UK: Telegraph Media Group Limited; 2013. A few extra tablets can cause cumulative paracetamol overdose. [online] http://www.telegraph.co.uk/health/healthnews/8907129/A-few-extra-tablets-can-cause-cumulative-paracetamol-overdose.html. [Accessed on Oct 10, 2013] [Google Scholar]
- 38.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187(1):185–194. [PubMed] [Google Scholar]
- 39.Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12(6):439–442. doi: 10.1002/jat.2550120613. [DOI] [PubMed] [Google Scholar]
- 40.Moore M, Thor H, Moore G, Nelson S, Moldeus P, Orrenius S. The toxicity of acetaminophen and N-acetyl-p-benzoquinone-imine in isolated hepatocytes is associated with thiol depletion and increased cytosolic Ca2+ J Biol Chem. 1985;260(24):13035–13040. [PubMed] [Google Scholar]
- 41.Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(3):185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerman HJ, Seeff LB. Enzymes in hepatic disease. In: Goodly EL, editor. Diagnostic Enzymology. Philadelphia, USA: Lea & Febiger; 1970. pp. 1–8. [Google Scholar]
- 43.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. London: Churchill Livingstone; 2003. pp. 368–370. [Google Scholar]
- 44.Recknagel RO, Lombardi B. Studies on biochemical changes in subcellular particles of rat liver and their relationship to a new hypothesis regarding the pathogenesis of carbon tetrachloride induced fat accumulation. J Biol Chem. 1961;236:564–569. [PubMed] [Google Scholar]
- 45.Yuan G, Gong Z, Li J, Li X. Ginkgo biloba extract protects against alcohol-induced liver injury in rats. Phytother Res. 2007;21(3):234–238. doi: 10.1002/ptr.2054. [DOI] [PubMed] [Google Scholar]
- 46.Romero G, Lasheras B, Sainz Suberviola L, Cenarruzabeitia E. Protective effects of calcium channel blockers in carbon tetrachloride-induced liver toxicity. Life Sci. 1994;55(13):981–990. doi: 10.1016/0024-3205(94)00632-6. [DOI] [PubMed] [Google Scholar]
- 47.Recknagel RO, Glende EA., Jr . Free radicals involved in hepatotoxicity of carbon tetrachloride. In: Miquel J, Quintanitha A, Weber H, editors. Handbook of free radicals and antioxidants in biomedicine, Vol. III. Florida: CRC Press; 1988. pp. 3–16. [Google Scholar]
- 48.Huang KC. The pharmacology of Chinese herbs. 2nd ed. Washington DC: CRC Press; 1999. pp. 74–85. [Google Scholar]