Abstract
Objective
To study the detailed pharmacognostic profile of galls of Quercus infectoria Olivier (Q. infectoria olivier) (Fagaceae), an important medicinal plant used in the Indian system of medicine.
Methods
Samples of galls of Q. infectoria were studied by macroscopical, microscopical, physiochemical, phytochemical, fluorescence analysis and othjer methods for standardization as recommended by WHO.
Results
Macroscopically, the crude drug is globose with horny appearances on external surface (1.4-2.3 cm in length and 1-1.5 cm in diameter), with greyish-brown to brownish-black in colour externally and dark brown buff colored. Surface is smooth with numerous horny protuberances giving rough touch, and with unpleasant odour. Microscopically, a wide zone of radially elongated parenchyma cells between upper and lower epidermis were found. The vascular strands were present at all places and radially elongated sclerides touched the lower epidermis. In physico-chemical studies, the moisture, total ash, acid insoluble ash, alcohol soluble, water soluble, petroleum ether, chloroform extractive value and tannin content were found to be 2.790, 5.020, 0.110, 38.780, 41.210, 0.402, 1.590 and 49.200 percentage respectively. Preliminary phytochemical screening showed the presence of phenols, flavonoids, steroids, triterpenes, tannins, saponins and alkaloids.
Conclusions
The results of the present study serve as a valuable source of information and provide suitable standards for identification of this medicinally important plant drug material for future investigations and applications.
Keywords: Quercus infectoria, Gall extracts, Traditional medicine, Pharmacognostic study, Microscopic studies.
1. Introduction
Quercus infectoria (Q. infectoria) (Family: Fagaceae) commonly known as gall oak, which is a small shrub found in Greece, Asia Minor and Iran. It is a small tree or shrub growing to 4 to 6 feet tall, crooked, with smooth and bright leaves, acorn long and narrow, scaly and downy. The oak tree prefers partial shade or partial sun to full sun, and requires moist soil. It can grow in semi-shade or no shade and requires moist soil. The flowers are monoecious and are pollinated by wind. The gall arising in the branches of the tree is called as ‘majuphal’ in Sanskrit and ‘machakai’ in Kannada (both are local languages in India). In India, the galls of Q. infectoria are used since ages as a home remedy for sore throat and chronic diarrhea in both rural and urban areas. It is also used as an ingredient in Ayurvedic preparations[1].
The gall, extracts of Q. infectoria, are extensively used in traditional medication as karkatasringi for the preparation of Balachaturbhadra, Shringyadichurna, Karkatadichurna, Kantkaryavaleha[2]–[4]. Traditionally, Pistacia integerrima is used as karkatasringi in the preparation of traditional medications. Sometimes, Rhus succedanea and Q. infectoria are used as substitutes for Pistacia integerrima in the preparation of karkatasringi, which is used in the treatment of coughs, phthisis, asthma, dysentery, skin diseases, menorrhagia, intestinal hemorrhage, eczema, spermatorrhea intertrigo, impetigo, hemorrhages, chronic diarrhea and trichomoniasis[5]. They are also used as an antidote, febrifuge, ophthalmic, haemostatic agent, ointments and suppositories[6]–[8]. The galls of Q. infectoria have been known to pocess medicinal properties, such as astringent, anti-inflammatory, antiviral, antidiabetic, larvicidal, antibacterial, antiulcerogenic and gastroprotective activities[9]–[16]. The gastroprotective effect against ethanol-induced gastric damage is also attributed to the aqueous extract from Quercus ilex root bark[17]. Ethanolic extracts of Quercus coccifera and Quercus aegilops fruit have been showed have a very high curative rate after ethanol-induced gastric damage in rats suggesting their use in treatment of stomach pain[18]. In view of its diverse medicinal applications and in order to ensure the quality, authenticity and assay[19], and in view of lack of pharmacognostic study, the present investigation was undertaken with an objective to evaluate galls of Q. infectoria on various pharmacognostic parameters, such as macroscopic, microscopic, physiochemical, fluorescence and phytochemical studies of the plant.
2. Materials and methods
2.1. Plant material
The galls of Q. infectoria were collected locally from Bangalore, Karnataka (Southern India) in May 2010 and authenticated at Regional Research Institute (Ayurveda), Pune. And a voucher specimen of the plant was deposited for future reference. The collected sample was dried under shade, packed in a paper bag and stored at ambient temperature until use.
2.2. Chemicals and instruments
Compound microscope, glass slides, cover slips, watch glass and other common glass ware were the basic apparatus and instruments used for the study. Microphotographs were taken using a Leica DMLS microscope attached with Leitz MPS 32 camara. Solvents viz petroleum ether, benzene, chloroform, acetone, ethanol (95%), n-butanol and reagents viz phloroglucinol, glycerine, HCl, chloral hydrate and sodium hydroxide were procured from Sisco Research Laboratories (SRL), Bangalore, India.
2.3. Pharmacognostic study
Fresh leaves and galls were taken for morphological and histological studies. Coarse poweder was used to study the microscopic characters, physicochemical and phytochemical investigations. For microscopic studies, transverse sections were prepared and stained as per standard procedure[20]–[22]. The powder microscopy was performed according to the method of Khandelwal[22].
2.4. Physicochemcial and phytochemical analysis
Physicochemical values such as % of ash values and extractive values were determined according to the well established protocols[23],[24]. Phytochemical screening was carried out using standard procedure described by Khandelwel[22].
2.5. Florescence analysis
Powdered gall extracts were treated with various chemical reagents and exposed to visible, ultraviolet light (short UV) to study their fluoresce behaviour[25],[26].
3. Results
3.1. Macroscopic characteristics
Q. infectoria is a small tree or shrub growing to 4 to 6 feet tall, crooked, with smooth and bright leaves, acorn long and narrow, scaly and downy. The branches often slender and drooping. Leaves are elliptical, glabrescent and up to 4 cm long. Petioles are up to 4 mm long. Flowers are in axillary fascicles, pedicels filiform. Fruits are baccate and 8 mm in diameter. It becomes black while ripening. Root is cylindrical, branched and shows fibrous fracture, 6-10 cm long and 4-8 mm in thickness. The crude drug is globose with horny appearances on external surface with size of 1.4-2.3 cm in length and 1-1.5 cm in diameter, with Greyish-brown to brownish-black in colour externally and dark brown buff coloured. Surface is smooth with numerous horny protuberances giving rough touch, and with unpleasant odour (Table 1). The galls are globular (2 inch), with uneven surface with pores (indicates infection), with hallow structures and inner surface is yellow (Figure 1).
Table 1. Macroscopic characteristics of crude drug and its powder form of gall extract of Q. infectoria Olivier.
| Q. infectoria Olivier | Characteristics | |
| Crude Drug | Shape | Globose with horny appearances on external surface |
| Size | 1.4-2.3 cm in length and 1-1.5 cm in diameter | |
| Surface | Smooth with numerous horny protuberances giving rough touch | |
| Colour | Greyish-brown to brownish-black in colour externally and dark brown buff coloured | |
| Odour | Not characteristic | |
| Taste | Bitter astringent but at end sweetish sensation | |
| Fracture | Short granular | |
| Powder | Nature | Mixture of coarse and fine |
| Colour | Creamish-white | |
| Touch | Rough to smooth | |
| Odour | Not characteristic | |
| Taste | Bitter astringent | |
Figure 1. Macroscopic characteristics of gall extract of Q. infectoria.
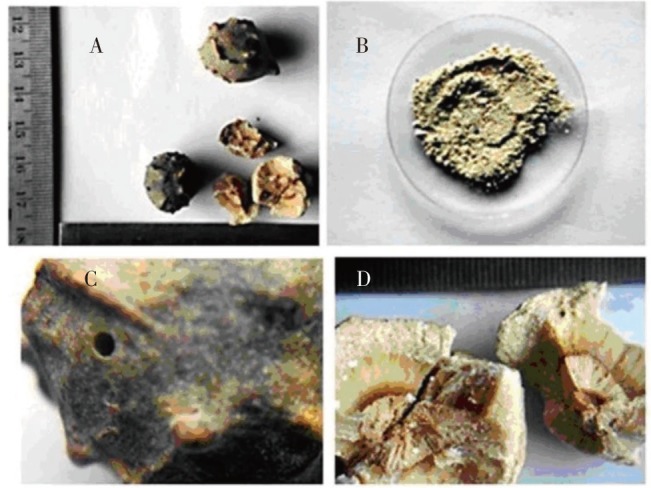
A: Crude drug; B: Powder form, C: External surface showing pore of insect exit, D: Internal surface in broken drugs.
3.2. Microscopic characteristics
Usually upper and lower epidermis cover the whole section, but with the upper epidermis ruptures is replaced by a metaderm composed of 1-2 layers of suberised cells (Figure 2A). This is followed by a wide zone of parenchyma (Figure 2D), the cells of middle and inner layers being larger in size and somewhat radially elongated (Figure 2F). Number of these cells contain tannin and many of them are having pitted wall. However, morphology of gall shows the pores which means exist of insects, but at places remains of insects, dark brown in colour and few remains of larva are also observed (Figure 1C and 1D). Vascular strands are present at places. Radially elongated sclereids were found touching the lower epidermis (Figure 2F). They are 4-7 layered and are interrupted with parenchyma cells at places. Deposition of colouring matter was more concentrated towards the lower epidermis (Figure 2C and 2E).
Figure 2. Microscopic characteristics of leaf gall of Q. infectoria.

A: Upper epidermis, B: Lower epidermis, C: Outline of lowermost portion, D: Parenchyma cells in middle portion, E: Upper epidermis, hypodermis and parenchyma cells, F: Radially elongated sclereids with septate wall.
3.3. Powder characters
Mixture of coarse and fine, with creamish-white colour. It is with no characteristic odour with bitter taste (Table 1).
3.4. Preliminary phytochemical screening
Preliminary phytochemical screening revealed the presence of saponins, alkaloids, tannins, glycosides, triterpenes, sterols, phenolic compounds, carbohydrates, and flavonoids in various extracts (Table 2)
Table 2. Preliminary phytochemical screening of leaf gall of Q. infectoria.
| Chemical constituents | Water extract | Alcohol extract | Petroleum ether extract | Chloroform extract |
| Phenols | + | + | - | - |
| Flavonoids | + | + | - | - |
| Steroids | - | - | + | + |
| Triterpenes | - | + | + | + |
| Tannins | + | + | - | - |
| Saponins | + | + | - | - |
| Alkaloids | + | + | - | + |
| Glycosides | - | - | - | - |
| Carbohydrates | + | + | - | - |
+ Denotes the presence of the respective class of compounds.
3.5. Physiochemical constants
The percentage of total ash, acid insoluble ash, sulphated ash and water soluble ash were shown in Table 3. The ash values of a drug give an idea of the earthy matter or the inorganic composition and other impurities present along with the drug. The loss on drying and foreign matter was 9.50 and 0.10, respectively. The extractive values are primarily useful for the determination of exhausted or adulterated drugs. The water soluble, alcohol soluble and ether soluble extractive values were shown in Table 4 and the results of fluorescence analysis of the drug powder were presented in Table 5.
Table 3. Hysiochemical parameters of leaf gall of Q. infectoria.
| Parameters | Value |
| Foreign matter (% w/w) | 0.1 |
| Loss on drying (% w/w) | 9.5 |
| Total ash value (% w/w) | 5.02 |
| Acid insoluble ash (% w/w) | 0.11 |
| Water soluble ash (% w/w) | 2.22 |
| Sulphated ash (% w/w) | 0.21 |
| Swelling index (mL) | 2 |
| Foaming index (mL) | - |
Table 4. Extractive values of leaf gall of Q. infectoria.
| Extract | Yield (% w/w) |
| Water | 41.215%, 15.47% |
| Methaholic | 38.785%, 21.29% |
| Chloroform | 1.59% |
| Pet ether | 0.40% |
Table 5. Florescence analysis of powdered gall extract of Q. infectoria.
| Treatment | Visible | Long Wavelength UV Light | Short Wavelength UV Light |
| 1N NaOH (Aqueous) | Royal Red | Brown | Dark Brown |
| 1N NaOH (Alcoholic) | Light Golden Yellow | Fluorescent Yellow | Fluorescent Dull Green |
| 1N HCl | Orange Yellow | Light Brown | Greenish Yellow |
| Acetic Acid | Yellow | Fluorescent Light Green | Fluorescent Light Green |
| Ethyl Alcohol | Yellow | Fluorescent Orange | Fluorescent Dull Green |
| H2SO4 (50%) | Golden Yellow | Light Brown | Fluorescent Green |
| Powder as such | Reddish-brown | Yellow | Orange Yellow |
4. Discussion
For measuring the quality, purity and ensuring the authenticity of the drugs used, standardization is very essential. The cost effective and time saving method is the microscopic evaluation to establish the right identification of the drug material. As Karkatashringiis are currently being used in the treatment of various disease conditions, and till date there is no report on standardization of the material (Galls of Q. infectoria). The present work was undertaken to lay down the standards that could be useful for establishing the authenticity of the drug material. The micro and macro standards obtained here can be identifying parameters to substantiate and authenticate the drug. The preliminary phytochemical screening will be useful in finding the chemical nature of the drug. In this study, the preliminary phytochemical screening ascertained the presence of steroids, triterpenes, tannins, saponins and alkaloids. The total ash value, fluoresecence analysis and extractive values will be helpful in identification and authentication of the plant material[27],[28] along with the microscopic method, which is the cheapest method to establish the correct identification of the source material[29]. Also the extractive methods are useful to evaluate the chemical constituents of the crude drug[30]. The present study on pharmacognostical evaluation of leaf gall of Q. infectoria will provide useful information for its identification of this medicinally important plant. Macro, micro and physiochemical standards discussed here can be considered as the identified parameters to substantiate and authenticate the drug. Thus exploring the usefulness of pharmacognostic evaluation to substantiate and authenticate drug.
Acknowledgments
We acknowledge Dr. R. Chenraj Jain, President, Jain University Trust., Dr. N Sundararajan, Vice Chancellor, Jain University and Prof. K.S. Shantamani, Chief Mentor, Jain University, Bangalore for their kind support and encouragement. DBL thank SASTRA University and Jain University, for providing facilities for carrying out this work. The Financial supported by SASTRA University to BLD in the form of TRR research grants is gratefully acknowledged. DBL acknowledges the financial support by Tamil Nadu Science and Technology (Grant No. TNSCST/S&T Projects/VR/MS/2012-13/203) of Tamilnadu State Council for Science and Technology to carry out this work.
Comments
Background
Many plant drugs used in medicinal folklore are being evaluate, due to their specific healing properties, health action and non-toxic effects. In this regard, as galls of Q. infectoria Olivera are currently being used in the treatment of various disease conditions, the pharmacognostic parameters, such as macroscopic, microscopic, physiochemical, fluorescence and phytochemical studies of the drug are the most cost effective and time saving methods to identify parameters to substantiate and authenticate the drug.
Research frontiers
Studies are being performed in order to establish the authenticity of standard drug that is used to treat various diseases. The total ash value, fluresecence analysis and extractive values will be helpful in identification and authentication of the plant material.
Related reports
The powder microscopy was performed according to the method of Khandelwal[22]. Physicochemical values such as % of ash values and extractive values were determined according to the well established protocols[23],[24]. Phytochemical screening was carried out using standard procedure described by Khandelwel[22]. Powdered gall extracts were treated with various chemical reagents and exposed to visible, ultraviolet light (short UV) to study their fluoresce behaviour[25],[26].
Innovations and breakthroughs
Pharmacogostic studies regarding galls are very scarce. This study has established the micro and macro standards obtained for the first time, preliminary phytochemical screening. The total ash value, fluresecence analysis and extractive values will be helpful in identification and authentication of the plant material for the use of drug purpose.
Applications
As this drug material is used for various diseases, the established standards of the drug (gall) by this study can be used as an useful information for identifying parameters to substantiate and authenticate the drug of this medicinally important plant. Thus exploring the usefulness of pharmacognostic evaluation to substantiate and authenticate drug.
Peer review
This is an interesting study where the authors have undertaken to lay down the standards that could be useful for establishing the authenticity of the drug material, i.e. the galls of Q. infectoria on various pharmacognostic parameters, such as macroscopic, microscopic, physiochemical, fluorescence and phytochemical studies of the plant. The results are interesting and suggests that the galls of Q. infectoria have a characteristic pharmacognostic aspects that are unique and as the identifying parameters to substantiate and authenticate the drug. Thus exploring the usefulness of pharmacognostic evaluation to substantiate and authenticate drug.
Footnotes
Foundation Project: Supported by Tamil Nadu Science and Technology of Tamilnadu State Council for Science and Technology (Grant No. TNSCST/S&T Projects/VR/MS/2012-13/203).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Dhiman AK. Ayurvedic drug plants. Delhi, India: Daya Publishing House; 2006. [Google Scholar]
- 2.Deshpande AP, Javalgekar RR, Rande S. Dravyagunvidhyan. Pune, India: Anmola Prakhashan; 1989. pp. 57–60. [Google Scholar]
- 3.Sarin YK. Illustrated manual of herbal drugs used in Ayurveda. New Delhi, India: CSIR and ICMR; 1996. pp. 178–179. [Google Scholar]
- 4.Kirtikar KR, Basu BD. Indian Medicinal Plants. Vol I. Dehradun, India: Bishen Singh Mahendra Pal Singh; 1984. [Google Scholar]
- 5.Upadhye AS. Standardization and preclinical studies on ‘Kakadshringi’: Leaf galls used in Ayurvedic system of medicine. Pharm Anal Acta. 2010 doi: 10.4172/2155-9872.1000012. [DOI] [Google Scholar]
- 6.Muhammad HS, Muhammad S. The use of Lawsonia inermis linn. (henna) in the management of burn wound infections. Afr J Biotechnol. 2005;4:934–937. [Google Scholar]
- 7.Perumal Samy R, Gopalakrishnakone P, Sarumathi M, Ignacimuthu S. Wound healing potential of Tragia involucrate extract in rats. Fitotherapia. 2006;77:300–302. doi: 10.1016/j.fitote.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Kudi AA, Ngbede JE. In vitro antibacterial activity of aqueous garlic (Allium sativum Linn.) extract on isolates from surface wounds. J Food Agric Environ. 2006;4:15–16. [Google Scholar]
- 9.Kaur G, Hamid H, Ali A, Alam MS, Athar M. Anti-inflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J Ethnopharmacol. 2004;90:285–292. doi: 10.1016/j.jep.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Dar MS, Ikram M, Fakouhi T. Pharmacology of Quercus infectoria. J Pharm Sci. 1976;65:1791–1794. doi: 10.1002/jps.2600651224. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JK, Kong TW, Baek NI, Pyun YR. Alpha-glycosidase inhibitory activity of hexagalloyl glucose from the galls of Quercus infectoria. Planta Med. 2000;66:273–274. doi: 10.1055/s-2000-8569. [DOI] [PubMed] [Google Scholar]
- 12.Redwane A, Lazrek HB, Bouallam S, Markouk M, Amarouch H, Jana M. Larvicidal activity of extracts from Quercus lusitanica var. infectoria galls (Oliv.) J Ethnopharmacol. 2002;79:261–263. doi: 10.1016/s0378-8741(01)00390-7. [DOI] [PubMed] [Google Scholar]
- 13.Basri DF, Fan SH. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J Pharmacol. 2005;37:26–29. [Google Scholar]
- 14.Hwang JK, Shim JS, Chung JY. Anticariogenic activity of some tropical medicinal plants against Streptococcus mutans. Fitoterapia. 2004;75:596–598. doi: 10.1016/j.fitote.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, Shimotohno K. Inhibitory effects of Sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother Res. 2000;14:510–516. doi: 10.1002/1099-1573(200011)14:7<510::aid-ptr646>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Sawangjaroen N, Sawangjaroen K, Poonpanang P. Effects of Piper longum fruit, Piper sarmentosum root and Quercus infectoria nutgall on caecal amoebiasis in mice. J Ethnopharmacol. 2004;91:357–360. doi: 10.1016/j.jep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Gharzouli K, Khennouf S, Amira S, Gharzouli A. Effects of aqueous extracts of Quercus ilex (L.) root bark, Punica granatum (L.) fruit peel and Artemisia-alba Asso leaves on ethanol-induced gastric damage in rats. Phytother Res. 1999;13:42–45. doi: 10.1002/(SICI)1099-1573(199902)13:1<42::AID-PTR383>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Alkofahi A, Atta AH. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J Ethnopharmacol. 1999;67:341–345. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 19.Torey A, Sasidharan S, Yeng C, Latha LY. Standardization of Cassia spetabilies with respect to authenticity, assay and chemical constituent's analysis. Molecules. 2010;15:3411–3420. doi: 10.3390/molecules15053411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brain KR, Turner TD. The practical evaluation of phytopharmaceuticals. Bristol: Wright-Scientechnica; 1975. [Google Scholar]
- 21.Pandya DJ, Desai TR, Nadpara NP, Mehta HA, Modi AM. Pharmacognostic study and establishment of quality parameters of leaves of Bombax insigne Linn. Int J Pharmacogn Phytochem Res. 2010;2:1–5. [Google Scholar]
- 22.Khandelwal KR. Practical pharmacognosy. Pune, India: Nirali publication; 2008. pp. 149–164. [Google Scholar]
- 23.Ministry of Health and Family Welfare . Indian Pharmacopeia. 4th ed. New Delhi: Government of India, Ministry of Health and Welfare, Controller of Publications; 1996. pp. A53–A54. [Google Scholar]
- 24.WHO . Quality control methods for medicinal plant material. Geneva: WHO; 1992. pp. 22–34. [Google Scholar]
- 25.Edwin S, Joshi SB, Jain DC. Comparative pharmacognostic studies on root powder of Plumbago zeylanica and Plumbago rosea. Indian J Nat Prod. 2008;2:27–29. [Google Scholar]
- 26.Pratt RJ, Chase CR. Flourescence of powdered vegetable drug with particular reference to development of a system of identification. J Am Pharm Assoc. 1949;38:324–333. doi: 10.1002/jps.3030380612. [DOI] [PubMed] [Google Scholar]
- 27.Nayak BS, Patel KN. Pharmacognostic studies of the Jatropha curcas leaves. Int J Pharm Tech Res. 2010;2:140–143. [Google Scholar]
- 28.Kumar S, Kumar V, Prakash O. Pharmacognostic study and anti-inflammatory activity of Callistemon lancelolatus leaf. Asian Pac J Trop Biomed. 2011;1:177–181. doi: 10.1016/S2221-1691(11)60022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokoski CJ, Kokoski RJ, Salma FJ. Fluorescence of powdered vegetable drug under ultraviolet radiation. J Am Pharm Assoc. 1958;47:715–717. doi: 10.1002/jps.3030471010. [DOI] [PubMed] [Google Scholar]
- 30.Thomas S, Patil DA, Patil AG, Chandra N. Pharmacognostic evaluation and physiochemcial analysis of Averrhoa carambola L. fruit. J Herb Med Toxicol. 2008;2:51–54. [Google Scholar]


