Abstract
Objective
To identify the biological forms, sporozoite rate and molecular characterization of the Anopheles stephensi (An. stephensi) in Hormozgan and Sistan-Baluchistan provinces, the most important malarious areas in Iran.
Methods
Wild live An. stephensi samples were collected from different malarious areas in southern Iran. The biological forms were identified based on number of egg-ridges. Molecular characterization of biological forms was verified by analysis of the mitochondrial cytochrome oxidase subunit I and II (mtDNA-COI/COII). The Plasmodium infection was examined in the wild female specimens by species-specific nested–PCR method.
Results
Results showed that all three biological forms including mysorensis, intermediate and type are present in the study areas. Molecular investigations revealed no genetic variation between mtDNA COI/COII sequences of the biological forms and no Plasmodium parasites was detected in the collected mosquito samples.
Conclusions
Presence of three biological forms with identical sequences showed that the known biological forms belong to a single taxon and the various vectorial capacities reported for these forms are more likely corresponded to other epidemiological factors than to the morphotype of the populations. Lack of malaria parasite infection in An. stephensi, the most important vector of malaria, may be partly due to the success and achievement of ongoing active malaria control program in the region.
Keywords: Anopheles stephensi, Mysorensis, Type, Intermediate, mtDNA markers, Molecular systematic, Iran
1. Introduction
Malaria is the most important vector borne disease in the world with 0.8-1 million deaths annually[1]. Also in Iran, malaria is one of the most important health problems that more than 2 million people of the country live in high risk area and are at risk[2]. In spite of elimination of malaria from most parts of Iran, the disease is still one of the infectious diseases in the country with more than average 15 000 annual cases in the last decade. Most of these cases occurred in south and southeast of the country where malaria control programs are still practiced. Recently the trend of malaria showed a notable decrease in malaria cases, but still cases are reported from Sistan-Baluchistan, Hormozgan and Kerman provinces. Five proven malaria vectors Anopheles stephensi (An. stephensi), Anopheles culicifacies s.l., Anopheles fluviatilis s.l., Anopheles dthali and Anopheles superpictus s.l. have been recorded in the southern endemic foci of the country including Hormozgan and Sistan-Baluchistan provinces[3]. Among them, An. stephensi is the primary vector of malaria in the region and other species act as a secondary vector and significantly prefer human blood[4]–[6]. The sporozoite rates in India were reported to be 3.6% for An. stephensi[7].
Based on egg-float ridge numbers, An. stephensi comprises three biological forms including type, intermediate and mysorensis[8]. An. stephensi type is an urban form whereas the mysorensis and intermediate forms are rare in urban areas[9]–[11]. These three forms have shown different vectorial capacities, each one has its own characteristics in malaria transmission in a particular region[4],[11]. These facts compel the medical entomologists to study different populations of An. stephensi independently in each malaria focus.
Emergence of new powerful tools such as molecular methods enable researches to use molecular markers to solve problems in malaria epidemiology such as identification of closely related species (species complexes, siblings) or populations, detection of pathogens in vectors, and blood feeding behavior of vectors[12]–[16].
Mitochondrial markers, especially cytochrome oxidase subunit I and II (COI and COII), are amongst the most powerful and reliable molecular markers to distinguish closely related species, or biological forms. Both COI and COII have been used in other arthropods for different purposes[14],[17],[18].
Direct microscopy method for detection of malaria parasites in Anopheles salivary glands was routine. This method needs a high level of technical specialty and quick dissection and test. Because of degeneration of parasites in a short time after the dissection of mosquitoes, direct detection of malaria parasites would be impossible.
During the time, other biochemical-based methods like ELISA were employed[19],[20]. Technical difficulties, need for several reagents and instruments, their low sensitivity and specificity lead researchers to develop molecular methods, which have wide range usage in this field and have been employed by several researchers[21],[22].
Several malaria control programs such as Indoor residual spraying, Insecticides treated nets, rapid disease detection and treatment, environment management and disruption of mosquitoes larval breeding places have been designed to decrease the burden of the disease in southern provinces in Iran[2],[23]. However, the key point for disease control programs is the continuous monitoring and periodically evaluation of mentioned programs. Determination of Plasmodium parasite species, sporozoite infection rate, as well as Anopheles species composition and distribution are crucial factors for evaluating control programs. This study was conducted to reveal the biological forms, sporozoite rate, and molecular characteristics of An. stephensi populations originated from different parts of the provinces. The results of this study will be useful to evaluate the ongoing malaria control programs, and will help decision making persons involved in these programs.
2. Materials and methods
2.1. Study area and mosquito collection
Hormozgan and Sistan-Baluchistan provinces as the main malarious areas are located in southern part of Iran. These provinces are located in northern coast of Persian Gulf and Oman sea (Figure 1), where the weather is warm and humid enough for Anopheles species to be active throughout the year. This situation makes An. stephensi the main vector responsible for transmission of malaria to human in southern Iran.
Figure 1. Map of Iran and location of Hormozgan and Sistan-Baluchistan Provinces.
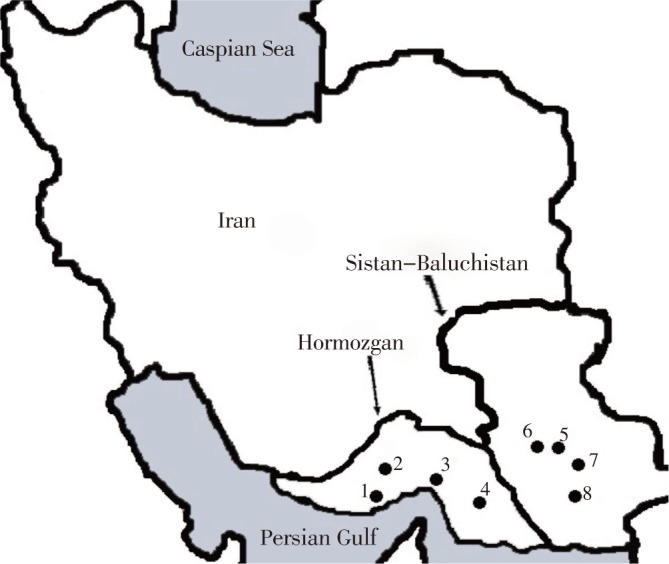
Study areas: 1: Bandar-Abbas, 2: Harmoodar, 3: Minab, 4: Bashagard, 5: Iranshahr, 6: Bampoor, 7: Kahiri, 8: Sarbaz.
Wild caught samples were collected form Bandar-Abbas (capital city of Hormozgan Provice), Harmoodar, Minab, Bashagard, Iranshahr, Bampoor, Kahiri and Sarbaz (Figure 1) by hand-catch indoors and pit shelters outdoors. Collected samples were identified using the standard key[24]. For molecular investigations 120 samples (65 from Sistan-Baluchistan and 55 from Hormozgan provinces) were collected twice during the transmission season (May-September 2010).
2.2. Determination of biological forms
Alive collected samples were transferred to Bandar-Abbas and Iranshahr insectariums. The gravid or blood fed females were kept individually in glass tubes with a dump paper at the bottom allowed to lay eggs. The rest of the mosquitoes were colonized using the standard protocol. They fed on guinea pigs and again females were kept individually to lay eggs. At least 15 females from every region were kept to lay eggs. A total of 10 eggs per female were collected and the egg-float ridge numbers were counted using Stereo-Microscope and categorized based on the described criteria for the biological forms[8].
2.3. Molecular investigations
2.3.1. DNA extraction
Genomic DNA was extracted individually from 120 wild-caught samples using G-Spin DNA Extraction Kit (Intron, South Korea) according to supplied procedure by manufacturer.
2.3.2. Mitochondrial markers amplification
Amplification of the mtDNA COI (877 bp), or COII (640 bp) regions were conducted using universal primers (Table 1). The PCR program was determined as: initial denaturation for 4 min at 94 °C, 32 cycles of 1 min denaturation at 94 °C, 1 min annealing at 55 °C, and 2 min extension at 72 °C, followed by a final extension for 7 min at 72 °C[25].
Table 1. List of primers used for amplification of COI and COII genes from mtDNA of the An. stephensi and detection of malaria sporozoites in mosquitoes.
| Primer name | sequence 5′-3′ | Expected amplicon length (bp) |
| COI | Forward 5_-TTG ATT TTT TGGTCA TCC AGA AGT-3 | 877 |
| Reverse 5-TAG AGC TTA AAT TCA TTG CAC TAA TC-3 | ||
| COII | Forward 5_- ATG GCA ACA TGA GCA AATT-3 | 640 |
| Reverse 5_-CCA CCC TTT CTG AAC ATT GAC C-3 | ||
| rPLU5 | Forward 5′ CCTGTTGTTGCCTTAAACTTC 3′ | 1 200 |
| rPLU6 | Reverse 5′ TTAAAATTGTTGCAGTTAAAACG 3′ | |
| rFAL1 | Forward 5′ TTAAACTGGTTTGGGAAAACCAAATATATT 3′ | 205 |
| rFAL2 | Reverse 5′ ACACAATGAACTCAATCATGACTACCCGTC 3′ | |
| rVIV1 | Forward 5′ CGCTTCTAGCTTA ATCCACATAACTGATAC 3′ | 120 |
| rVIV2 | Reverse 5′ CTTCCAAGCCGAAGCAAAGAAAGTCCTTA 3′ |
2.3.3. Detection of sporozoite infection in mosquitoes
DNA specimens of head-thoracic portion of female mosquito were tested for malaria parasites genomic DNA using the nested PCR based on the 18S rRNA gene marker described by Snounou[21]. As above, PCR were carried out by Eppendorf thermo-cycler using Maxime PCR PreMix Kit (Intron biotechnology, Korea). After a Plasmodium spp. specific nested PCR with the rPLU5 and rPLU6 primers, the primary PCR product of Plasmodium positive samples was separately amplified with the two species-specific primer pairs rFAL1/2 and rVIV1/2 (Table 1) to identify the species. A total of 5 µL of DNA and 2 µL of primary PCR product were used for the primary and secondary PCR reactions, respectively. In order to thrift time and materials, equal proportion (1 µL) of 10 extracted DNA templates of the head-thoracic portion of female mosquito were pooled and 5 µL of it was used as DNA template for the PCR. After an initial denaturation carried out at 95 °C for 5 min, 25 cycles were programmed as follows: 945 °C for 1 min, 58 °C for 2 min, 72 °C for 2 min and final extension at 72 °C for 5 min. Similar program was used for the second step except for 30 cycles. Detection of PCR products was done by 1.0% or 2.5% agarose gel electrophoresis and ethidium bromide staining for the first and second step, respectively. In all PCR assays, ddH2O and DNA of male mosquitos were used as negative controls. Also, DNA of Plasmodium falciparum donated from Department of Parasitology of Tehran University of Medical Sciences was used as positive control.
3. Results
3.1. Biological forms
All three biological forms of An. stephensi, type, intermediate and mysorensis, were found in the study areas based on egg-float ridge numbers. The type form was found in Bandar-abbas, the city in the coastal area as well as in Harmoodar district, a sub-urban area located in a hilly region of Hormozgan Province. Intermediate form presented in Minab and Bashagard districts in northern and eastern parts of Hormozgan. All the specimens collected from Sistan-Baluchistan were An. stephensi mysorensis (Table 2).
Table 2. Details of study area condition and the mean of Egg-float ridge number of An. stephensi biological forms from Hormozgan and Sistan-Baluchistan provinces, Iran.
| Location | Geographical locationLatitude (°N) Longitude(°E) | Site | Setting | Geographical condition | Ridge number (mean) | Biological form | |
| Bandar-Abbas (Hormozgan) | 27°11′3.88″ | 56°15′17.92″ | Bandar-Abbas | Urban | coastal | 18.50±1.72 | Type |
| Harmoodar (Hormozgan) | 27°26′48.28″ | 56°2′21.12″ | Harmoodar | Semi-urban | plain | 18.10±1.69 | Type |
| Minab (Hormozgan) | 27°8′43.12″ | 57°5′25.63″ | Minab | urban | coastal | 15.00±0.92 | Intermediate |
| Bashagard (Hormozgan) | 26°34′23.43″ | 58°53′29.49″ | Sardasht | Semi-urban | mountainous | 15.20±0.89 | Intermediate |
| Bashagard (Hormozgan) | 26°30′9.11″ | 58°26′45.21″ | Molkan | rural | hilly | 15.40±0.85 | Intermediate |
| Iranshahr (Sistan-Baluchistan) | 27°12′15.97″ | 60°40′5.69″ | Iranshahr | urban | plain | 13.20±0.95 | mysorensis |
| Iranshahr (Sistan-Baluchistan) | 27°7′46.20″ | 60°52′34.74″ | Kahiri | rural | hilly | 13.10±1.05 | mysorensis |
| Bampoor (Sistan-Baluchistan) | 27°11′41.81″ | 60°27′21.30″ | Bampoor | Semi-urban | plain | 12.90±0.65 | mysorensis |
| Sarbaz (Sistan-Baluchistan) | 26°40′51.24″ | 61°12′47.99″ | Angoori | rural | mountainous | 13.00±0.90 | mysorensis |
3.2. COI and COII gene sequences
All studied An. stephensi specimens showed bands of 877 and 640 bp length for the COI and COII regions, respectively. Three samples of PCR products for COI and COII genes from each biological form were sequenced. Analysis of the sequences showed 100% homology to the previously submitted sequences of these genes from An. stephensi in other studies[26] (COI, AY877426; COII, AY883830, AY883836). Further analysis also showed no sequence variation (substitutions or indels) between the sequences of the type, mysorensis, and Intermediate biological forms in both loci of COI and COII.
3.3. Plasmodium infection in mosquito samples
None of the mosquitoes were infected by malaria parasite. However, the PCR results were positive for positive controls. The size of PCR products were around 1 200 base pairs for the positive control.
4. Discussion
An. stephensi acts as an efficient malaria vector in several parts of South and South-west Asia such as Middle East region and Indian subcontinent[7]. This species has three biological forms including type, intermediate and mysorensis forms. Type form acts as an important malaria vector in urban areas in India, whereas other two forms are none or weak vectors[27]–[29]. In this study, the authors have found type form in urban as well as semi-urban areas. This is the first report on presence of type form in semi-urban areas. The role of type form in transmission of malaria has been reported previously in Hormozgan Province[4],[23]. In other malaria endemic parts of Iran such as Baluchistan, and neighboring countries of Pakistan and Afghanistan, mysorensis plays as a good vector of malaria in either urban or rural areas[30]. Based on ecological and biological data, mosquitoes of An. stephensi are the main vector of malaria parasites in Minab and Bashagard districts. These areas demographically are mixed of rural and semi-urban areas and this study showed that only intermediate form present there. Hence, it can be concluded that in contrast with situation in India, intermediate form is a good vector in northern and eastern parts of Hormozgan Province. Also, in contrast with India, mysorensis has a significant role in malaria transmission and indeed is the most important vector of malaria[4],[31].
Different molecular markers have been introduced to identify different closely Anopheles species especially sibling or biological forms of single species. These markers play a great role in the field of molecular entomology and will help to develop applicable and reliable mosquitoes molecular keys. Such keys could be complementary to the routine morphological keys[12]. However, our findings showed that the three biological forms have no difference in the mtDNA COI/COII genes. This is in concordance with the previous reports indicating identical sequences in various loci such as ITS2-rDNA, RAPD markers, and mtDNA genes of the three biological forms or various populations of An. stephensi[26],[32].
In this study, none of the wild caught female An. stephensi specimens were infected with malaria parasites. This could be due to low prevalence of the disease in the study areas at the time of sample collection[23]. The molecular method we used here has been widely used in many laboratories and showed high sensitivities and specificities[33]. However, since the introduction of this method, several new molecular methods have been introduced to improve the ability and sensitivities of pathogen detection in arthropod vectors[13],[16],[33],[34]. This ability can help the staff of health section to judge the ongoing control programs, and determine the need for stop, improvement or development of control programs. The control programs by the national center of malaria and probably the climate condition had resulted in low cases of malaria at the time of study, and these could be an explanation for our parasite-free mosquitoes at the current season situation.
Based on epidemiological surveys, it could be concluded that different biological forms of An. stephensi have different transmission capacities, but it will be useful to evaluate this environmental-based finding with laboratory-based experiments.
Acknowledgments
We gratefully thank the staff of Bandar-Abbas and Iranshahr National Institute of Health Research Centers for their contributions in laboratory and fields of Hormozgan and Sistan-Baluchistan Provinces. This work was financially supported by Tehran University of Medical Sciences (TUMS) (Grant No. 89-01-27-10448).
Comments
Background
Malaria remains an important vector-borne disease globally and is a threat for human life. An. stephensi is the primary vector of malaria in Iran. There are three biological forms of An. stephensi.
Research frontiers
This manuscript explains the molecular characteristics of An. stephensi from different populations in southern parts of Iran. The authors also determined the sporozoite rate based on molecular approach.
Related reports
An. stephensi has three biological forms including type, intermediate and mysorensis forms. The findings of this study showed that the three biological forms have no difference in the mtDNA COI/COII genes. This is in concordance with the previous reports indicating identical sequences in various loci such as ITS2-rDNA, RAPD markers, and mtDNA genes of three biological forms or various populations of An. stephensi.
Innovations and breakthroughs
In this study, the authors tried to describe the biological forms of An. stephensi using molecular approach. There is morphological and molecular information on this subject. Also, the information about sporozoite rate of An. stephensi is useful for further researches.
Applications
It is very interesting to know if there is any molecular approach to separate these biological forms. It is also important if there is any correlation between traditional methods and modern methods. This manuscript may increas knowledge of molecular and medical entomology.
Peer review
This is a good study that revealed the biological forms, sporozoite rate, and molecular characteristics of An. stephensi populations originated from different parts of the provinces in Iran. The data may represent a useful contribution to our knowledge on biological forms of An. stephensi and their sporozoite rate in southern Iran.
Footnotes
Foundation Project: This work was financially supported by Tehran University of Medical Sciences (TUMS) (Grant No. 89-01-27-10448).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Enayati A, Hemingway J. Malaria management: past, present, and future. Annu Rev Entomol. 2010;55:569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- 2.Hanafi-Bojd AA, Vatandoost H, Oshaghi M, Haghdoost AA, Shahi M, Sedaghat MM, et al. et al. Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 2012;121(2):85–92. doi: 10.1016/j.actatropica.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Hanafi-Bojd AA, Azari-Hamidian S, Vatandoost H, Charrahy Z. Spatio-temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pac J Trop Med. 2011;4(6):498–504. doi: 10.1016/S1995-7645(11)60134-X. [DOI] [PubMed] [Google Scholar]
- 4.Vatandoost H, Oshaghi MA, Abaie MR, Shahi M, Yaaghoobi F, Baghaii M, et al. et al. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan province, southern Iran, 2002. Acta Trop. 2006;97(2):196–203. doi: 10.1016/j.actatropica.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Chavshin AR, Oshaghi MA, Vatandoost H, Pourmand MR, Raeisi A, Enayati AA, et al. et al. Identification of bacterial microflora in the midgut of the larvae and adult of wild caught Anopheles stephensi: a step toward finding suitable paratransgenesis candidates. Acta Trop. 2012;121(2):129–134. doi: 10.1016/j.actatropica.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Swami KK, Srivastava M. Blood meal preference of some anopheline mosquitoes in command and non-command areas of Rajasthan, India. J Arthropod Borne Dis. 2012;6(2):98–103. [PMC free article] [PubMed] [Google Scholar]
- 7.Korgaonkar NS, Kumar A, Yadav RS, Kabadi D, Dash AP. Mosquito biting activity on humans & detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, India. Indian J Med Res. 2012;135:120–126. doi: 10.4103/0971-5916.93434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subbarao SK, Vasantha K, Adak T, Sharma VP, Curtis CF. Egg-float ridge number in Anopheles stephensi: ecological variation and genetic analysis. Med Vet Entomol. 1987;1(3):265–271. doi: 10.1111/j.1365-2915.1987.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 9.Oshaghi M, Yaaghoobi F, Vatandoost H, Abaei M, Akbarzadeh K. Anopheles stephensi biological forms, geographical distribution, and malaria transmission in malarious regions in Iran. Pak J Biol Sci. 2006;9(2):294–298. [Google Scholar]
- 10.Nagpal BN, Srivastava A, Dash AP. Resting behaviour of Anopheles stephensi type form to assess its amenability to control malaria through indoor residual spray. J Vector Borne Dis. 2012;49(3):175–180. [PubMed] [Google Scholar]
- 11.Mehravaran A, Vatandoost H, Oshaghi MA, Abai MR, Edalat H, Javadian E, et al. et al. Ecology of Anopheles stephensi in a malarious area, southeast of Iran. Acta Med Iran. 2012;50(1):61–65. [PubMed] [Google Scholar]
- 12.Loaiza JR, Bermingham E, Sanjur OI, Scott ME, Bickersmith SA, Conn JE. Review of genetic diversity in malaria vectors (Culicidae: Anophelinae) Infect Genet Evol. 2012;12(1):1–12. doi: 10.1016/j.meegid.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Steenkeste N, Incardona S, Chy S, Duval L, Ekala MT, Lim P, et al. et al. Towards high-throughput molecular detection of Plasmodium: new approaches and molecular markers. Malar J. 2009;8:86. doi: 10.1186/1475-2875-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hein GL, French R, Siriwetwiwat B, Amrine JW. Genetic characterization of North American populations of the wheat curl mite and dry bulb mite. J Econ Entomol. 2012;105(5):1801–1808. doi: 10.1603/ec11428. [DOI] [PubMed] [Google Scholar]
- 15.An H, Jung G, Kim CB. Molecular taxonomy of a phantom midge species (Chaoborus flavicans) in Korea. Anim Syst Evol Divers. 2012;28(1):36–41. [Google Scholar]
- 16.Naddaf SR, Kishdehi M, Siavashi MR. Comparison of PCR-based diagnosis with centrifuged-based enrichment method for detection of borrelia persica in animal blood samples. Iran J Arthropod Borne Dis. 2011;5(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon MJ, Song JH, Ahn KJ. Molecular phylogeny of the marine littoral genus Cafius (Coleoptera: Staphylinidae: Staphylininae) and implications for classification. Zool Scr. 2012;41(2):150–159. [Google Scholar]
- 18.Chen R, Jiang LY, Qiao GX. The effectiveness of three regions in mitochondrial genome for aphid DNA barcoding: a case in Lachininae. PLoS One. 2012;7(10):46190. doi: 10.1371/journal.pone.0046190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fryxell RT, Nieman CC, Fofana A, Lee Y, Traoré SF, Cornel AJ, et al. et al. Differential Plasmodium falciparum infection of Anopheles gambiae ss molecular and chromosomal forms in Mali. Malar J. 2012;11:133. doi: 10.1186/1475-2875-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam MS, Chakma S, Khan WA, Glass GE, Mohon AN, Elahi R, et al. et al. Diversity of anopheline species and their Plasmodium infection status in rural Bandarban, Bangladesh. Parasit Vectors. 2012;5:150. doi: 10.1186/1756-3305-5-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61(2):315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 22.Rider MA, Byrd BD, Keating J, Wesson DM, Caillouet KA. PCR detection of malaria parasites in desiccated Anopheles mosquitoes is uninhibited by storage time and temperature. Malar J. 2012;11:193. doi: 10.1186/1475-2875-11-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanafi-Bojd A, Vatandoost H, Philip E, Stepanova ES, Abdi A, Safari R, et al. et al. Malaria situation analysis and stratification in bandar abbas county, southern Iran, 2004-2008. Iran J Arthroppod Borne Dis. 2010;4(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- 24.Azari-Hamidian S, Harbach RE. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae) Zootaxa. 2009;2078:1–33. [Google Scholar]
- 25.Crozier RH, Crozier YC. The mitochondrial genome of the honeybee Apis mellifera: complete sequence and genome organization. Genetics. 1993;133(1):97–117. doi: 10.1093/genetics/133.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshaghi MA, Yaaghoobi F, Abaie MR. Pattern of mitochondrial DNA variation between and within Anopheles stephensi (Diptera: Culicidae) biological forms suggests extensive gene flow. Acta Trop. 2006;99(2–3):226–233. doi: 10.1016/j.actatropica.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Alam MT, Bora H, Das MK, Sharma YD. The type and mysorensis forms of the Anopheles stephensi (Diptera: Culicidae) in India exhibit identical ribosomal DNA ITS2 and domain-3 sequences. Parasitol Res. 2008;103(1):75–80. doi: 10.1007/s00436-008-0930-7. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh SK, Tiwari S, Raghavendra K, Sathyanarayan TS, Dash AP. Observations on sporozoite detection in naturally infected sibling species of the Anopheles culicifacies complex and variant of Anopheles stephensi in India. J Biosci. 2008;33(3):333–336. doi: 10.1007/s12038-008-0052-5. [DOI] [PubMed] [Google Scholar]
- 29.Sharma RS. Urban malaria and its vectors Anopheles stephensi and Anopheles culicifacies (Diptera: Culicidae) in Gurgaon, India. Southeast Asian J Trop Med Public Health. 1995;26(1):172–176. [PubMed] [Google Scholar]
- 30.Rowland M, Mohammed N, Rehman H, Hewitt S, Mendis C, Ahmad M, et al. et al. Anopheline vectors and malaria transmission in eastern Afghanistan. Trans R Soc Trop Med Hyg. 2002;96(6):620–626. doi: 10.1016/s0035-9203(02)90331-7. [DOI] [PubMed] [Google Scholar]
- 31.Vatandoost H, Hanafi-Bojd AA. Indication of pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pac J Trop Med. 2012;5(9):722–726. doi: 10.1016/S1995-7645(12)60114-X. [DOI] [PubMed] [Google Scholar]
- 32.Djadid ND, Gholizadeh S, Aghajari M, Zehi AH, Raeisi A, Zakeri S. Genetic analysis of rDNA-ITS2 and RAPD loci in field populations of the malaria vector, Anopheles stephensi (Diptera: Culicidae): implications for the control program in Iran. Acta Trop. 2006;97(1):65–74. doi: 10.1016/j.actatropica.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Swain S, Mohanty A, Mahapatra N, Parida SK, Marai NS, Tripathy HK, et al. et al. The development and evaluation of a single step multiplex PCR for simultaneous detection of Anopheles annularis group mosquitoes, human host preference and Plasmodium falciparum sporozoite presence. Trans R Soc Trop Med Hyg. 2009;103(11):1146–1152. doi: 10.1016/j.trstmh.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Oshaghi MA, Ravasan NM, Hide M, Javadian EA, Rassi Y, Sedaghat MM, et al. et al. Development of species-specific PCR and PCR-restriction fragment length polymorphism assays for L.infantum/L.donovani discrimination. Exp Parasitol. 2009;122(1):61–65. doi: 10.1016/j.exppara.2009.01.015. [DOI] [PubMed] [Google Scholar]


