Abstract
Objective
To investigate the effects of different dietary fat and oils (differing in their degree of saturation and unsaturation) on lipid peroxidation in liver and blood of rats.
Methods
The study was conducted on 50 albino rats that were randomly divided into 5 groups of 10 animals. The groups were fed on dietary butter (Group I), margarine (Group II), olive oil (Group III), sunflower oil (Group IV) and corn oil (Group V) for 7 weeks. After 12 h of diet removal, livers were excised and blood was collected to measure malondialdehyde (MDA) levels in the supernatant of liver homogenate and in blood. Blood superoxide dismutase activity (SOD), glutathione peroxidase activity (GPx), serum vitamin E and total antioxidant capacity (TAC) levels were also measured to determine the effects of fats and oils on lipid peroxidation.
Results
The results indicated that no significant differences were observed in SOD activity, vitamin E and TAC levels between the five groups. However, there was significant decrease of GPx activity in groups IV and V when compared with other groups. The results indicated that feeding corn oil caused significant increases in liver and blood MDA levels as compared with other oils and fats. There were positive correlations between SOD and GPx, vitamin E and TAC as well as between GPx and TAC (r: 0.743; P<0.001) and between blood MDA and liver MDA (r: 0.897; P<0.001). The results showed also negative correlations between blood MDA on one hand and SOD, GPx, vitamin E and TAC on the other hand.
Conclusions
The results demonstrated that feeding oils rich in polyunsaturated fatty acids (PUFA) increases lipid peroxidation significantly and may raise the susceptibility of tissues to free radical oxidative damage.
Keywords: Vegetable oils, Butter, Margarine, Polyunsaturated fatty acids (PUFA), Lipid peroxidation, Malondialdehyde (MDA), Superoxide dismutase activity (SOD), Glutathione peroxidase activity (GPx)
1. Introduction
Lipids are an essential component of the diet as they are among the major sources of energy second to carbohydrates[1]–[5]. Lipids are required for the absorption and transport of lipid-soluble vitamins through the bloodstream[6]–[10]. As an important constituent of cell membranes, lipids also play specific roles in membrane signaling events. Thus cell development certain lipids are indicators of cellular events, and lipid concentration can represent physiological conditions of cells[11]–[13]. The incidence of cardiovascular disease is correlated with diets high in saturated fatty acids (SFA). Animal fats, which contain higher proportions of SFA, increase the risk of vascular system diseases. Numerous studies indicate that butter elevates the level of total cholesterol, low density lipoprotein (LDL) and triacylglycerols. It has also been reported that consumption of dietary butter contributes to hypercholesterolemia due to its high content of SFA[14]. Margarine made from corn or sunflower oils is much lower in SFA than butter. Substitution of margarine for dietary butter reduces total cholesterol and LDL levels[15]. By reducing serum cholesterol levels without any effect on high-density lipoprotein (HDL) cholesterol levels, olive oil rich in monounsaturated fatty acids shows protective effects against arteriosclerosis[16],[17]. Recently, consumption of sunflower and corn oils has increased. These oils are quite rich in linoleic acid which is an essential PUFA. Although sunflower and corn oils reduce cholesterol synthesis and thus its level, they are considered as risk factors for the sensitivities to free radical formation because of their high contents of PUFA. It is we known that PUFA are more susceptible to lipid peroxidation than SFA[18]–[20].
In organisms, endogenous and exogenous free radicals can damage structures of lipids, proteins, carbohydrates and nucleic acids by interacting with them and can subsequently produce new free radicals[21]. Among all biomolecules, lipids are the most sensitive molecules to free radicals. Double bonds in fatty acids form peroxide products by reacting with free radicals and lipid radicals can be formed subsequently upon removal of electrons[22]. As a result of lipid peroxidation, malondialdehyde (MDA, a genotoxic harmful degradative byproduct of lipid peroxidation) can be formed in cell membranes. MDA shows both mutagenic and carcinogenic effects by changing membrane properties[23],[24]. Organisms protect themselves from harmful effects of free radicals by antioxidant defense mechanisms. The antioxidant system involves both enzymatic and non-enzymatic antioxidants. The fast step in the enzymatic system is superoxide dismutase (SOD), which catalyzes the dismutation of superoxide anion (O2−) to H2C2. The conversion of H2O to H2O2 by either glutathione peroxidase (GPx) or catalase forms the second step of enzymatic system. Superoxide dismutase and GPx enzyme activities and the balance between them are very crucial protection against oxidative stress[25]–[27]. Lipid-soluble vitamin E is a non-enzymatic antioxidant which plays a significant role in the protection of the cell membrane and against LDL cholesterol as well. It can reduce free radicals and it has the most action to break the chain reaction in lipid peroxidation[27]–[29]. The measure of total antioxidant capacity (TAC), which is the cumulative action of all the antioxidants present in plasma, provides an insight into the delicate balance in vivo between oxidants and antioxidants[30],[31].
The objectives of this study were to demonstrate the influence of dietary butter, margarine, olive oil, sunflower oil and corn oil on liver and blood lipid peroxidation in albino rats by measuring MDA levels in blood and liver, and to assess the antioxidant activity in these animals by measuring SOD and GPx activities as well as, vitamin E and TAC levels in blood to determine the dietary oils most susceptible to lipid peroxidation.
2. Materials and methods
2.1. Fats and oils
Corn oil, sunflower oil, olive oil, butter and margarine were obtained from local market in Kingdom of Saudi Arabia. Fatty acids were transesterified into FAME using N-trimethylsulfoniumhydroxide (Macherey-Nagel, Düren, Germany) according to the procedure reported by Ramadan et al[32]. FAME were identified on a Shimadzu GC-14A equipped with flame ionisation detector and C-R4AX chromatopac integrator (Kyoto, Japan). The flow rate of the carrier gas helium was 0.6 mL/min and the split value with a ratio of 1:40. A sample of 1 µL was injected on a 30 m×0.25 mm×0.2 µm film thickness Supelco SPTM-2380 (Bellefonte, PA, USA) capillary column. The injector and flame ionisation detector temperature was set at 250 °C. The initial column temperature was 100 °C programmed by 5 °C/min until 175 °C and kept 10 min at 175 °C, then 8 °C/min until 220 °C and kept 10 min at 220 °C. A comparison between the retention times of the samples with those of authentic standard mixture (Sigma, St. Louis, MO, USA; 99% purity specific for GLC), run on the same column under the same conditions, was made to facilitate identification. Reagents and chemicals used in the study were of the highest purity available.
2.2. Experimental animal protocol
Fifty white albino rats of both sexes were used in this study. The animals were obtained from faculty of pharmacy, King Saud University (Kingdom of Saudi Arabia). All animals were kept under normal healthy conditions and fed on a basal diet for one week. The animals were randomly allocated into five groups (each group of 10) of approximately equal average body weight (100-150 g). Utmost care was taken to provide equal physical and environmental housing conditions (namely size of units, light, temperature and aeration). The units were illuminated 24 h a day. Tap water supply for all rats was adjusted by calculating the volume of fluid intake per day per rat as follows: the volume of fluid intake was estimation daily per cage (every cage contained five rats) for the whole duration of the work. The volume of fluid intake was calculated per day and divided by the number of rats per cage to get the average volume of fluid intake per day per rat. All experimental procedures used were in accordance with the published ethical guidelines for the animal use and care.
In the first week, all groups were fed a basal diet composed of 89.2% dry matter, 20.93% crude protein, 4.91% etude cellulose, 5.31% ash, 2.83% crude oil, 0.73% methionine-cysteine, 1.01% lysine, 0.9% calcium and 0.68% phosphor with a 2 830 kcal/kg metabolic energy. The selected lipids were added to respective diets as 5.4% in the 2nd and 3rd week, 6% in the 4th and 5th week, and 7% in the 6th and 7th week. The diets differed in the nature of lipids (namely dietary butter for Group I, margarine for Group II, olive oil for Group III, sunflower oil for Group IV or corn oil for Group V, and were prepared fresh every week. Margarine and butter were added to each diet after solubilization at low temperature. Basal diet was mixed with lipids completely and rats were provided with this diet ad libitum. Rats were sacrificed after 12 h of food deprivation. Blood samples were collected from hearts of the rats into plain tubes (to obtain serum for malondialdehyde), heparinezed tubes (to obtain heparinezed plasma for SOD and GPx activities, and TAC) and EDTA-tubes (to obtain EDTA-plasma for vitamin E assay). Samples were kept at -70 °C until analysis. After sacrifices, livers were excised immediately, washed thoroughly with ice-cold 0.9% NaCl, and kept at -70 °C in phosphate buffer (pH 7.4) until analysis.
Analysis of liver MDA level was carried out according to Ohkawa et al[33]. Fractions after homogenization and centrifugation of tissue samples diluted 10-fold with 1% KCI. Serum MDA was determined and measured according to the method of Yoshioka et al.[34], using spectrophotometer (Milton Roy 3000 ARRAY double beam spectrophotometer, USA). SOD and GPx activities were assayed spectrophotometrically within 3 d of sampling by commercial kits. Total blood SOD activity was determined by inhibition of formazan dye (505 nm) employing the xanthin-xanthin oxidase enzymatic method to generate superoxide radicals[35] and expressed as U/mg of haemoglobin. GPx activity was measured based on nicotinamide adenine dinucleotide phosphate oxidation at 340 nm using curmen hydroperoxide as the substrate (Randox Laboratories, Crurnlin, UK) and expressed in U/L. Plasma vitamin E was assessed by the High Performance Liquid chromatographic (HPLC) system as described by Thumham et al[36]. The HPLC system consists of auto-sampler (GBC-LC 1610, Australia), bio-liquid dual pump (GBC-LC 1150; Australia), a C18 reversed phase column (3 µm spherosorb ODS-2, 15 cm×4.6 mm), and fluorescent photometer (Perkin Elmer, LC 30, UK). The excitation wavelength was 292 nm with emission wavelength of 318 nm). The mobile phase consists of methanol (HPLC-grade) at a flow rate of 1 mL/min. Briefly, plasma was deproteinized in the presence of ethanol containing retinol acetate as an internal standard. Vitamin E was extracted by using hexane then evaporated to dryness under a steam of nitrogen. The residues were dissolved in methanol and injected into the column. A linear calibration curve was done using different concentrations of α-tocopherol acetate (5, 10, 20, 40 and 100 µmol/L) as external standard used in calculation of the results. Finally, plasma TAC was assessed by a colorimetric method developed by Koracevic et al.[37], using the Trolox-Equivalent Anti-oxidant Capacity Kit (Randox Laboratories, Antrim, UK).
2.3. Statistical analysis
The data were expressed as means±SD. Data were analyzed using statistical package for social sciences (SPSS) (version 10.0 for Windows Smart Viewer) supplied by SPSS Inc. 2000 (Mapinfo Corp. Tokyo, New York, USA). Differences between groups were evaluated by one-way analysis of variance (ANOVA) followed by student's test. Results were considered statistically significant at P<0.05. The strength of the association between all the measured parameters was determined by the Pearson correlation coefficient method.
3. Results
3.1. Fatty acid profile of oils and fats
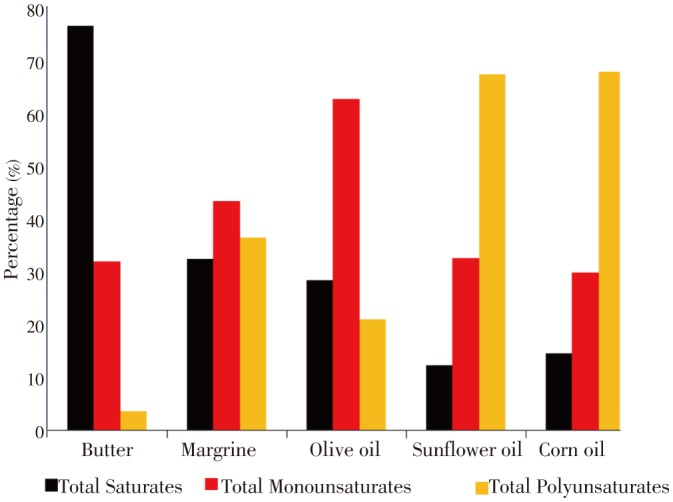
Fatty acid profile of the oils and fats under study are presented in Table 1. According to the results, 18 fatty acids were identified in the different oils and fats, where in the analysis of FAME gave the proportion of linoleic and oleic, as the major fatty acids in vegetable oils and margrine, which comprising together more than 65% of total identified FAME. Concerning SFA (especially palmitic and stearic), butter was characterized by appreciable level of saturates (ca. 68% of total fatty acids). Corn oil recorded the highest levels of PUFA and olive oil contained the highest amount of monounsaturated fatty acids (Figure 1). A striking feature of the vegetable oils and margarine was the high level of PUFA, especially linoleic acid (C18:2n-6). The nutritional value of linoleic acid is due to its metabolism at tissue levels which produce the hormone-like prostglandins. The activity of these includes lowering of blood pressure and constriction of smooth muscle[20],[38]. The PUFA have anti-inflammatory properties that are mediated by the production of anti-inflammatory eicosanoids. Moreover, interest in the PUFA as health-promoting nutrients has expanded dramatically in recent years. A rapidly growing literature illustrates the benefits of PUFA, in alleviating cardiovascular, inflammatory, heart diseases, atherosclerosis, autoimmune disorder, diabetes and other diseases[39],[40].
Table 1. Fatty acid profile of vegetable oils, margarine and butter under study.
| Butter | Margarine | Olive oil | Sunflower oil | Corn oil | |
| C 4:0 | 1.80±0.15 | ND | ND | ND | ND |
| C 6:0 | 2.20±0.22 | ND | ND | ND | ND |
| C 8:0 | 1.20±0.13 | ND | ND | ND | ND |
| C 10:0 | 2.80±0.14 | ND | ND | ND | ND |
| C 12:0 | 3.70±0.23 | 3.7±0.33 | ND | ND | 0.18±0.03 |
| C 14:0 | 12.50±0.44 | 2.2±0.15 | ND | ND | 0.07±0.02 |
| C 16:0 | 35.10±1.44 | 11.9±0.77 | 21.33±1.35 | 6.62±0.33 | 9.46±0.77 |
| C 16:1 | 3.20±0.23 | 3.2±0.23 | 3.38±0.35 | 0.11±0.02 | 0.09±0.02 |
| C 18:0 | 9.00±0.26 | 11.1±0.58 | 3.66±0.43 | 3.11±0.24 | 2.90±0.05 |
| C 18:1 | 25.30±2.35 | 35.4±2.45 | 52.37±3.25 | 28.6±2.06 | 26.1±1.22 |
| C 18:2 | 2.80±0.61 | 29.1±1.87 | 18.20±1.44 | 59.9±3.65 | 60.0±3.85 |
| C 18:3 | 0.40±0.05 | 3.4±0.33 | 0.55±0.07 | 0.11±0.05 | 0.44±0.06 |
| C 20:0 | NDa | ND | 0.35±0.09 | 0.26±0.03 | 0.32±0.07 |
| C 20:1 | ND | ND | 0.16±0.02 | 0.27±0.04 | 0.33±0.03 |
| C 20:2 | ND | ND | ND | ND | 0.03±0.01 |
| C 22:0 | ND | ND | ND | 0.66±0.03 | 0.03±0.01 |
| C 22:1 | ND | ND | ND | 0.10±0.02 | 0.04±0.02 |
| C 24:0 | ND | ND | ND | 0.26±0.03 | 0.010±0.002 |
aND= not detected.
Figure 1. Levels of saturated, monounsaturated and polyunsaturated fatty acids in oils and fats under study.
3.2. Nutritional assessment in rats
The obtained results during feeding study showed the effects of feeding diets containing different dietary fats and oils on the body weight gain, food intake and feed efficiency of rats. The results indicated that there were no significant differences in food consumption between groups by measuring each day's food intake (data not shown).
3.3. Liver and blood analysis
The results obtained from this study are represented in Tables 2, 3 and 4. No significant differences (P>0.05) were observed in SOD activity, vitamin E and TAC levels between the five groups. However, there was significant decrease of GPx activity in Groups IV and V when compared with other groups (P<0.001). The results indicated that the corn oil feeding caused significant increases in liver (P<0.001) and blood MDA (P<0.001) levels when compared with other oils. There were significant positive correlations between; SOD and GPx (P<0.001), between SOD and vitamin E (P<0.001), between SOD and TAG (P<0.001), between GPx and vitamin E (P<0.001), between GPx and TAC (P<0.001), between vitamin E and TAC (P<0.001) and between blood MDA and liver MDA (P<0.001). The results of our study showed significant negative correlations between blood MDA in one hand and SOD (P<0.001), GPx (P<0.001), vitamin E (P<0.001) and TAC (P<0.001) on the other hand. Also, there were significant negative correlations between liver MDA in one hand and SOD (P<0.001), GPx (P<0.001), vitamin E (P<0.001) and TAC (P<0.001) on the other hand.
Table 2. Blood SOD, GPx, vitamin E, TAC, MDA and liver MDA in studied groups.
| Parameter Groups | SOD (U/mg Hb) | GPx (U/L) | vitamin E (µmol/L) | TAC (mmol/L) | Blood MDA (µmol/L) | Liver MDA (pmol/mg protein) |
| Butter (group I) | 2.90±0.85 | 68.31±5.61 | 14.07±1.71 | 2.97±0.23 | 9.19±1.53 | 8.48±0.77 |
| Margarine (group II) | 2.67±0.68 | 66.22±6.83 | 13.25±1.65 | 2.90±0.18 | 9.03±1.64 | 8.51±0.78 |
| Olive oil (group III) | 2.77±0.69 | 70.48±7.05 | 12.54±1.69 | 3.05±0.23 | 9.74±1.56 | 8.37±0.78 |
| Sunflower oil (group IV) | 2.37±0.78 | 58.30±4.16 | 14.61±1.66 | 2.95±0.21 | 9.11±1.51 | 8.49±0.80 |
| Corn oil (group V) | 2.15±0.70 | 53.85±4.41 | 12.55±1.64 | 2.98±0.20 | 12.33±1.98 | 12.44 ±1.25 |
The values are expressed as mean±SD.
Table 3. Analysis of variance (F-Test) of the studied parameters in all groups.
| parameters | Group I | Group II | Group III | Group IV | Group V | F | P |
| Plasma SOD (U/mg Hb) | 2.90±0.85 | 2.67±0.68 | 2.77±0.69 | 2.37±0.78 | 2.15±0.70 | 1.75 | >0.01 |
| Plasma GPx (U/L) | 68.31±5.61 | 66.22±6.83 | 70.48±7.05 | 58.30±4.16 | 53.85±4.41 | 15.14 | <0.001 |
| Plasma vit E (µmol/L) | 14.07±1.71 | 13.25±1.65 | 12.54±1.69 | 14.61±1.66 | 12.55±1.64 | 3.03 | >0.01 |
| Plasma TAC (mmol/L) | 2.97±0.23 | 2.90±0.18 | 3.05±0.23 | 2.95±0.21 | 2.98±0.20 | 0.95 | >0.01 |
| Serum MDA (µmol/L) | 9.19±1.53 | 9.03±1.64 | 9.74±1.56 | 9.11±1.51 | 12.33±1.98 | 7.16 | <0.001 |
| Liver MDA (pmol/mg protein) | 8.48±0.77 | 8.51±0.78 | 8.37±0.78 | 8.49±0.80 | 12.44±1.25 | 48.25 | <0.001 |
P>0.01: non significant, P<0.001: highly significant. The values are expressed as mean±SD.
Table 4. Correlation between the studied parameters in all groups.
| Data | Plasma SOD | Plasma GPx | Plasma Vitamin E | Plasma TAC | Serum MDA | Liver MDA |
| Plasma SOD | r=+0.838 | r=+0.832 | r =+0.937 | r=-0.865 | r=-0.670 | |
| Plasma GPx | r=+0.838 | r=+0.536 | r =+0.743 | r=-0.800 | r=-0.795 | |
| Plasma Vitamin E | r=+0.832 | r=+0.536 | r=4-0.841 | r=-0.862 | r=-0.610 | |
| Plasma TAC | r=+0.937 | r=+0.743 | r=+0.841 | r=-0.796 | r=-0.571 | |
| Serum MDA | r=-0.865 | r=-0.800 | r=-0.862 | r=-0.796 | r=+0.897 |
4. Discussion
Vegetable oils with high PUFA levels were hitherto thought as the healthiest lipids because of their cholesterol lowering effects. However, recent studies indicate that PUFA are more susceptible to lipid peroxidation than SFA. Lipid peroxidation usually results in decreasing membrane fluidity, cell injury and may cause the formation of atherosclerotic plaques [16],[41]. Antioxidant enzymes (SOD and GPx) scavenge lipid peroxides and free radicals and detoxify them. No significant differences were detected when the activities of SOD and GPx were compared in the studied groups. In a similar study, although soyabean or olive oil diets modified the liver microsomal fatty acid phospholipid composition, SOD and GPx activities remained unchanged[42]. The level of the natural antioxidant (vitamin E) also determined which is important for the maintenance of good health. Although several studies have reported that vitamin E level can be sensitive to the composition of the diet, the results presented in our study indicated that vitamin E level was not affected by the type of dietary oils. The probability of lipid peroxidation increases with increasing number of double bonds in fatty acids.
As a result of the degradation of lipid peroxides, MDA is used as an indicator of lipid peroxidation[25]. Therefore, to better assess the effects of the dietary oils on lipid peroxidation, we measured the level of MDA in rats fed on dietary oils differed in the degree of fatty acid saturation. The results indicated that the highest level of MDA was observed in the group fed with corn oil which contains the highest amount of PUFA among the lipids supplemented to the diets. MDA levels were found to increase with increasing amounts of corn oil supplemented to a semi-synthetic diet[19]. In agreement with our results, Sarraga and Reguciro[43] also indicated that lipid peroxidation was higher in broilers fed sunflower oil composed of highest amount of PUFA among lipids tested, and it was very low in the group fed olive oil. The present results demonstrated significant negative correlation between blood and liver MDA (as a product of lipid peroxidation) on one hand and the antioxidant parameters on the other hand indicating that the high level of lipid peroxidation is accompanied by a decrease in the activities of the enzymes involved in antioxidant defense mechanism. There are numerous harmful effects of MDA, for example, by crosslinking with the membrane components, MDA causes inactivation of enzymes and receptors in membrane and thus changes membrane properties. Malondialdehyde also causes mutations by reacting with guanine nucleotidein DNA[12],[44].
In the light of evidence presented here, it is suggested that corn oil and, to a lesser degree, sunflower oil feeding increases lipid peroxidation significantly and thus challenge the antioxidant defense system and may increase the susceptibility of tissues to degradation products of lipid peroxides.
Acknowledgments
This research was funded by Qassum University, Kingdom of Saudi Arabia (Grant No. 559).
Comments
Background
Lipids are among the major sources of energy second to carbohydrates and are required for the absorption and transport of lipid-soluble vitamins through the bloodstream. As an important constituent of cell membranes, lipids also play specific roles in membrane signaling events and thus cell development certain lipids are indicators of cellular events and lipid concentration can represent physiological conditions of cells. Changing lifestyle increased liver injury and causes altered liver functions. Therefore, there is need to investigate the impact of different fat and oils on lipid peroxidation in liver and blood.
Research frontiers
The study showed that there were no significant differences in SOD activity, vitamin E and TAC levels between studied animal groups. There was significant decrease of GPx activity in some groups. Feeding corn oil caused significant increases in liver and blood MDA levels as compared with other oils and fats. There were positive correlations between SOD and GPx, vitamin E and TAC as well as between GPx and TAC and between blood MDA and liver MDA. The study showed also negative correlations between blood MDA on one hand and SOD, GPx, vitamin E and TAC on the other hand.
Related reports
Lipids are the most sensitive molecules to free radicals. As a result of lipid peroxidation, MDA can be formed in cell membranes. MDA shows both mutagenic and carcinogenic effects by changing membrane properties. Organisms protect themselves from harmful effects of free radicals by antioxidant defense mechanisms. The antioxidant system involves both enzymatic and non-enzymatic antioxidants. The fast step in the enzymatic system is SOD, which catalyzes the dismutation of superoxide anion (O2−) to H2C2. The conversion of H2O to H2O2 by either GPx or catalase forms the second step of enzymatic system. Superoxide dismutase and GPx enzyme activities and the balance between them are very crucial protection against oxidative stress. Lipid-soluble vitamin E is a non-enzymatic antioxidant which plays a significant role in the protection of the cell membrane and against LDL cholesterol as well. It can reduce free radicals and it has the most action to break the chain reaction in lipid peroxidation. The measure of TAC, which is the cumulative action of all the antioxidants present in plasma, provides an insight into the delicate balance in vivo between oxidants and antioxidants.
Innovations and breakthroughs
The results demonstrated that feeding oils rich in polyunsaturated fatty acids (PUFA) increases lipid peroxidation significantly and may raise the susceptibility of tissues to free radical oxidative damage.
Applications
From the literature review it has been found that lipid peroxidation, MDA (a genotoxic harmful degradative byproduct of lipid peroxidation) can be formed in cell membranes. The results support the use of monounsaturated oils and fats to reduce lipid oxidation and to reduce the susceptibility of tissues to free radical oxidative damage.
Peer review
This is a promising research work in which authors have studied the influence of dietary butter, margarine, olive oil, sunflower oil and corn oil on liver and blood lipid oxidation in albino rats by measuring MDA levels in blood and liver.
Footnotes
Foundation Project: Supported by Qassum University, Kingdom of Saudi Arabia (Grant No. 559).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Mohdaly AA, Smetanskaa I, Ramadan MF, Sarhanb MA, Mahmoud A. Antioxidant potential of sesame (Sesamum indicum) cake extract in stabilization of sunflower and soybean oils. Ind Crop Prod. 2011;34:952–959. [Google Scholar]
- 2.Ramadan MF, Zayed R, Abozid M, Asker MM. Apricot and pumpkin oils reduce plasma cholesterol and triacylglycerol concentrations in rats fed a high-fat diet. Grasasy Aceites. 2011;62:443–452. [Google Scholar]
- 3.Ramadan MF. Functional properties, nutritional value, and industrial applications of niger oilseeds (Guizotia abyssinica Cass.) Crit Rev Food Sci Nutr. 2012;52:1–8. doi: 10.1080/10408398.2010.486083. [DOI] [PubMed] [Google Scholar]
- 4.Ramadan MF. Antioxidant characteristics of phenolipids (quercetin-enriched lecithin) in lipid matrices. Ind Crop Prod. 2012;36:363–369. [Google Scholar]
- 5.Elsanhoty RM, Al-Turki IA, Ramadan MF. Screening of medium components by Plackett-Burman design for carotenoid production using date (Phoenix dactylifera) wastes. Ind Crop Prod. 2012;36:313–320. [Google Scholar]
- 6.Ramadan MF, Elsanhoty RM. Lipid classes, fatty acids and bioactive lipids of genetically modified potato Spunta with Cry V gene. Food Chem. 2012;133:1169–1176. [Google Scholar]
- 7.Ramadan MF. Physalis peruviana pomace suppresses high-cholesterol diet-induced hypercholesterolemia in rats. Grasasy Aceites. 2012;63:411–422. [Google Scholar]
- 8.Abd El-Gleel W, Hassanien MF. Antioxidant properties and lipid profile of Diplotaxis harra, Pulicaria incisa and Avicennia marina. Acta Alimentaria. 2012;41:143–151. [Google Scholar]
- 9.Ramadan MF, Asker MM, Tadros M. Antiradical and antimicrobial properties of cold-pressed black cumin and cumin oils. Eur Food Res Technol. 2012;234:833–844. [Google Scholar]
- 10.Hassanien MF. Tcool and phytosterol composition of edible oils in the Egyptian market. J Food Proc Preserv. 2012;36:531–538. [Google Scholar]
- 11.Wolfrum C, Spener F. Fatty acids as regulators of lipid metabolism. Eur J Lipid Sci Technol. 2000;102:746–762. [Google Scholar]
- 12.Hur S, Nam DM, Williamson K, Ahn M. Effect of dietary fats on blood cholesterol and lipid and the development of atherosclerosis in rabbits. Nutr Res. 2009;25:925–935. [Google Scholar]
- 13.Ramadan MF, Amer MM, Awad A. Coriander (Coriandrum sativum L.) seed oil improves plasma lipid profile in rats fed diet containing cholesterol. Eur Food Res Technol. 2008;227:1173–1182. [Google Scholar]
- 14.Chisholm A, Mann J, Sutherland W, Duncan A, Frampton C. Effect of lipoprolein profile of replacing butter with margarine in a low fat diet. Brit Med J. 1996;312:931–934. doi: 10.1136/bmj.312.7036.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Law MR. Plant sterol and stanol margarines and health. West J Med. 2000;173:43–47. doi: 10.1136/ewjm.173.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kris-Etherton PM. Monounsaturated fatty ticids and risk of cardiovascular disease. Circulation. 1999;100:1253–1258. doi: 10.1161/01.cir.100.11.1253. [DOI] [PubMed] [Google Scholar]
- 17.Ramadan MF. Healthy blends of high linoleic sunflower oil with selected cold pressed oils: Functionality, stability and antioxidative characteristics. Ind Crops Prod. 2013;43:65–72. [Google Scholar]
- 18.Howel TJ, Mac-Dougall DE, Jones P. Phytosterol partially explain differences in cholesterol metabolism caused by corn or olive oil feeding. J Lipid Res. 1998;39:892–900. [PubMed] [Google Scholar]
- 19.Lu YF, Lu S. Influence of dietary fat saturation on lipid peroxidation of serum and low density lipoprotein in rats. Nutr Res. 2002;22:463–472. [Google Scholar]
- 20.Ramadan MF, Amer MM, Awad A. Changes of lipid profile by dietary vegetable oil blends in rats with hypercholesterolemia. Food Sci Technol Int. 2009;15:119–130. [Google Scholar]
- 21.Halliwell B. Free radicals antioxidants and human disease: curiosity, cause or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 22.Mates JM, Pérez-Gómez C, de Castro IN. Antioxidant enzymes and human diseases. Clinic Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 23.Girotti AW. Lipid hydroperoxide generation turnover and effect action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 24.Nielsen F, Mikkelsen B, Nielsen A, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effect of life-style factors. Clinic Chem. 1997;43:1204–1214. [PubMed] [Google Scholar]
- 25.Gerbhart R. Oxidative stress, plant derived antioxidants and liver fibrosis. Planta Medica. 2002;68:289–296. doi: 10.1055/s-2002-26761. [DOI] [PubMed] [Google Scholar]
- 26.DC-Haan IB, Cristiano F, Tannello R, Bladier C, Kelner MJ, Kola L. Elevation of ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. J Hum Mol Gene. 1996;5:283–292. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- 27.Hefnawy HT, Ramadan MF. Protective effects of Lactuca sativa ethanolic extract on carbon tetrachloride induced oxidative damage in rats. Asian Pac J Trop Dis. 2013;3:277–285. [Google Scholar]
- 28.Milne GL, Seal JR, Havrilla CM, Wijtmans M, Porter NA. Identification and analysis of products formed from phospholipids in the free radical oxidation of human low density lipoproteins. J Lipid Res. 2005;46:307–319. doi: 10.1194/jlr.M400311-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Ramadan MF, Asker MM, Ibrahim ZK. Functional bioactive compounds and biological activities of Spirulina platensis lipids. Czech J Food Sci. 2008;26:211–222. [Google Scholar]
- 30.Miller NJ, Paganga C, Wiseman S, Nielen W, Tijburg L, Catherina A, et al. et al. Total antioxidant activity of low density lipoproteins and the relationship with a-tocoferol status. FEBS Letters. 1995;365:164–166. doi: 10.1016/0014-5793(95)00448-i. [DOI] [PubMed] [Google Scholar]
- 31.Ramadan MF. Quercetin increases antioxidant activity of soy lecithin in a triolein model system. LWT-Food Sci Technol. 2008;41:581–587. [Google Scholar]
- 32.Ramadan MF, Kinni SG, Seshagiri M, Mörsel J-T. Fat-soluble bioactives, fatty acid profile and radical scavenging activity of Semecarpus anacardium seed oil. J Am Oil Chem Soc. 2010;87:885–894. [Google Scholar]
- 33.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 34.Yoshioka T, Kawada K, Shimada T, Mori M. Lipid per oxidation in maternal and cord blood and protective mechanism against activated oxygen toxicity in blood. Am J Obstet Gynacol. 1979;135:372–376. doi: 10.1016/0002-9378(79)90708-7. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clinic Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 36.Thurnham DI, Smith E, Flora PS. Concurrent liquid-chrotnatographic assay of retinol, alpha-tocopherol, beta-carotene, alpha-carotene, lycopene, and beta- cryptoxanthin in plasma, with tocopherol acetate as internal standard. Clinic Chem. 1988;34:377–381. [PubMed] [Google Scholar]
- 37.Koracevic D, Koraacevic G, Djordjevic S, Andrejevic S. Method for the measurement of antioxidant activity in human fluids. J Clinic Path. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohdaly AA, Sarhan MA, Mahmoud A, Ramadan MF, Smetanska I. Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection. Food Chem. 2010;123:1019–1026. [Google Scholar]
- 39.Vijaimohan K, Jainu M, Sabitha KE, Subramaniyam S, Anandhan C, Shyamala Devi CS. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci. 2006;79:448–454. doi: 10.1016/j.lfs.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Ramadan MF, Wahdan KM. Blending of corn oil with black cumin (Nigella sativa) and coriander (Coriandrum sativum) seed oils: Impact on functionality, stability and radical scavenging activity. Food Chem. 2012;132:873–879. [Google Scholar]
- 41.Oluba OM, Adeyemi O, Ojieh GC, Isiosio IO. Fatty acid composition of Citrullus lanatus (egusi melon) and its effect on serum lipids and some serum enzymes. Inter J Cardio Res. 2008;5:2–10. [Google Scholar]
- 42.Yuan YV, Kilts DD, Godin DV. Variations in dietary fat and cholesterol intakes modify antioxidant status of SRR and WKY rats. J Nutr. 1998;128:1620–1630. doi: 10.1093/jn/128.10.1620. [DOI] [PubMed] [Google Scholar]
- 43.Sarraga JC, Regueiro JA. Membrane lipid oxidation and proteolylic activity in thigh muscles from broilers fed different diets. Meat Sci. 1999;52:3–9. doi: 10.1016/s0309-1740(98)00170-3. [DOI] [PubMed] [Google Scholar]
- 44.Otteneder M, Plaslaras JP, Marnett LJ. Reaction of malondialdehyde-DNA adducts with hydrmines - Development of a facile assay for quantification of malondialdehyde equivalents in DNA. Chem Res Toxicol. 2002;15:312–318. doi: 10.1021/tx010105v. [DOI] [PubMed] [Google Scholar]


