Abstract
AIM: To identify molecular biologic differences between two gastric adenocarcinoma subgroups presenting different prognoses through the analysis of microRNA and protein expression.
METHODS: Array technologies were used to generate 1146 microRNAs and 124 proteins expression profiles of samples from 60 patients with gastric cancer. For the integrative analysis, we used established mRNA expression data published in our previous study. Whole mRNA expression levels were acquired from microarray data for 60 identical gastric cancer patients. Two gastric adenocarcinoma subgroups with distinct mRNA expression profiles presented distinctly different prognoses. MicroRNA and protein expression patterns were compared between gastric cancer tissue and normal gastric tissue and between two different prognostic groups. Aberrantly expressed microRNA, associated mRNA, and protein in patients with poor-prognosis gastric cancer were validated by quantitative reverse transcription polymerase chain reaction and immunochemistry in independent patients.
RESULTS: We obtained the expression data of 1146 microRNAs and 124 cancer-related proteins. Four microRNAs were aberrantly expressed in the two prognostic groups and in cancer vs non-cancer tissues (P < 0.05). In the poor-prognosis group, miR-196b, miR-135b, and miR-93 were up-regulated and miR-29c* was down-regulated. miR-196b expression positively correlated with Homeobox A10 (HOXA10) expression (r = 0.726, P < 0.001), which was significantly increased in poor-prognosis patients (P < 0.001). Comparing gastric cancer with non-cancer tissues, 46/124 proteins showed differential expression (P < 0.05); COX2 (P < 0.001) and cyclin B1 (P = 0.017) were clearly over-expressed in the poor-prognosis group.
CONCLUSION: Co-activation of miR-196b and HOXA10 characterized a poor-prognosis subgroup of patients with gastric cancer. Elucidation of the biologic function of miR-196b and HOXA10 is warranted.
Keywords: Gastric cancer, Gene expression, Microarray, MicroRNA, miR-196b, Homeobox A10
Core tip: Using an integrative analysis of the tumor transcriptome and protein expression profiles obtained by microarray techniques, we have elucidated the molecular biologic characteristics of a poor prognosis gastric cancer subgroup. Overexpression of miR196b and Homeobox A10 are implicated in gastric cancer, particularly in tumors with poor prognosis features. We anticipate this integrative approach will contribute to the characterization of cancer heterogeneity and the development of personalized therapy through the identification of cancer targets.
INTRODUCTION
Gastric cancer is the second leading cause of cancer-related deaths worldwide[1]. Surgery is the only curative treatment strategy and conventional chemotherapy has shown limited efficacy for the treatment of patients with advanced disease. Understanding the molecular mechanisms governing carcinogenesis, progression, and prognosis in gastric cancer is a prerequisite for the construction of a convincing management strategy. It is widely acknowledged that there are favorable and poor-prognosis subtypes of cancer, although the treatment strategy is generally determined by clinical and histopathologic stage rather than disease type. Patients with gastric cancer are also clinically heterogeneous, which suggests an underlying molecular diversity[2,3]. Molecular subtypes of gastric cancer have been suggested through analysis of gene or protein expression profiles and oncogenic signaling pathways[4-9]. In a previous study, we identified distinct gastric cancer subclasses by analyzing gene expression profiles and we described two gastric cancer subtypes that were strongly associated with prognosis[10].
MicroRNAs play a role in the pathogenesis of various human cancers[11]. Some microRNAs function as oncogenes and others as tumor suppressors, although their mechanisms remain to be elucidated[12,13]. The relationship between microRNA expression profile and gastric cancer prognosis has been actively explored[14,15], as has the pathogenesis of gastric cancer[16,17]. Overexpression of miR-196b has been linked to leukemia and several solid cancers including gastric cancer and may represent a useful gastric cancer marker[18,19]. A role for miR-196b as an oncogene or tumor suppressor has not yet been confirmed[20], nor has its role in gastric carcinogenesis and progression.
Homeobox A10 (HOXA10) is transcription factor involved in the proliferation of hematopoietic stem cells and progenitor cells. Its over-expression is associated with cancer development and poor prognosis in patients with acute myeloid leukemia and solid cancers[21-23]. HOXA10 is a neighboring gene of miR-196b but these genes have no known function in gastric cancer pathogenesis.
In this study, we generated microRNA and protein expression profiles using samples from 60 patients with gastric cancer to identify molecular biologic differences between previously identified good- and poor-prognosis patient subgroups.
MATERIALS AND METHODS
Patients and samples
Tumor specimens and clinical data were obtained from patients with primary gastric adenocarcinoma who underwent curative gastrectomy as the primary treatment between 1999 and 2007 at Severance Hospital and Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea. Three independent patient cohorts were used for each experiment: Group 1 (frozen tissue samples from 60 patients, each of whom provided a cancer sample and 8 of whom provided a non-cancer sample) for microRNA, and protein microarray analysis (Table 1); Group 2 (12 paired cancer and non-cancer frozen tissue samples from 12 patients) for validation tests using quantitative reverse transcription polymerase chain reaction (qRT-PCR); and Group 3 (paraffin-embedded samples from 368 patients with gastric cancer) for tissue microarray immunohistochemistry (IHC). Frozen tissue samples were examined by pathologists at the time of collection and were stored below -80 °C at a tissue bank until the analysis. Samples were collected after obtaining written informed consent from the patients. The study was approved by the institutional review board of Yonsei University Health System.
Table 1.
Clinicopathologic factors of patients with gastric cancer participating in the microarray analysis n (%)
| Characteristic | Group 1 (n = 60) | miR-196b high (n = 28) | miR-196b low (n = 29) | P value | HOXA10 high (n = 29) | HOXA10 low (n = 28) | P value |
| Age, yr | |||||||
| Median (range) | 63 (32-83) | 66 (34-78) | 58 (32-83) | 0.134 | 64 (34-83) | 60 (32-78) | 0.134 |
| > 65 | 15 (52) | 9 (32) | 15 (52) | 9 (32) | |||
| Sex | |||||||
| Male | 41 (68) | 19 (68) | 20 (69) | 0.928 | 18 (62) | 21 (75) | 0.294 |
| Histologic type | |||||||
| Diffuse | 27 (45) | 9 | 17 | 0.066 | 8 | 18 | 0.007 |
| Intestinal | 23 (38) | 14 | 9 | 16 | 7 | ||
| Mixed | 10 (17) | 5 | 3 | 5 | 3 | ||
| T stage | |||||||
| T1/T2 | 26 (43) | 10 (36) | 14 (48) | 0.337 | 11 (38) | 13 (46) | 0.516 |
| T3/T4 | 34 (57) | 18 (64) | 15 (52) | 18 (62) | 15 (54) | ||
| N stage | |||||||
| N0/N1 | 36 (60) | 15 (54) | 19 (66) | 0.358 | 16 (55) | 18 (64) | 0.483 |
| N2/N3 | 24 (40) | 13 (46) | 10 (34) | 13 (45) | 10 (36) | ||
| AJCC stage1 | |||||||
| I/II | 23 (38) | 11 (39) | 12 (41) | 0.872 | 11 (38) | 12 (43) | 0.705 |
| III/IV | 37 (62) | 17 (61) | 17 (59) | 18 (62) | 16 (57) |
Based on the American Joint Committee on Cancer staging manual 6th edition. HOXA10: Homeobox A10; AJCC: American Joint Committee on Cancer.
MicroRNA microarray
Total RNA was extracted from fresh frozen tissues using a mirVanaTM miRNA isolation kit (Ambion, Austin, TX, United States). Microarray experiments were performed according to the manufacturer’s protocols (Illumina microRNA Expression Profiling Assay; Illumina, San Diego, CA, United States). The panel contained 1146 miRNA assays described in the Sanger Institute miRBase Release 12.0 (from miR-1 to miR-1827)[24]; 200 ng RNA was used for labeling and hybridization. Chips were scanned with an Illumina BeadArray Reader (Illumina, San Diego, CA, United States) and intensity values were analyzed using BeadStudio version 3.1.3. A cut-off detection P value of 0.01 was used to determine whether a microRNA probe was significantly detected. Microarray data were normalized using the quantile normalization method. MicroRNA microarray data is available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) public database (accession number GSE26595).
Protein expression assay
Reverse-phase protein array (RPPA) was performed according to the manufacturer’s protocol and the data were analyzed as previously described[25]. Frozen tissues were lysed in RPPA lysis buffer and complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Protein-containing samples were diluted to a uniform protein concentration and transferred into 384-well plates. The lysate was printed onto nitrocellulose-coated glass slides and probed using 124 antibodies. Signal intensity was measured by scanning the slides: slides were scanned, analyzed, and quantified using the customized Microvigene software (VigeneTech, Inc., Carlisle, MA, United States) to measure spot intensity.
Analysis of microarray data
The microarray expression level was transformed into a log 2 base before further analysis. Cluster analysis was performed after median centering using the Cluster 3.0 program and visualized in Treeview (Eisen Lab. CA, United States). BRB-ArrayTools (version 3.6; Biometrics Research Branch, National Cancer Institute, MD, United States) were used for microarray data analysis[26]. The previously generated gene expression data from the poor-prognosis group[10] were obtained from the NCBI GEO public database (accession number GSE13861).
Quantitative RT-PCR and analysis
Quantitative RT-PCR was carried out according to the manufacturer’s protocols (miRURY LNA microRNA PCR System, Exiqon, Vedbaek, Denmark) and was performed in duplicate. The RT reactions were incubated for 30 min at 50 °C followed by heat inactivation at 85 °C for 5-10 min. cDNA templates were diluted and added to the PCR master mix. Cycling conditions were as follows: 37 °C for 10 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 20 s and 60 °C for 60 s. RT-PCR was performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, United States) with a 96-well block module. Relative amounts of microRNA were calculated from the threshold cycle (CT) number using the expression of U6 snRNA for normalization.
Tissue microarray construction and IHC staining
Paraffin-embedded tissue microarray blocks were created using gastric cancer tissue specimens obtained from 368 patients. A tissue microarray set contained 14 slides with 30 tissue cores per slide. Sections 4 μm thick were deparaffinized and treated to block endogenous peroxidase activity. After antigen retrieval, the primary antibody - anti-HOXA10 antibody (polyclonal, dilution 1:75; Santa Cruz Biotechnology, Santa Cruz, CA, United States) - was applied to the sections. The sections were incubated with a secondary antibody (horse radish peroxidase-rabbit/mouse), developed using a NovaRED substrate kit (VECTOR Laboratory, Burlingame, CA, United States) and counterstained with Harris hematoxylin. Stained slides were examined with a Zeiss Axio Imager M2 microscope with an AxioCam HRc camera and photographed using AxioVision 4.8.2 software (Zeiss, Jena, Germany). Strong cytoplasmic staining was considered as positive expression. Positive expression was defined as staining stronger compared to smooth muscle. Negative expression was defined as staining positivity lower than or similar to smooth muscle.
Statistical analysis
Statistical analysis was performed with PASW Statistics 18 (SPSS Inc., San Diego, United States) and graphical interpretations were generated with GraphPad Prism 5 (GraphPad Software, San Diego, CA, United States). The Kaplan-Meier method was used to estimate patient prognosis; differences between genotypes were compared using the log-rank test. Differences between groups were analyzed using the two-tailed Student’s t test and the χ2 test. Associations between the expression levels of the two targets were analyzed using the Pearson correlation coefficient. Generally, differences were considered statistically significant at P < 0.05.
RESULTS
Gene expression microarray-established gastric cancer subgroups C1 and C2
In our previous study[10], two gastric cancer subgroups [a poor-prognosis group C1 (n = 32) and a good-prognosis group C2 (n = 28)] were established through unsupervised hierarchical clustering of gene expression data from the 60 patients with gastric cancer in Group 1. Genes with expression levels that were at least 2-fold different in at least 15 tissues, relative to the median value across tissues, were selected for hierarchical clustering analysis (2077 gene features) (Figure 1A). Following comparison of gene expression profiles of samples from C1, C2, and non-cancer patients, unique gene sets were identified for the C1 and C2 groups that contained 2755 and 1437 genes, respectively (P < 0.001). C1 patients had significantly poorer relapse-free survival than C2 patients (P = 0.0019; log-rank test). This was independent of disease stage, indicating that the molecular features reflected in gene expression patterns might be strong independent predictors of clinical outcome (Figure 1B). Gene expression data for 5922 transcription factor (TF) genes were selected and C1-specific TF genes were extracted (Figure 1C). A total of 413 up-regulated and 326 down-regulated TF genes are shown in Figure 1D; the top 20 up- and down-regulated TF genes are listed in Figure 1E.
Figure 1.
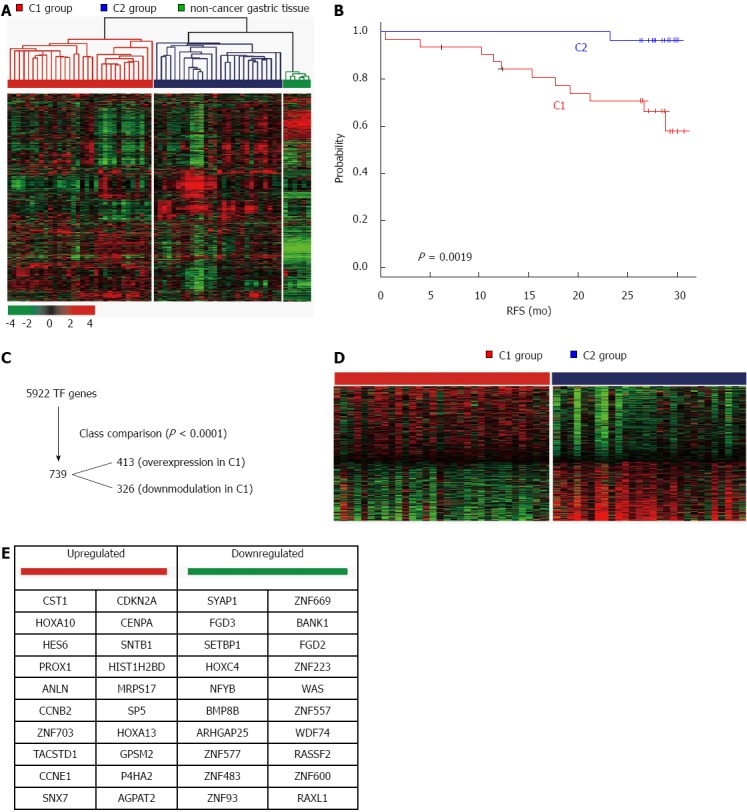
Two prognostic groups of patients with gastric cancer, C1 and C2, have distinct mRNA expression patterns. A: mRNA expression patterns of gastric cancer and non-cancer gastric tissue are depicted in a heat map. Hierarchical clustering revealed two subtypes of gastric cancer, C1 and C2. The data are presented as a matrix in which rows represent individual mRNAs and columns represent each tissue. The color red represents relatively high expression levels and green reflects lower expression levels; B: Kaplan-Meier plot of relapse-free survival (RFS) revealed C1 and C2 represented poor- and good-prognosis gastric cancer tissues, respectively; C: Workflow of C1-specific 739 transcription factors (TFs) selected from a total 5,922 TFs; D: Hierarchical clustering of gene expression for 739 TF genes; E: List of top 20 up- and down-regulated TF genes in the C1 group.
microRNA expression profile of gastric cancer
A total of 410 out of 1146 probes in the microRNA expression assay were significantly detected (P < 0.01) and further processed (Figure 2A). Though unsupervised clustering analysis revealed two major subtypes, these did not appear to have any prognostic or clinico-pathologic value. Subsequently, 164 microRNAs significantly (P < 0.05 in the t test) aberrantly expressed in gastric cancer tissue were identified (Figure 2A). Non-cancer tissue showed relatively homogenous microRNA expression patterns, in contrast to the highly heterogeneous expression patterns observed in cancer tissues.
Figure 2.
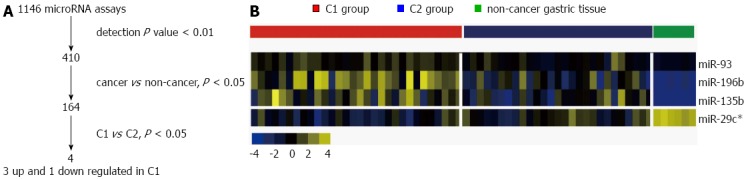
Aberrant microRNA expression in gastric cancer. A: Flowchart of the data analysis to identify poor-prognosis C1-specific microRNAs. Four microRNAs (miR-196b, miR-135b, miR-93, and miR-29c*) were selected; B: Expression patterns of the four microRNAs are shown in a heat map for C1, C2, and non-cancer gastric tissues. The color yellow represents relatively high expression levels and blue reflects relatively low levels.
Aberrant microRNA expression in C1 and C2 patients
MicroRNA expression data were compared between non-cancer and cancer tissues and between C1 and C2 samples. Four microRNAs were uniquely dysregulated (P < 0.05) among non-cancer, C1, and C2 samples (Figure 2A). miR-196b, miR-135b, and miR-93 were up-regulated and miR-29c* was down-regulated in the C1, poor-prognosis group (Figure 2B). Log-transformed expression levels are shown in Figure 3A. miR-196b showed the most significantly different expression patterns between cancer and non-cancer samples (P < 0.001), and between the C1 and C2 groups (P < 0.001).
Figure 3.
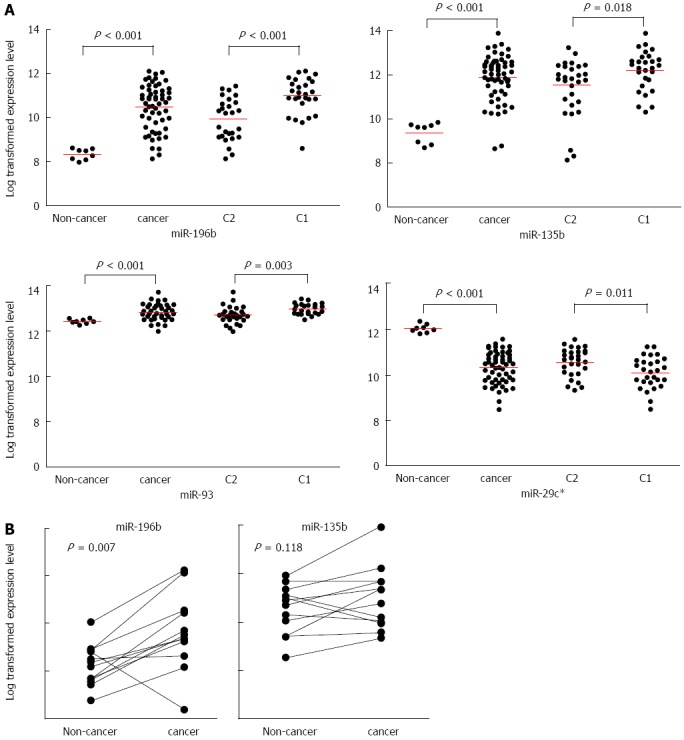
The expression levels of four microRNAs. A: Four microRNAs expressed differentially in gastric tissues. Scatter plots of gene expression levels with a bar indicating the mean; B: The expression level of miR-196b and miR-135b were validated in a quantitative reverse transcription polymerase chain reaction study of 12 patients samples.
Validation of microRNA expression by quantitative RT-PCR
To validate the microRNA expression microarray data, qRT-PCR was performed using RNA from the 12 patients in Group 2. Using CT numbers, miR-196b was determined to be up-regulated in gastric cancer tissue compared with non-cancer tissue (P = 0.007; two-sample paired t test; Figure 3B). The up-regulation of miR-135b in gastric cancer samples was not statistically significant (P = 0.118).
Correlation between HOXA10 and miR-196b expression
HOXA9 and HOXA10 are neighboring genes to miR-196b (Figure 4A) and are regarded as miR-196b-associated genes. Therefore, the expression level of HOXA9 and HOXA10 and their correlation with miR-196b expression were evaluated. Analysis of microRNA and mRNA expression data from gastric cancer tissue samples obtained from patients in Group 1 showed that HOXA10 mRNA expression was positively correlated with miR-196b expression (Figure 4B; r = 0.726, P < 0.001). HOXA10 expression was up-regulated in gastric cancer compared with non-cancer tissue and was higher in C1 compared with C2 patients (Figure 4C). Overexpression of miR-196b was significantly associated with shorter overall survival (OS; P = 0.022; Figure 4D). Overexpression of HOXA10 exhibited a tendency to poor OS (P = 0.202; Figure 4D) but HOXA9 did not exhibit any significant association with miR-196b or prognosis (Figure 4B and D). HOXA10 protein-positive expression was identified in 56 of 368 gastric cancer tissue samples (15.2%) from patients in Group 3 (Figure 5A and B). In non-cancer gastric tissue, body glands were stained variably. In contrast to well and moderately differentiated adenocarcinoma, poorly differentiated adenocarcinoma and signet ring cell carcinomashowed less positive staining for HOXA10 (29.4% vs 8.4%, P < 0.001).
Figure 4.
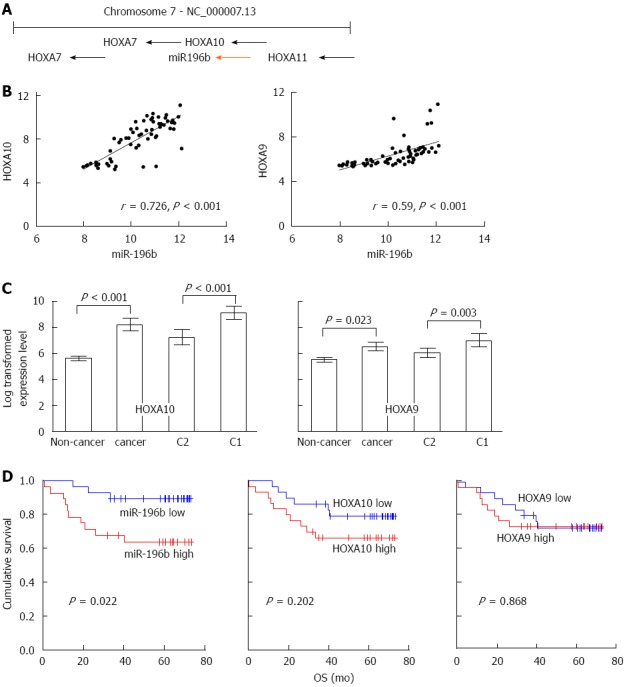
The correlation between miR-196b and homeobox A10 expression in gastric cancer. A: miR-196b, homeobox A10 (HOXA10), and HOXA9 are neighbor genes and located on chromosome 7p15.2; B: Expression of miR-196b and HOXA10 were positively correlated (r = 0.726; P < 0.001). The correlation between miR-196b and HOXA9 expression was less significant; C: HOXA10 and HOPXA9 expression level in gastric cancer vs non-cancer tissues and in the C1 vs the C2 group. Results are presented as means with 95%CI; D: Kaplan–Meier plot of overall survival according to the expression level of miR-196b, HOXA10, and HOXA9.
Figure 5.
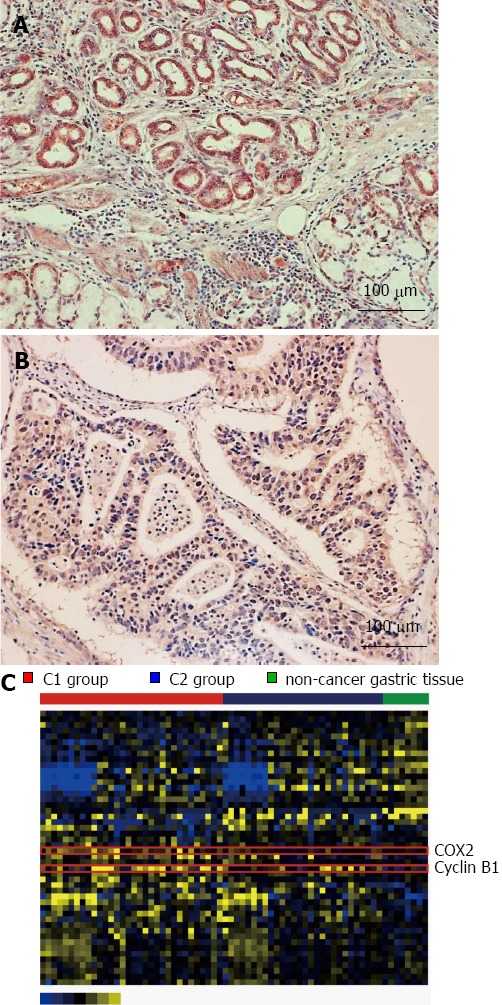
Aberrant protein expression in gastric cancer. A: Representative image from the homeobox A10 (HOXA10) immunohistochemical assay (× 200) of gastric cancer tissue. The moderately differentiated adenocarcinoma shows positive for HOXA10 expression. A strong cytoplasmic immunoreaction was noted; B: Negative for HOXA10 expression; C: The expression pattern of 48 proteins from Reverse-phase protein array (RPPA) was depicted using a heat map. The color yellow represents relatively high expression levels and blue reflects relatively low levels.
Protein expression profile of gastric cancer
The unsupervised clustering analysis revealed that protein expression was highly variable compared with mRNA and microRNA expression in non-cancer and cancer tissues. Two-group two-sample t tests comparisons were performed using cancer and non-cancer samples to identify gastric cancer-specific proteins. Univariate analyses revealed 48 out of 124 proteins with significantly different levels (P < 0.05) between cancer and non-cancer tissues (Figure 5B). Among these 48 proteins, the expression of COX2 and cyclin B1 was significantly increased in gastric cancer vs non-cancer tissue (both P < 0.001), and up-regulated in the C1 compared with C2 groups (P < 0.001 for COX2 and P = 0.016 for cyclin B1).
DISCUSSION
We previously identified two prognostic subgroups of patients with gastric cancer through gene expression microarray technology[10]. Our findings suggested that their gene expression signatures reflected clinical differences between good- and poor-prognosis patients independent of tumor-node-metastasis stage and histology. The molecular biologic mechanisms responsible for these clinical differences were unknown. In an effort to understand these molecular mechanisms, we obtained microRNA and protein expression data from tumor tissue samples using high-throughput microarray techniques.
First, two-group comparison analysis revealed 164 microRNAs that showed different expression profiles in the cancer and non-cancer tissue samples. Supervised clustering (C1 vs C2 vs non-cancer) using these 164 microRNAs revealed that non-cancer tissues manifested relatively homogenous microRNA expression. A comparison of the microRNA expression revealed that three microRNAs (miR-196b, miR-135b, and miR-93) were up-regulated in C1 vs C2 and in cancer vs non-cancer tissues. Conversely, miR-29c* was down-regulated in C1 samples. We suggest, therefore, that dysregulation of those four microRNAs might characterize C1, our previously identified poor-prognosis gastric cancer subgroup. The accumulated knowledge about microRNA-associated genes and cancers is summarized in Table 2.
Table 2.
microRNAs differentially expressed in gastric cancer tissues; non-cancer tissue and C1 and C2 groups (P < 0.05)
| Name | Chromosome location | Function | Related gene | Related cancer | References |
| Expression level: C1 > C2 > non-cancer | |||||
| hsa-miR-196b | 7p15.2 | Tumor suppressor, progression | HOXA, HOXC8 | T-cell ALL, MLL-rearranged leukemia, ALL, stomach, lung, pancreas | 18,19,27,28 |
| hsa-miR-135b | 1q32.1 | Tumorigenesis | FOXO1, APC | Leukemia, anaplastic large cell lymphoma, pancreas, colon, prostate, osteosarcoma | 29-31 |
| hsa-miR-93 | 7q22.1 | Tumorigenesis, progression, prognosis | E2F1, ZBTB4, FUS1, TP53INP1, ITGB8, VEGFA, CDKN1A | Kidney, breast, colon, liver, stomach, lung, osteosarcoma, adult T-cell leukemia | 17,32-37 |
| Expression level: C1 < C2 < non-cancer | |||||
| hsa-miR-29c* | 1q32.2 | Prognosis | Malignant pleural mesothelioma | 38 | |
ALL: Acute lymphoblastic leukemia; MLL: Mixed lineage leukemia gene; HOXA10: Homeobox A10.
The overexpression of miR-196b has previously been linked to leukemia and several solid cancers[18,19]. The increased expression of miR-196b contributes to the development of leukemia and the relationship between differential miR-196b expression and clinical and biologic features has been studied in leukemia[18,39-41]. miR-196b expression is also increased in pancreatic ductal adenocarcinoma and bronchial squamous cell carcinoma, although its role in these cancers has not been established[27,28]. Tsai et al[19] reported that a lack of promoter methylation in miR-196b resulted in its overexpression in gastric cancer cell lines and patient samples, and they proposed that miR-196b overexpression might provide a useful tumor marker. However, it has not yet been determined whether miR-196b functions as an oncogene or a tumor suppressor[20], and its role in gastric carcinogenesis and progression has not yet been identified.
In recent in vitro study, a leukemia oncogene was found to promote the expression of miR-135b, with elevated miR-135b expression decreasing chemosensitivity, suggesting the contribution of miR-135b to the oncogenic activity of the leukemia oncogene[29]. It has been suggested that oncogenic kinase-linked miR-135b contributes to tumorigenesis through modulation of the tumor-immune phenotype and microenvironment[29]. Others have identified miR-135b as a novel biomarker for pancreatic ductal adenocarcinoma[30]. In chronically inflamed or genetic models of colon cancer, miR-135b was suggested along with other microRNAs, to regulate signaling pathways related to mitogen-activated protein kinase, phosphoinositide 3-kinase, WNT, and transforming growth factor (TGF)-β, all of which are known to be involved in cell transformation[31]. However, there are as of yet no data relating to the pathogenesis of gastric cancer.
miR-93 is a well-known constituent of the miR-106b approximately 25 cluster, paralogs of which have oncogenic activity in several cancers, including gastric cancer. Up-regulated miR-93 in gastric cancer leads to cell cycle activation and impaired apoptosis[17,32]. miR-29c* is, however, poorly understood with no known target and it has only been shown to be an independent favorable prognostic factor in malignant pleural mesothelioma[38].
Information about each of these microRNAs provides support for the proposal that our poor-prognosis microRNA signature - up-regulation of miR-196b, miR-135b, and miR-93 and down-regulation of miR-29c* - is relevant. As miR-93 has largely been investigated in gastric cancer and miR-29c* is poorly understood, we focused our attention on miR-196b and miR-135b. The qRT-PCR assay demonstrated that miR-196b was significantly overexpressed in cancer vs non-cancer tissue while miR-135b was not (Figure 3B). Therefore, the role of miR-196b in gastric cancer was further investigated.
Using correlation analysis, we identified that HOXA10 levels were also elevated in gastric cancer. The expression of miR-196b appears to be most positively associated with HOXA10 expression. Our previous gene expression microarray data indicated that HOXA10 was ranked as the second most up-regulated TF in gastric cancer (Figure 1E), as it was significantly elevated in gastric cancer vs non-cancer and in poor-prognosis C1 vs good-prognosis C2. Evidence is accumulating that HOXA10 is involved in the proliferation of hematopoietic stem cells and progenitor cells, leading to cancer development through activating the target genes coding for TGFβ2, dual-specificity protein phosphatase 4, and integrin-β3[21,42,43]. Up-regulation of HOX genes is associated with a tumor stem-like cell phenotype of glioblastoma, and high HOXA10 protein expression has been associated with resistance to chemotherapy[44]. HOXA10 is also over-expressed in a poor-prognosis subset of patients with acute myeloid leukemia and in several solid cancers[21-23]. On the basis of these findings, we postulate that HOXA10 may induce gastric carcinogenesis and function as a marker of poor prognosis. Recently, it was reported that upregulation of HOXA10 was correlated with favorable prognosis and inversely correlated with the depth of invasion in gastric cancer[45]. Different patient populations and detection methods for HOXA10 seems to be responsible for inconsistent results. More reliable evidences should be accumulated to elucidate the role of HOXA10 in gastric cancer.
miR-196b is located in the HOXA cluster on chromosome 9 and expression levels of pri-miR-196b are highly correlated with its neighboring gene HOXA10[18]. A previous study showed that expression patterns of miR-196b were inversely correlated with the methylation status of promoter CpG-rich regions in gastric cancer cell lines and primary gastric cancer samples[19]. Suzuki et al[46] analyzed genome-wide profiling of chromatin signatures combining microRNA expression and suggested that DNA demethylation can alter the chromatin signatures of numerous microRNAs in cancer. Elevated expression of miR-196b in gastric cancer cells may result from the preferential unmethylated status of CpG islands and from other TFs. We suggest that HOXA10 expression might also be governed by miR-196b modulation; however, the exact mechanism of miR-196b promoter activity modulation by TFs remains to be elucidated.
In the present study, miR-196b and HOXA10 were co-activated in gastric cancer samples, especially in the poor-prognosis group. miR-196b and HOXA10 are also closely related to the development and progression of hematopoietic stem cells. This suggests that gastric cancer co-activated by miR-196b and HOXA10 might share the same pathogenesis as leukemia. Chronic inflammation caused by Helicobacter pylori (H. pylori) is associated with gastric carcinogenesis and there is recent evidence that H. pylori infection recruits bone marrow-derived cells (BMDCs) in the gastric epithelial mucosa that participate in gastric preneoplasia[47,48]. Over-expression of miR-196b and HOXA10 may mediate the development of hematopoietic progenitor cells and gastric cancer; thus, it is necessary to elucidate the mechanism and function of the co-activation of miR-196b and HOXA10 in gastric cancer.
RPPA is a quantitative assay that analyzes large samples for known proteins and their active forms. We evaluated the expression of 124 proteins to understand the physiology and pathogenesis of gastric cancer at the protein level. The limitations of this platform, measuring only small numbers of known antibodies, were taken into consideration when interpreting the data. As shown in Figure 5B, protein expression patterns are highly heterogeneous, even between gastric cancers, indicating that a few tumor targets will apply to almost all patients with gastric cancer and that most markers are likely to only apply to selected patients. In the present study, COX2 and cyclin B1 were significantly elevated in the C1 group compared with the C2 group. In combination with gene expression array data, the HOXA10 promoter has a nuclear factor-κB (NFκB) binding site, and NFκB controls COX2 expression, leading to inflammation and carcinogenesis. The elucidation of this relationship might explain the mechanism of HOXA10 over-expression in gastric cancer.
In conclusion, using an integrative analysis of tumor transcriptome and protein expression data by microarray techniques, we have elucidated the molecular biologic characteristics of a poor-prognosis gastric cancer subgroup. We postulate that the overexpression of miR-196b and HOXA10 are implicated in gastric cancer, particularly in tumors with poor-prognosis features. The personalized cancer therapy targets aberrations that drive tumor growth and survival. Administering the right drug for the right person consequently improves clinical outcomes and decreases toxicity. We anticipate that this approach will contribute to the characterization of cancer heterogeneity and the development of personalized therapy through the identification of cancer targets.
ACKNOWLEDGMENTS
The authors would like to acknowledge Yiling Lu, Department of Systems Biology, University of Texas MD Anderson Cancer Center, for performing the RPPA experiment.
COMMENTS
Background
Gastric cancer is clinically heterogenous, which suggest underlying molecular diversity. Advances in high-throughput technologies such as microarray for gene or protein expression profiles have reinforced the identification of molecular biologic characteristics of cancer. It is of great clinical value to perform integrative analysis of data from diverse high-throughput technologies simultaneously, to elucidate gastric cancer subtype harboring poor prognosis.
Research frontiers
MicroRNAs play a role in the pathogenesis of various human cancers by regulating the expression of target gene post transcriptionally. Microarray and bioinformatic technologies enable the investigation of the trans-correlation of hundreds of microRNAs and tens of thousands of genes.
Innovations and breakthroughs
MiR-196b was the most significantly up-regulated microRNA in gastric cancer and was significantly correlated with poor prognosis. The expression of miR-196b appeared to be most positively associated with homeobox A10 (HOXA10) expression. The expression of miR-196b and HOXA10 seems to be regulated by identical biologic mechanism and characterize a poor-prognosis gastric cancer subtype.
Applications
An integrative approach that simultaneously evaluates the expression profiles of mRNA, microRNA, and protein in cancer tissue will contribute to characterizing cancer heterogeneity and developing personalized therapy through the identification of cancer targets.
Terminology
A microarray is a multiplex lab-on-a-chip and assays large amounts of biological material using high-throughput screening methods. The types of microarrays include DNA microarray, microRNA microarray, protein microarray, and tissue microarray. Bioinformatics is the analysis of biological data including gene and protein expression and regulation data.
Peer review
This study generated and analyzed gene, microRNA, and protein expression profiles from 60 patients with gastric cancer and identified the overexpression of miR-196b and HOXA10 as a characteristic of a poor-prognosis gastric cancer subtype. Further investigation to elucidate the molecular biologic function of miR-196b and HOXA10 in gastric cancer is necessary.
Footnotes
Supported by The Faculty Research Grant of Yonsei University College of Medicine (6-2011-0113); the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, No. 2010-0024248
P- Reviewers: Lu XM, Tez M, Zhou L S- Editor: Wen LL L- Editor: A E- Editor: Ma S
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Shah MA, Ajani JA. Gastric cancer--an enigmatic and heterogeneous disease. JAMA. 2010;303:1753–1754. doi: 10.1001/jama.2010.553. [DOI] [PubMed] [Google Scholar]
- 3.Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90:114–33; discussion 133. doi: 10.1002/jso.20214. [DOI] [PubMed] [Google Scholar]
- 4.Tay ST, Leong SH, Yu K, Aggarwal A, Tan SY, Lee CH, Wong K, Visvanathan J, Lim D, Wong WK, et al. A combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypes. Cancer Res. 2003;63:3309–3316. [PubMed] [Google Scholar]
- 5.Kim B, Bang S, Lee S, Kim S, Jung Y, Lee C, Choi K, Lee SG, Lee K, Lee Y, et al. Expression profiling and subtype-specific expression of stomach cancer. Cancer Res. 2003;63:8248–8255. [PubMed] [Google Scholar]
- 6.Chen X, Leung SY, Yuen ST, Chu KM, Ji J, Li R, Chan AS, Law S, Troyanskaya OG, Wong J, et al. Variation in gene expression patterns in human gastric cancers. Mol Biol Cell. 2003;14:3208–3215. doi: 10.1091/mbc.E02-12-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HS, Cho SB, Lee HE, Kim MA, Kim JH, Park do J, Kim JH, Yang HK, Lee BL, Kim WH. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007;13:4154–4163. doi: 10.1158/1078-0432.CCR-07-0173. [DOI] [PubMed] [Google Scholar]
- 8.Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, Tan SH, Wu J, Lee MH, Ooi CH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–85, 485.e1-11. doi: 10.1053/j.gastro.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5:e1000676. doi: 10.1371/journal.pgen.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850–1857. doi: 10.1158/1078-0432.CCR-10-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond SM. MicroRNAs as oncogenes. Curr Opin Genet Dev. 2006;16:4–9. doi: 10.1016/j.gde.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Zhang Y, Zhang Y, Ding J, Wu K, Fan D. Survival prediction of gastric cancer by a seven-microRNA signature. Gut. 2010;59:579–585. doi: 10.1136/gut.2008.175497. [DOI] [PubMed] [Google Scholar]
- 16.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, et al. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 17.Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Popovic R, Riesbeck LE, Velu CS, Chaubey A, Zhang J, Achille NJ, Erfurth FE, Eaton K, Lu J, Grimes HL, et al. Regulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalization. Blood. 2009;113:3314–3322. doi: 10.1182/blood-2008-04-154310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai KW, Hu LY, Wu CW, Li SC, Lai CH, Kao HW, Fang WL, Lin WC. Epigenetic regulation of miR-196b expression in gastric cancer. Genes Chromosomes Cancer. 2010;49:969–980. doi: 10.1002/gcc.20804. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia S, Kaul D, Varma N. Functional genomics of tumor suppressor miR-196b in T-cell acute lymphoblastic leukemia. Mol Cell Biochem. 2011;346:103–116. doi: 10.1007/s11010-010-0597-0. [DOI] [PubMed] [Google Scholar]
- 21.Shah CA, Wang H, Bei L, Platanias LC, Eklund EA. HoxA10 regulates transcription of the gene encoding transforming growth factor beta2 (TGFbeta2) in myeloid cells. J Biol Chem. 2011;286:3161–3176. doi: 10.1074/jbc.M110.183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamatoji M, Kasamatsu A, Yamano Y, Sakuma K, Ogoshi K, Iyoda M, Shinozuka K, Ogawara K, Takiguchi Y, Shiiba M, et al. State of homeobox A10 expression as a putative prognostic marker for oral squamous cell carcinoma. Oncol Rep. 2010;23:61–67. [PubMed] [Google Scholar]
- 23.Li B, Jin H, Yu Y, Gu C, Zhou X, Zhao N, Feng Y. HOXA10 is overexpressed in human ovarian clear cell adenocarcinoma and correlates with poor survival. Int J Gynecol Cancer. 2009;19:1347–1352. doi: 10.1111/IGC.0b013e3181a83f1d. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, Kornblau SM. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 26.Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-ArrayTools. Cancer Inform. 2007;3:11–17. [PMC free article] [PubMed] [Google Scholar]
- 27.Mascaux C, Laes JF, Anthoine G, Haller A, Ninane V, Burny A, Sculier JP. Evolution of microRNA expression during human bronchial squamous carcinogenesis. Eur Respir J. 2009;33:352–359. doi: 10.1183/09031936.00084108. [DOI] [PubMed] [Google Scholar]
- 28.Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E, Hahn SA. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26:4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 29.Matsuyama H, Suzuki HI, Nishimori H, Noguchi M, Yao T, Komatsu N, Mano H, Sugimoto K, Miyazono K. miR-135b mediates NPM-ALK-driven oncogenicity and renders IL-17-producing immunophenotype to anaplastic large cell lymphoma. Blood. 2011;118:6881–6892. doi: 10.1182/blood-2011-05-354654. [DOI] [PubMed] [Google Scholar]
- 30.Munding JB, Adai AT, Maghnouj A, Urbanik A, Zöllner H, Liffers ST, Chromik AM, Uhl W, Szafranska-Schwarzbach AE, Tannapfel A, et al. Global microRNA expression profiling of microdissected tissues identifies miR-135b as a novel biomarker for pancreatic ductal adenocarcinoma. Int J Cancer. 2012;131:E86–E95. doi: 10.1002/ijc.26466. [DOI] [PubMed] [Google Scholar]
- 31.Necela BM, Carr JM, Asmann YW, Thompson EA. Differential expression of microRNAs in tumors from chronically inflamed or genetic (APC(Min/+)) models of colon cancer. PLoS One. 2011;6:e18501. doi: 10.1371/journal.pone.0018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–1681. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du L, Schageman JJ, Subauste MC, Saber B, Hammond SM, Prudkin L, Wistuba II, Ji L, Roth JA, Minna JD, et al. miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol Cancer Res. 2009;7:1234–1243. doi: 10.1158/1541-7786.MCR-08-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du WW, Yee AJ, Ang LC, He C, Shan SW, et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-β8. Oncogene. 2011;30:806–821. doi: 10.1038/onc.2010.465. [DOI] [PubMed] [Google Scholar]
- 35.Kim K, Chadalapaka G, Lee SO, Yamada D, Sastre-Garau X, Defossez PA, Park YY, Lee JS, Safe S. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31:1034–1044. doi: 10.1038/onc.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeung ML, Yasunaga J, Bennasser Y, Dusetti N, Harris D, Ahmad N, Matsuoka M, Jeang KT. Roles for microRNAs, miR-93 and miR-130b, and tumor protein 53-induced nuclear protein 1 tumor suppressor in cell growth dysregulation by human T-cell lymphotrophic virus 1. Cancer Res. 2008;68:8976–8985. doi: 10.1158/0008-5472.CAN-08-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montanini L, Lasagna L, Barili V, Jonstrup SP, Murgia A, Pazzaglia L, Conti A, Novello C, Kjems J, Perris R, et al. MicroRNA cloning and sequencing in osteosarcoma cell lines: differential role of miR-93. Cell Oncol (Dordr) 2012;35:29–41. doi: 10.1007/s13402-011-0059-z. [DOI] [PubMed] [Google Scholar]
- 38.Pass HI, Goparaju C, Ivanov S, Donington J, Carbone M, Hoshen M, Cohen D, Chajut A, Rosenwald S, Dan H, et al. hsa-miR-29c* is linked to the prognosis of malignant pleural mesothelioma. Cancer Res. 2010;70:1916–1924. doi: 10.1158/0008-5472.CAN-09-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Oliveira JC, Scrideli CA, Brassesco MS, Morales AG, Pezuk JA, Queiroz Rde P, Yunes JA, Brandalise SR, Tone LG. Differential miRNA expression in childhood acute lymphoblastic leukemia and association with clinical and biological features. Leuk Res. 2012;36:293–298. doi: 10.1016/j.leukres.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Coskun E, von der Heide EK, Schlee C, Kühnl A, Gökbuget N, Hoelzer D, Hofmann WK, Thiel E, Baldus CD. The role of microRNA-196a and microRNA-196b as ERG regulators in acute myeloid leukemia and acute T-lymphoblastic leukemia. Leuk Res. 2011;35:208–213. doi: 10.1016/j.leukres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Schotte D, De Menezes RX, Akbari Moqadam F, Khankahdani LM, Lange-Turenhout E, Chen C, Pieters R, Den Boer ML. MicroRNA characterize genetic diversity and drug resistance in pediatric acute lymphoblastic leukemia. Haematologica. 2011;96:703–711. doi: 10.3324/haematol.2010.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Lu Y, Huang W, Papoutsakis ET, Fuhrken P, Eklund EA. HoxA10 activates transcription of the gene encoding mitogen-activated protein kinase phosphatase 2 (Mkp2) in myeloid cells. J Biol Chem. 2007;282:16164–16176. doi: 10.1074/jbc.M610556200. [DOI] [PubMed] [Google Scholar]
- 43.Bei L, Lu Y, Bellis SL, Zhou W, Horvath E, Eklund EA. Identification of a HoxA10 activation domain necessary for transcription of the gene encoding beta3 integrin during myeloid differentiation. J Biol Chem. 2007;282:16846–16859. doi: 10.1074/jbc.M609744200. [DOI] [PubMed] [Google Scholar]
- 44.Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, de Tribolet N, Regli L, Wick W, Kouwenhoven MC, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26:3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 45.Sentani K, Oue N, Naito Y, Sakamoto N, Anami K, Oo HZ, Uraoka N, Aoyagi K, Sasaki H, Yasui W. Upregulation of HOXA10 in gastric cancer with the intestinal mucin phenotype: reduction during tumor progression and favorable prognosis. Carcinogenesis. 2012;33:1081–1088. doi: 10.1093/carcin/bgs121. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H, Takatsuka S, Akashi H, Yamamoto E, Nojima M, Maruyama R, Kai M, Yamano HO, Sasaki Y, Tokino T, et al. Genome-wide profiling of chromatin signatures reveals epigenetic regulation of MicroRNA genes in colorectal cancer. Cancer Res. 2011;71:5646–5658. doi: 10.1158/0008-5472.CAN-11-1076. [DOI] [PubMed] [Google Scholar]
- 47.Varon C, Dubus P, Mazurier F, Asencio C, Chambonnier L, Ferrand J, Giese A, Senant-Dugot N, Carlotti M, Mégraud F. Helicobacter pylori infection recruits bone marrow-derived cells that participate in gastric preneoplasia in mice. Gastroenterology. 2012;142:281–291. doi: 10.1053/j.gastro.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 48.Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol. 2008;26:2876–2882. doi: 10.1200/JCO.2007.15.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]


