Abstract
AIM: To compare effectiveness, safety, and cost of photodynamic therapy (PDT) and radiofrequency ablation (RFA) in treatment of Barrett’s dysplasia (BD).
METHODS: Consecutive case series of patients undergoing either PDT or RFA treatment at single center by a single investigator were compared. Thirty-three patients with high-grade dysplasia (HGD) had treatment with porfimer sodium photosensitzer and 630 nm laser (130 J/cm), with maximum of 3 treatment sessions. Fifty-three patients with BD (47 with low-grade dysplasia -LGD, 6 with HGD) had step-wise circumferential and focal ablation using the HALO system with maximum of 4 treatment sessions. Both groups received proton pump inhibitors twice daily. Endoscopic biopsies were acquired at 2 and 12 mo after enrollment, with 4-quadrant biopsies every 1 cm of the original BE extent. A complete histological resolution response of BD (CR-D) was defined as all biopsies at the last endoscopy session negative for BD. Fisher’s exact test was used to assess differences between the two study groups for primary outcomes. For all outcomes, a two-sided P value of less than 0.05 was considered to indicate statistical significance.
RESULTS: Thirty (91%) PDT patients and 39 (74%) RFA were men (P = 0.05). The mean age was 70.7 ± 12.2 and 65.4 ± 12.7 (P = 0.10) year and mean length of BE was 5.4 ± 3.2 cm and 5.7 ± 3.2 cm (P = 0.53) for PDT and RFA patients, respectively. The CR-D was (18/33) 54.5% with PDT vs (47/53) 88.7% with RFA (P = 0.001). One patient with PDT had an esophageal perforation and was managed with non-surgical measures and no perforation was seen with RFA. PDT was five times more costly than RFA at our institution. The two groups were not randomized and had different BD grading are the limitations of the study.
CONCLUSION: In our experience, RFA had higher rate of CR-D without any serious adverse events and was less costly than PDT for endoscopic treatment of BD.
Keywords: Barrett’s esophagus, Dysplasia, Photodynamic therapy, Radiofrequency ablation, Cost comparison
Core tip: Barrett’s esophagus containing dysplasia confers an elevated risk for developing esophageal adenocarcinoma. Photodynamic therapy (PDT) and radiofrequency ablation (RFA) have both been shown in randomized controlled trials to eradicate Barrett’s dysplasia (BD) and reduce the risk for disease progression. We compared the effectiveness, safety, and cost of PDT and RFA in managing BD in consecutive case series performed at single center by single endoscopist. We found that RFA had significantly higher rate of complete histological resolution of Barrett’s dysplasia and it was five times less costly than PDT at our institute compared to PDT.
INTRODUCTION
Adenocarcinoma of the esophagus and gastroesophageal junction (GEJ) is a devastating disease with 5-year survival rate of less than 15%[1]. Since 1970s, the incidence of this cancer has increased by more than 500% and it is considered one of the most rapidly increasing cancers in western society[2,3]. Barrett’s esophagus (BE), defined as metaplasia of the esophageal epithelium, in which squamous mucosa is replaced by a specialized columnar epithelium containing goblet cells, is a premalignant condition of the esophagus with increased risk of developing esophageal adenocarcinoma[4]. BE is based on the endoscopic findings of columnar epithelium lining the distal esophagus and diagnosis is confirmed by the presence of specialized intestinal metaplasia in esophageal biopsy specimens[5]. BE, sometimes also referred as intestinal metaplasia (IM), is caused by chronic inflammation and injury due to gastroesophageal reflux disease (GERD). BE may be present in as many as 10%-15% of patients with GERD and is associated with a significantly elevated risk of esophageal adenocarcinoma[4,6-9].
Barrett’s dysplasia (BD) is a histological diagnosis that one or more clones of epithelial cells have acquired genetic alterations rendering them neoplastic and prone to malignancy. Most recent study suggests the annual rate of cancer development for patients with BE is 0.12%[10]. While this rate increases to 6% for patients with BE and HGD[11]. Even among experienced pathologists, the extent of inter-observer agreement for the diagnosis of BD is a major problem[12-14]. Despite several imperfections such as sampling errors and inter-observer variability, pathologic classification of dysplasia on endoscopic biopsy specimens is the single most predictive variable for progression to cancer in patients with BD[15]. The degree of dysplasia has been shown to correlate with the risk of development cancer. The optimal care of patients with BD is unclear. In uncontrolled studies, satisfactory results have been noted in BE with HGD who were managed with esophagectomy[16], intensive endoscopic surveillance[17] and various endoscopic ablation techniques[18,19]. Although current guidelines[11] endorse various strategies, the relative efficacy and safety of the promising endoscopic ablation treatment modalities remain unclear. There is no previous head-to-head comparison of photodynamic therapy (PDT) vs radiofrequency ablation (RFA) exists. Therefore, we compare efficacy, safety, and cost-effectiveness of PDT vs RFA in patients with BD, in IRB-approved, prospectively collected BE outcome database at single center.
MATERIALS AND METHODS
Study population
The institutional review board at The Methodist Hospital, Houston, Texas approved this protocol. All patients signed an informed consent form prior to being enrolled in the study. We retrospective evaluated prospective collected database of all patients with a diagnosis of BE containing dysplasia [low-grade dysplasia (LGD) and high-grade dysplasia (HGD)] between May 2000 and June 2009 to ascertain eligibility for surveillance and therapeutic intervention. Inclusion criteria were: age > 18 years of age and non-nodular BD at enrollment. Exclusion criteria were: active esophagitis, esophageal stricture preventing passage of a therapeutic endoscope, any history of esophageal cancer, esophageal varices, and uncontrolled coagulopathy. All patients with BD were counseled about antireflux measures and received twice daily oral proton pump inhibitors throughout the study. All HGD patients underwent surgical consultation and based on age, performance status and co-morbidities were either ineligible for surgery or were offered surgery and refused after multidisciplinary meeting with gastroenterologist and surgeon regarding the risk and benefits related to endoscopic therapy and surgery.
Interventions
All endoscopic procedures (biopsy and ablation) were performed on an outpatient basis using intravenous conscious sedation (narcotic and benzodiazepine) or monitored anesthesia care (propofol). Endoscopic biopsies for baseline dysplastic grade confirmation, as well as for surveillance after ablative therapy, were performed using jumbo forceps in at least four quadrants every 1 cm of the BE segment. Post-ablative biopsies always encompassed the entire original extent of BE. Additional directed biopsies were obtained and placed in a separate container if any other visible abnormalities were noted during surveillance. Specimens from each level were fixed in formalin and embedded in paraffin to allow mapping of lesions. The blocks were sectioned, applied to glass slides, and stained with hematoxylin and eosin. All slides were independently evaluated by two gastrointestinal pathologists with expertise in the field of BE. Each specimen was assessed for the presence of BE, and if present, the worst pathologic grade noted per specimen as follows: non-dysplastic BE, LGD, HGD, or cancer. The worst pathologic grade used as the grade for that patient for that biopsy session. In cases of discordance between independent pathology readings, an open consensus diagnosis was obtained. Patients with visible nodule(s) who had endoscopic mucosal resection (EMR) were excluded from this study group.
Eligible patients with HGD who were enrolled between May 2000 and late 2007 were offered PDT, consisting of an intravenous photosensitizing agent (2.0 mg/kg, porfimer sodium, Axcan Pharma, Birmingham, AL) 40-50 h prior to endoscopy. During endoscopy, laser light (630 nm) was applied to the BE segment using a laser catheter without centering balloon (dose 130 J/cm). A maximum of 7 cm of BE was treated per session. In longer segments, a second PDT session was performed 3 mo later to treat the remaining segment. PDT patients had upper endoscopy and biopsies at 2 and 12 mo after the primary PDT and then annually thereafter, provided no HGD or adenocarcinoma was found on biopsy. If HGD was detected, PDT was repeated for a maximum of 3 total PDT sessions.
Eligible patients with LGD or HGD who were enrolled between September 2007 and June 2010 were offered RFA, consisting of step-wise treatment with the HALO ablation system (Covidien Inc., Mansfield, MA). In cases where the BE segment was > 3 cm, the primary ablation treatment was performed with the HALO360 ablation catheter, a balloon-based electrode which creates a circumferential 3-cm long ablation zone (40 W/cm2, 12 J/cm2). In cases where the BE segment was < 3 cm, the primary ablation treatment was performed with the HALO90 ablation catheter, an endoscope-mounted electrode which creates a focal 1.3 cm by 2.0 cm ablation zone (40 W/cm2, 12 J/cm2). After primary ablation, patients had endoscopy every 2 mo with assessment of endoscopic response to therapy. If residual BE was detected visually, RFA touch-up with HALO-90 was performed (maximum 2 additional sessions). Upon achieving a complete visual response or exceeding the maxium allowed sessions, biopsies were performed to confirm a histological complete response. Thereafter, patients were biopsied at two and 12 mo, and then annually provided no BD or BE detected.
Post-ablation care
After ablation, all patients were provided with double dose proton pump inhibitor medication to enable healing of the ablation zone and ensure long-term control of GERD. Patients were also provided with pain medication and anti-emetics to use as needed for 1-2 wk after therapy. PDT patients were educated to avoid exposure of eyes and skin to direct sunlight and high intensity visible light for at least 30 d or longer in patients with lighter skin color.
Outcome measures
The primary outcomes for this study were complete histological response of BD (CR-BD), defined as no evidence of dysplasia at last available biopsy session. Detection of cancer during any follow-up was considered a censoring event for the primary outcome. Secondary outcomes include the occurrence of adverse events, occurrence of subsquamous intestinal metaplasia at the last biopsy visit, and cost calculated per procedure and per patient.
Statistical analysis
The primary analysis was intention-to-treat (ITT), with all lost-to-follow-up patients being considered failures for the primary outcomes. The secondary analysis was per-protocol (PP), evaluating those patients with data available. Fisher’s exact test and Student’s t test were used to compare baseline variables. Fisher’s exact test was used to assess differences between the two study groups for primary outcomes. For all outcomes, a two-sided P value of less than 0.05 was considered to indicate statistical significance. The statistical analysis was performed using the SPSS 12.0.1 software for Windows. The costs of the PDT and RFA were calculated with a non-bias formula by the Methodist Hospital Cost Center.
RESULTS
There were 86 patients who fulfilled the study criteria and signed informed consent. Thirty-three patients underwent primary PDT (all with HGD). Fifty-three patients underwent primary RFA (6 with HGD and 47 with LGD). See Table 1 for baseline patient characteristics and Figure 1 for patient flow. Mean baseline BE length values were similar between groups (PDT: 5.4 ± 3.2, RFA: 5.7 ± 3.2, P = 0.53), while PDT patients tended to be older (PDT: 70.7 ± 12.2, RFA: 65.4 ± 12.8, P = 0.10) and more likely to have HGD (PDT: 33/33, RFA: 6/53) as entry diagnosis (P < 0.0001).
Table 1.
Characteristics factors of the patients n (%)
| Variable | PDT (n = 33) | RFA (n = 53) |
| Age (yr) | ||
| Mean ± SD | 70.7 ± 12.2 | 65.4 ± 12.7 |
| Range | 49-80 | 54-80 |
| Sex | ||
| Female | 3 (9) | 14 (26) |
| Male | 30 (91) | 39 (74) |
| Body-mass index | ||
| Mean ± SE | 27.8 ± 0.7 | 31.7 ± 1.3 |
| Range | 21-38 | 23-47 |
| Length of Barrett's esophagus (cm) | ||
| Mean ± SD | 5.4 ± 3.2 | 5.7 ± 3.2 |
| Range | 1.0-8.0 | 1.0-9.0 |
PDT: Photodynamic therapy; RFA: Radiofrequency ablation.
Figure 1.
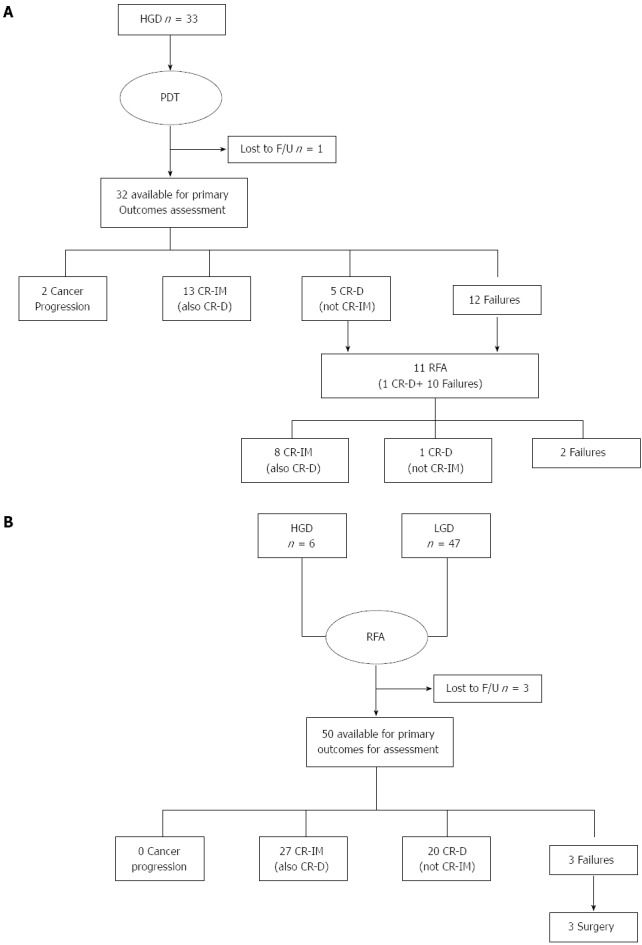
Patient flow. HGD: High-grade dysplasia; PDT: Photodynamic therapy; CR-D: Complete histological resolution of Barrett’s dysplasia; IM: Intestinal metaplasia.
The average length of follow-up from primary ablative therapy to primary outcome biopsy session was 44 mo (range 24-60 mo) for PDT and 33 mo (range 24-48 mo) for RFA. PDT patients had an average of 1.4 PDT sessions, while RFA had an average of 2.6 sessions. CR-D was achieved in 18/33 (54.5%) PDT and 47/53 (88.7%) RFA patients (P = 0.0001), Table 2. Eight PDT patients demonstrated an initial complete response, but then recurred (1 HGD, 7 LGD) at later biopsy sessions and were considered failures for the primary outcomes with PDT. After recurrence, these patients could not undergo further PDT due to exceeding the maximum PDT sessions achieved or intolerance to therapy, therefore all underwent salvage therapy with RFA with 100% CR-BD (post-hoc analysis). Two PDT patients developed cancer during follow-up at 18 and 24 mo. Both were considered failures for the primary endpoint of this study and both underwent esophagectomy. Surgical pathology showed T1NOMO lesions. No RFA patient developed cancer progression.
Table 2.
Outcomes measures n (%)
| PDT | RFA | |
| Primary outcomes | ||
| Complete Response IM | ||
| ITT | 13/33 (39.4) | 27/53 (50.9) |
| PP | 13/32 (40.6) | 35/61 (57.4) |
| Complete Response Dysplasia | ||
| ITT | 18/33 (54.5) | 47/53 (88.7) |
| PP | 18/32 (56.3) | 56/61 (91.8) |
| Secondary outcomes | ||
| Complications | ||
| Perforation | 1/32 (3.1) | 0/50 (0.00) |
| Stricture | 9/32 (28.1) | 2/50 (4.0) |
| Cost per session | $9449 | $1888 (HALO-360) |
| $1486 (HALO-90) |
PDT: Photodynamic therapy; RFA: Radiofrequency ablation; ITT: Intention-to-treat; PP: Per-protocol; IM: Intestinal metaplasia.
In the PDT cohort, two patients reported photosensitivity reactions (none required therapy), 9 (28.13%) developed a stricture necessitating serial endoscopic dilations (mean 3 per patient) with two requiring temporary stent placement. One patient developed an esophageal perforation that was managed by nonsurgical measures in the PDT cohort. In the RFA cohort, 2 patients (4.0%) developed a stricture managed with endoscopic dilation (one dilation per patient). No perforation was seen in the RFA patients. Subsquamous intestinal metaplasia (SSIM, “buried glands”) was seen in 4/32 (12.5%) of patients treated with PDT and 3/50 (6%) of those treated with RFA (P = 0.28).
The facility cost for a HALO-360 RFA session vs a PDT session was $1888 and $9449, respectively. The facility cost of a HALO-90 RFA was slightly lower at $1486. Cost per patient was also calculated, including primary ablation, follow-up ablation, and salvage ablation procedures, but excluding surgical salvage. According to a calculated cost analysis in our institution, PDT was approximately five times more costly than RFA per procedure.
DISCUSSION
Professional guidelines endorse various strategies for management of BE with dysplasia[11]. Due to lack of head to head comparison, relative efficacy of interventions like PDT, RFA, and cryotherapy remains unclear. Based on the finding that over 40% of patients with HGD undergoing esophagectomy have invasive cancer in their pathology specimen[16,20], esophagectomy is still been recommended for patients with HGD[11]. The prevalence of a missed cancer in patients may be less with recently used aggressive endoscopic biopsy protocols and better endoscopic visualization, however it is not been proven yet. Moreover, even when an esophagectomy is performed by experienced surgeons in high volume centers, it is associated with significant morbidity and mortality[16,20]. There are no RCTs comparing esophagectomy and endoscopic therapy for treatment of dysplastic BE[21]. Although debatable, intensive endoscopic surveillance with rigorous biopsy protocol can detect the progression from BD to cancer at the curative stage[17], but is associated with considerable anxiety for these patients with a negative impact to their quality of life. The accumulated evidence suggests that endoscopic resection (ER) therapy like endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) or endoscopic ablation modalities such as PDT and RFA have become attractive alternatives for the management of patients with BD[22-25]. ER of focal lesions can achieve complete eradication of HGD and IMC in 82.5%-95% of patients and also allows detection of occult invasive adenocarcinoma[21,26-28]. However, metachronous lesions or disease recurrence is been seen in up to 14% of patients within 12 mo, and 21.5% of patients over 5 years after ER[21,24,29]. International consortium of expert clinicians recommends ER for focal lesions followed by ablation of whole Barrett’s segment to decrease recurrence rate[30]. Endoscopic ablation modalities are based on the hypothesis that injury to the metaplastic and dysplastic epithelium will reverse the pathologic progression of BD to invasive cancer and restore a normal squamous epithelium. Despite the attractiveness of these endoscopic ablation technologies, no previous head-to-head comparison of PDT vs RFA exists in patients with BD.
In our study, we compared outcomes in patients treated with PDT and RFA in a single center by a single gastroenterologist in a prospectively collected database. These two modalities were compared with regard to complete eradication of BE and BD, adverse events, and costs. Our data shows that PDT and RFA eradicated dysplasia in 54% and 89%, respectively. Complete reversal of intestinal metaplasia was noted in 39% cases for PDT and 51% cases for RFA.
Overholt et al[31] compared PDT with Photofrin (PHO) plus omeprazole (PHOPDT) to omeprazole (OM) only to eradicate BE-HGD in a multicenter, randomized (2:1 randomization), pathology-blinded trial. At 5 years PHOPDT was significantly more effective than OM in eliminating HGD [77% (106/138) vs 39% (27/70), P < 0.0001]. A secondary outcome measure preventing progression to cancer showed a significant difference (P = 0.027) with about half the likelihood of cancer occurring in PHOPDT [21/138 (15%)] vs OM [20/70 (29%)], with a significantly (P = 0.004) longer time to progression to cancer favoring PHOPDT. In our experience, PDT eliminated HGD in 54.5% of patients compared to 77% on Overholt et al[31] study. In Overholt et al[31] study 36% of the patients developed stricture, and 69% had a photosensitivity reaction. Similarly, in our study 28% of patients developed strictures requiring serial endoscopic dilation. Shaheen et al[32] performed a multicenter, sham-controlled trial, and randomly assigned 127 patient with dysplastic BE in a 2:1 ratio to receive either RFA or a sham procedure (control group). In the intention-to-treat analyses, among patients with LGD, complete eradication of dysplasia occurred in 90.5% of those in the ablation group, as compared with 22.7% of those in the control group (P < 0.001)[32]. Among patients with HGD, complete eradication occurred in 81.0% of those in the ablation group, as compared with 19.0% of those in the control group (P < 0.001). Overall, 77.4% of patients in the ablation group had complete eradication of intestinal metaplasia, as compared with 2.3% of those in the control group (P < 0.001). Patients in the ablation group had less disease progression (3.6% vs 16.3%, P = 0.03) and fewer cancers (1.2% vs 9.3%, P = 0.045). We observed similar rates of complete response as illustrated in Table 2.
While making decisions about the management of precancerous conditions, potential benefits, risks and costs associated with different competing strategies are the most important considerations. For less severe disease like BE with LGD compared to BE with HGD, safety and cost of the suggested intervention becomes even more important[32]. According to the calculated direct cost comparison analysis, PDT was five times more costly than RFA in our institution. Detail cost effective analysis requires calculation for direct and indirect cost related to each intervention, as well as cost associated with complication and treatment failure. PDT patients are required to avoid sun and high intensity visible light during four weeks after the procedure and this may add significant indirect cost, in addition to high direct cost with PDT therapy. In our experience, side effects and complications of RFA were also less than PDT therapy.
In principle, a head-to-head comparison of PDT and RFA requires a randomized, blinded study design but this is currently impossible given the limited availability of PDT ablation technology for the esophagus. The strengths of our study are the very low drop-out rate, tight control of technique by a single experienced gastroenterologist (AE), and a rigorous biopsy protocol with review of all pathology by two well known gastroenterology pathologists and long follow up duration. Our study also has several limitations including the lack of randomization, the significant disproportion of HGD between the PDT and RFA groups respectively and difference in follow up time for both PDT and RFA group and the results of our study should be interpreted accordingly. PDT therapy was approved for HGD only and till RFA technology became available, only patients with BE-HGD were offered treatment with PDT. The implications of LGD vs HGD in Barrett’s patients are markedly different. LGD implies a risk of progression to cancer of less than 1% per patient-year[33], whereas the risk associated with HGD may be as high as 10% per patient-year[34,35].
In conclusion, in our experience both PDT and RFA were successful in eradicating dysplasia in BE. However, overall success rate of RFA was higher than PDT and RFA was very well tolerated without any major complications and fewer side effects. At our center, each session of RFA therapy was five times less costly than PDT therapy. However, for head-to-head comparison of PDT and RFA, prospective, randomized, blinded, multi-center studies are needed.
ACKNOWLEDGMENTS
The authors would like to thank the contributing GI pathologists, Mamoun Younes, MD, and Mary Schwartz, MD, for their expertise and contributions in reviewing all pathology specimens from this study.
COMMENTS
Background
The incidence of esophageal adenocarcinoma has increased by more than 500% since 1970 and it is considered one of the most rapidly increasing cancers in western society. Barrett’s esophagus (BE) is a premalignant condition and predisposes to adenocarcinoma of the esophagus and gastro-esophageal junction.
Research frontiers
Current guidelines endorse various strategies like esophagectomy, intensive endoscopic surveillance and various endoscopic resection and endoscopic ablation techniques for management of dysplastic BE. Significant controversies and disagreement exists in management of BE including the role of Barrett’s surveillance, the optimal means of intervention, and the stage at which intervention is indicated.
Innovations and breakthroughs
Various endoscopic ablation techniques like photodynamic therapy (PDT), radiofrequency ablation (RFA) and cryotherapy are currently available for treatment of dysplastic Barrett’s esophagus. Both PDT and RFA have been proven to be superior to eradicate dysplastic Barrett’s esophagus compared to routine antireflux measures and pharmacological anti-reflux measures in randomized trials. This study compared relative safety, efficacy and cost related to PDT and RFA therapy for dysplastic BE in a single center consecutive case series and found that RFA was more effective, safe and less costly than PDT therapy.
Applications
With better understanding of relative efficacy, safety and cost between PDT and RFA, this study will help physicians to determine optimal endoscopic ablation technique for treatment of dysplastic BE.
Peer review
It is a well written and accurate study. It shows a striking superiority of RFA vs PDT for Barret esophagus and, even that the study has not been randomized and the numer of patients is not very long, the results deserve its publication and must be known by practical endoscopists.
Footnotes
P- Reviewers: BordasJM, Herszenyi L, Pedrazzani C, Redondo-Cerezo E S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
References
- 1.Eloubeidi MA, Mason AC, Desmond RA, El-Serag HB. Temporal trends (1973-1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98:1627–1633. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 3.Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 4.Spechler SJ. Clinical practice. Barrett’s Esophagus. N Engl J Med. 2002;346:836–842. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ. The role of gastric carditis in metaplasia and neoplasia at the gastroesophageal junction. Gastroenterology. 1999;117:218–228. doi: 10.1016/s0016-5085(99)70571-8. [DOI] [PubMed] [Google Scholar]
- 6.Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, Russo MW, Sandler RS. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 7.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Talley NJ, Agréus L. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Cummings OW, Shaw M, Cumings MD, Wong RK, Vasudeva RS, Dunne D, Rahmani EY, Helper DJ. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology. 2002;123:461–467. doi: 10.1053/gast.2002.34748. [DOI] [PubMed] [Google Scholar]
- 10.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 11.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18–52; quiz e13. doi: 10.1053/j.gastro.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, Van Belle G, Lewin K, Weinstein WM, Antonioli DA, Goldman H. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 13.Skacel M, Petras RE, Gramlich TL, Sigel JE, Richter JE, Goldblum JR. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95:3383–3387. doi: 10.1111/j.1572-0241.2000.03348.x. [DOI] [PubMed] [Google Scholar]
- 14.Wani S, Falk GW, Post J, Yerian L, Hall M, Wang A, Gupta N, Gaddam S, Singh M, Singh V, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141:1179–186, 1186.e1. doi: 10.1053/j.gastro.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Sikkema M, Looman CW, Steyerberg EW, Kerkhof M, Kastelein F, van Dekken H, van Vuuren AJ, Bode WA, van der Valk H, Ouwendijk RJ, et al. Predictors for neoplastic progression in patients with Barrett’s Esophagus: a prospective cohort study. Am J Gastroenterol. 2011;106:1231–1238. doi: 10.1038/ajg.2011.153. [DOI] [PubMed] [Google Scholar]
- 16.Williams VA, Watson TJ, Herbella FA, Gellersen O, Raymond D, Jones C, Peters JH. Esophagectomy for high grade dysplasia is safe, curative, and results in good alimentary outcome. J Gastrointest Surg. 2007;11:1589–1597. doi: 10.1007/s11605-007-0330-9. [DOI] [PubMed] [Google Scholar]
- 17.Schnell TG, Sontag SJ, Chejfec G, Aranha G, Metz A, O’Connell S, Seidel UJ, Sonnenberg A. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 18.Chennat J, Konda VJ, Ross AS, de Tejada AH, Noffsinger A, Hart J, Lin S, Ferguson MK, Posner MC, Waxman I. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol. 2009;104:2684–2692. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 19.Ganz RA, Overholt BF, Sharma VK, Fleischer DE, Shaheen NJ, Lightdale CJ, Freeman SR, Pruitt RE, Urayama SM, Gress F, et al. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008;68:35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Heitmiller RF, Redmond M, Hamilton SR. Barrett’s esophagus with high-grade dysplasia. An indication for prophylactic esophagectomy. Ann Surg. 1996;224:66–71. doi: 10.1097/00000658-199607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Max Almond L, Barr H. Management controversies in Barrett’s oesophagus. J Gastroenterol. 2013:Jun 5; Epub ahead of print. doi: 10.1007/s00535-013-0816-z. [DOI] [PubMed] [Google Scholar]
- 22.Wolf DS, Dunkin BJ, Ertan A. Endoscopic Radiofrequency Ablation of Barrett’s Esophagus. Surg Technol Int. 2012;Dec 3; XXII. pii:sti22/33; Epub ahead of print. [PubMed] [Google Scholar]
- 23.Li YM, Li L, Yu CH, Liu YS, Xu CF. A systematic review and meta-analysis of the treatment for Barrett’s esophagus. Dig Dis Sci. 2008;53:2837–2846. doi: 10.1007/s10620-008-0257-3. [DOI] [PubMed] [Google Scholar]
- 24.Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 25.van Vilsteren FG, Pouw RE, Seewald S, Alvarez Herrero L, Sondermeijer CM, Visser M, Ten Kate FJ, Yu Kim Teng KC, Soehendra N, Rösch T, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–773. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Sharma P. How effective is endoscopic therapy in the treatment of patients with early esophageal cancer? Nat Clin Pract Gastroenterol Hepatol. 2009;6:70–71. doi: 10.1038/ncpgasthep1330. [DOI] [PubMed] [Google Scholar]
- 27.Hull MJ, Mino-Kenudson M, Nishioka NS, Ban S, Sepehr A, Puricelli W, Nakatsuka L, Ota S, Shimizu M, Brugge WR, et al. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol. 2006;30:114–118. doi: 10.1097/01.pas.0000180438.56528.a0. [DOI] [PubMed] [Google Scholar]
- 28.Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, Swan MP, Hopper AD, Kwan V, Bailey AA. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276–1283. doi: 10.1038/ajg.2010.1. [DOI] [PubMed] [Google Scholar]
- 29.Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoléon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24–29. doi: 10.1055/s-2006-945182. [DOI] [PubMed] [Google Scholar]
- 30.Bennett C, Vakil N, Bergman J, Harrison R, Odze R, Vieth M, Sanders S, Gay L, Pech O, Longcroft-Wheaton G, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012;143:336–346. doi: 10.1053/j.gastro.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, Bronner MP, Taylor SL, Grace MG, Depot M. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 32.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 33.Sharma P, Falk GW, Weston AP, Reker D, Johnston M, Sampliner RE. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566–572. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buttar NS, Wang KK, Sebo TJ, Riehle DM, Krishnadath KK, Lutzke LS, Anderson MA, Petterson TM, Burgart LJ. Extent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinoma. Gastroenterology. 2001;120:1630–1639. doi: 10.1053/gast.2001.25111. [DOI] [PubMed] [Google Scholar]


