Abstract
AIM: To evaluate the efficacy of computer-assisted color analysis of colorectal lesions using a novel auto-fluorescence imaging (AFI) system to distinguish neoplastic lesions from non-neoplastic lesions and to predict the depth of invasion.
METHODS: From January 2013 to April 2013, consecutive patients with known polyps greater than 5 mm in size who were scheduled to undergo endoscopic treatment at The Jikei University Hospital were prospectively recruited for this study. All lesions were evaluated using a novel AFI system, and color-tone sampling was performed in a region of interest determined from narrow band imaging or from chromoendoscopy findings without magnification. The green/red (G/R) ratio for each lesion on the AFI images was calculated automatically using a computer-assisted color analysis system that permits real-time color analysis during endoscopic procedures.
RESULTS: A total of 88 patients with 163 lesions were enrolled in this study. There were significant differences in the G/R ratios of hyperplastic polyps (non-neoplastic lesions), adenoma/intramucosal cancer/submucosal (SM) superficial cancer, and SM deep cancer (P < 0.0001). The mean ± SD G/R ratios were 0.984 ± 0.118 in hyperplastic polyps and 0.827 ± 0.081 in neoplastic lesions. The G/R ratios of hyperplastic polyps were significantly higher than those of neoplastic lesions (P < 0.001). When a G/R ratio cut-off value of > 0.89 was applied to determine non-neoplastic lesions, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were 83.9%, 82.6%, 53.1%, 95.6% and 82.8%, respectively. For neoplastic lesions, the mean G/R ratio was 0.834 ± 0.080 in adenoma/intramucosal cancer/SM superficial cancer and 0.746 ± 0.045 in SM deep cancer. The G/R ratio of adenoma/intramucosal cancer/SM superficial cancer was significantly higher than that of SM deep cancer (P < 0.01). When a G/R ratio cut-off value of < 0.77 was applied to distinguish SM deep cancers, the sensitivity, specificity, PPV, NPV, and accuracy were 80.0%, 84.4%, 29.6%, 98.1% and 84.1%, respectively.
CONCLUSION: The novel AFI system with color analysis was effective in distinguishing non-neoplastic lesions from neoplastic lesions and might allow determination of the depth of invasion.
Keywords: Autofluorescence imaging, Computer-aided diagnosis, Colon, Neoplasia, Colonoscopy
Core tip: Recently, a novel auto-fluorescence imaging (AFI) system with has higher resolution and higher flame rate than those offered by previously used AFI systems has become commercially available in Japan. We evaluated the efficacy of computer-assisted color analysis using the novel AFI system for distinguishing colorectal neoplasia and non-neoplasia and for predicting depth of invasion. The green/red ratios, which were obtained by dividing green color intensity by red color intensity, were significantly different between hyperplastic polyps, adenoma/intramucosal cancer/SM superficial cancer, and SM deep cancer. The novel AFI system was effective in distinguishing non-neoplastic lesions from neoplastic lesions and might have potential to predict the depth of invasion.
INTRODUCTION
Colonoscopy is the most effective method for the detection of colonic neoplastic lesions in the earlier and more curable stages. According to the National Polyp Study, colorectal cancer incidence was decreased by the endoscopic removal of polyps detected during colonoscopy screenings[1].
Accurate endoscopic determination of the histology of colorectal polyps could prevent unnecessary polypectomies and may allow the proposal of adequate surveillance recommendations. The strategy of “resect and discard” for diminutive colorectal polyps has been suggested by The American Society for Gastrointestinal Endoscopy[2]. However, a report evaluating real-time optical biopsy analysis of polyps with narrow band imaging (NBI) concluded that only 25% of community-based gastroenterologists assessed polyps with > 90% accuracy[3]. Therefore, easy and objective methods for distinguishing colorectal neoplastic lesions from non-neoplastic lesions are required.
We previously reported the effectiveness of auto-fluorescence imaging (AFI) systems in distinguishing colorectal non-neoplastic lesions from neoplastic lesion[4,5]; however, in that study, the number of lesions examined was limited.
In an AFI system, white light is separated into excitation light and green light and used to irradiate onto the colon mucosa. After blocking the reflected light, a false color image is displayed on the monitor based on the balance of autofluorescence intensity and green light intensity. Recently, a novel AFI system composed of light sources (EVIS LUCERA ELITE CLV-290SL; Olympus Medical Systems, Tokyo, Japan) and video processors (EVIS LUCERA ELITE CV-290; Olympus Medical Systems, Tokyo, Japan) was developed. The newer AFI systems feature two major improvements over previously available AFI systems (EVIS LUCERA CLV-260SL, EVIS LUCERA CV-260S; Olympus Medical Systems, Tokyo, Japan) The first improvement is a brighter lamp that allows a higher frame rate, although the overall brightness of the endoscopic images is the same as that in first-generation AFI systems. The use of a brighter lamp produces less flickering and color splitting in the endoscopic images. The second major improvement is in the image-processing algorithm used, specifically the noise-reduction algorithm, which results in higher resolution images with less noise interference (Figure 1). In addition, software that enables real-time color analysis has been developed and is available.
Figure 1.

Comparisons of images obtained with the previous autofluorescence imaging system and with the novel autofluorescence imaging system. The major improvement in the novel autofluorescence imaging (AFI) system is in the image-processing algorithm, specifically the noise-reduction algorithm, which results in higher resolution images with less noise interference. An AFI image from the previous AFI system (A: EVIS LUSERA ERITE CLV-290) is clearer than an image from the novel AFI system (B: EVIS LUSERA CLV-260).
The aim of this study was to prospectively evaluate the efficacy of computer-assisted color analysis of colorectal lesions by using the novel AFI system to attempt to distinguish non-neoplastic lesions from neoplastic lesions. In addition, if a the colorectal neoplasm invades the submucosal layer, which contains where autofluorescent substances such as, collagen, nicotinamide adenine dinucleotide hydrate (NADH), flavin adenine dinucleotide, lysosome granules, and porphyrin[6-8], autofluorescence would be expected to be attenuated. Therefore, we hypothesized that the AFI system would be useful in determining the depth of invasion of colorectal neoplasms. To verify our hypothesis, we evaluated the effectiveness of the novel system in permitting diagnosis of the depth of invasion of colorectal neoplasia.
MATERIALS AND METHODS
This study was approved by the ethical committee of our institution [registration number 24-188(6954)] and was registered in the University Hospital Medical Information Network (UMIN) in Japan under registration number 24-188(6954): UMIN R000011404. Written informed consent for examination and treatment was obtained from all patients prior to the procedures. From January 2013 to April 2013, consecutive patients with known polyps over 5 mm in size who were scheduled to undergo endoscopic treatment at The Jikei University Hospital were prospectively recruited for this study.
The exclusion criteria were refusal to participate, ages less than 19 or more than 91 years, and the presence of inflammatory bowel disease (IBD), familial adenomatous polyposis, or serrated sessile adenoma/polyp (SSA/P) lesions confirmed by pathological findings after endoscopic resection. Patients with SSA/P lesions were excluded because the histological diagnostic criteria for SSA/P are controversial in Japan at present[9].
Endoscopic procedure
All patients received total bowel cleansing with polyethylene glycol solutions (Niflec; Ajinomoto Pharmaceuticals Co. Ltd., Tokyo, Japan) after 12 h of nil per os (NPO) status. All colonoscopies were performed using the novel AFI system with a colonoscope designed for AFI observation (CF-FH260AZI; Olympus Medical Systems, Tokyo, Japan) by 5 endoscopists, each of whom possesses more than 5 years of experience in colonoscopy and treats more than 500 cases a year.
All endoscopic procedures were performed under conditions of CO2 insufflation[10]. First, colonoscopy was performed using the white light imaging (WLI) function of the AFI system. When colorectal lesions were detected, they were observed by switching to the NBI mode using a button on the control head of the endoscope. Subsequently, the lesions were observed by chromoendoscopy (CE) using 0.4% indigo carmine and the WLI mode. The region of interest (ROI) was then determined from the NBI findings without magnification or CE. We determined the placement of the ROI in regions where characteristic endoscopic findings for SM deep invasion, such as expansion appearance, a large nodule equal to or greater than 10 mm in size, redness, or demarcated depressed areas[11,12] were found, we determined the placement of the ROI based on the individual characteristics of the lesion (Figure 2). In lesions without these findings, the ROI was placed at the center of the lesion. After washing the indigo carmine with water insufflation, real-time color analysis of the ROI on the AFI images was conducted using a personal computer with software for color analysis connected to the endoscopy system. On the computer monitor, the ROI was placed using a cursor (10 × 10 pixels). The green/red (G/R) ratio, which is obtained by dividing the green color tone intensity by the red color tone intensity, was calculated automatically during the endoscopic procedures (Figure 3). Finally, magnifying endoscopy with NBI and CE using crystal violet was performed to estimate the depth of invasion of the encountered lesion[13].
Figure 2.
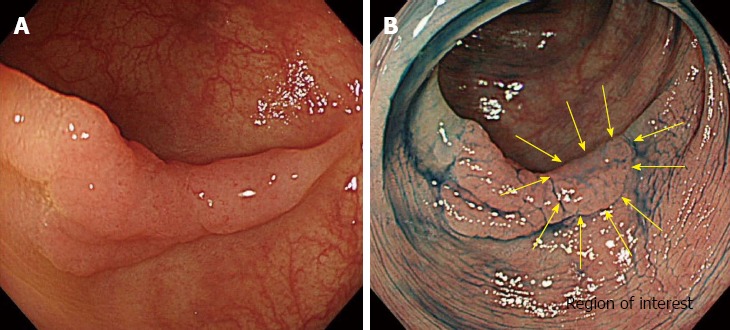
A determination of region of interest. A: A white light image of a flatelevated lesion located in the sigmoid colon; B: A depressed area observed on chromoendoscopy (the area surrounded by yellow arrows). The region of interest for color tone analysis was chosen by the endoscopist based on the findings of non-magnifying narrow-band imaging or chromoendoscopy.
Figure 3.

A method for real-time color intensity analysis calculated software. A: A personal computer with software that enables real-time color tone analysis was connected to the endoscopy system. B: The user interface of the personal computer for color tone analysis. The region of interest was chosen using the cursor (yellow square). The color tone intensity (blue square) and the green/red ratio (red square), which is obtained by dividing the green color tone intensity by the red color tone intensity, were calculated automatically.
If the estimated depth of invasion was limited to the mucosa or superficial SM (less than 1000 μm from the muscularis mucosae), the lesions were endoscopically removed. Lesions diagnosed as SM deep cancer (1000 μm or more from the muscularis mucosae) from magnified endoscopic findings were surgically resected.
Histological assessment
All tissue specimens were fixed in 10% buffered formalin and cut into 2-mm slices. The specimens were then examined microscopically to determine depth of invasion and histological type. Histological diagnoses were based on the Japanese classification system for cancer of the colon and rectum[14] and on the Vienna classification system[15]. The final pathological assessments were conducted by a pathologist who was blinded to this study and to the results of the color analyses.
Endoscopic diagnosis
The endoscopist assessed whether the lesions were neoplastic or non-neoplastic and determined the depth of invasion (SM deep or not) on the basis of the findings of magnified observation using NBI and CE. After completion the color analysis, the diagnoses were related to the study coordinator by the endoscopist.
Statistical analysis
Statistical analysis was performed using SPSS for Windows (SPSS, release 6.0, 1993; SPSS Inc., Chicago, Illinois, United States). Data are expressed as the mean ± SD. To determine differences in the mean G/R ratio, comparisons between groups were performed using one-way analysis of variance (ANOVA) followed by multiple comparison testing using the Bonferroni-Dunn method. Continuous variables were analyzed using a t test. A P value < 0.05 was considered statistically significant.
RESULTS
A total of 191 patients were recruited for this study. The following patients were excluded from this study based on the exclusion criteria: 89 patients who refused to participate, 9 patients who had SSA/P lesions, and 5 patients diagnosed with IBD. Finally, 88 patients with 163 lesions were enrolled in this study. The mean patient age was 63.0 ± 10.4 years, the male-to-female ratio was 1:0.51, and the mean lesion size was 15.0 ± 12.6 mm. Histologically, of the 163 lesions, there were 31 hyperplastic polyps (19.0%), 82 tubular/tubulovillous adenomas (50.3%), 40 mucosal or SM superficial cancers (24.5%), and 10 SM deep cancers (6.1%). Macroscopic types included 72 protruded (44.2%) and 91 flat elevated or depressed (55.8%) lesions. Tumor locations included 13 in the cecum (8.0%), 63 in the right colon (38.7%), 64 in the left colon (39.3%), and 23 in the rectum (14.1%). There were significant differences between the G/R ratios of the hyperplastic polyps (non-neoplastic lesions), adenoma/intramucosal cancer/SM superficial cancer, and SM deep cancer (P < 0.0001) (Figure 4A). The mean G/R ratios were 0.984 ± 0.118 in hyperplastic polyps and 0.827 ± 0.081 in neoplastic lesions. The G/R ratios of hyperplastic polyps were significantly higher than those of neoplastic lesions (P < 0.001) (Figure 4B), and the area under the receiver operating characteristic (ROC) curve sensitivity and 1-specificity of the G/R ratio in discriminating between hyperplastic polyps and neoplastic lesions was 0.8795. When a G/R ratio cut-off value of > 0.89 was applied to determine non-neoplastic lesions, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were 83.9%, 82.6%, 53.1%, 95.6%, and 82.8%, respectively (Figure 5A). Regarding the use of endoscopic diagnosis by experienced endoscopists to distinguish neoplastic lesions from non-neoplastic lesions using magnifying endoscopy with NBI and/or CE, the sensitivity, specificity, PPV, NPV, and accuracy were 93.5%, 97.0%, 87.9%, 98.5% and 96.3%, respectively. The accuracy of color analysis for distinguishing neoplastic lesions from non-neoplastic lesions was 90.1% for flat/depressed types and 70.8% for protruded types. The diagnostic accuracy for the flat/depressed type was significantly higher than that for the protruded type (Table 1).
Figure 4.
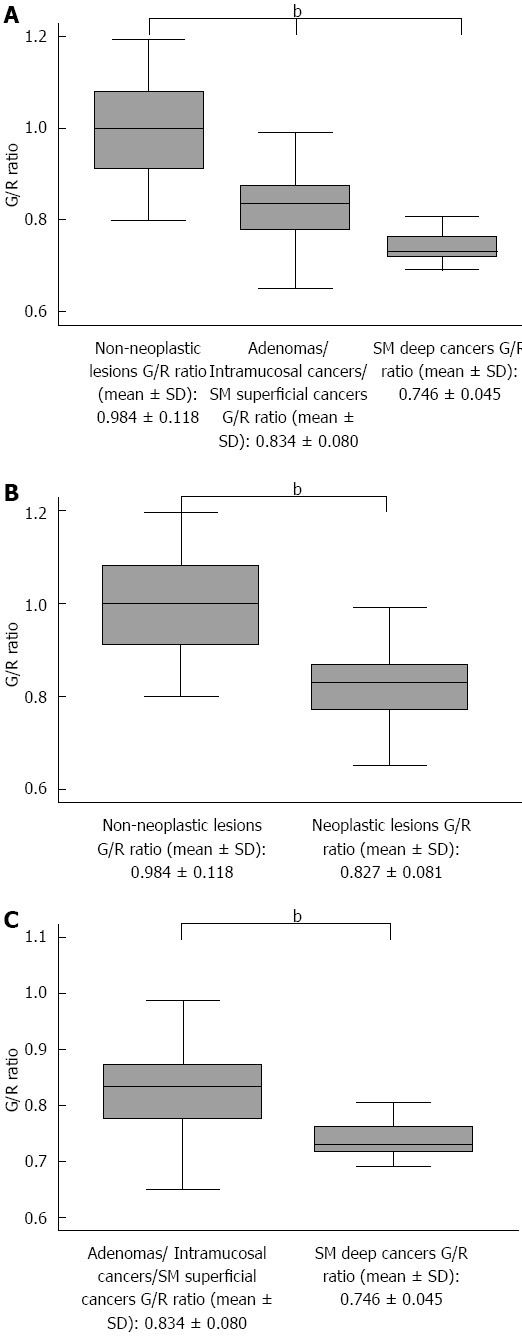
Distribution of green/red ratios. A: Between non-neoplastic lesions, adenoma/intramucosal cancer/submucosal (SM) superficial cancer, and SM deep cancer, there was a significant difference in the green/red (G/R) ratios of non-neoplastic lesions, adenomas/intramucosal cancer/SM superficial cancer, and SM deep cancer (bP < 0.01); B: Between non-neoplastic lesions and neoplastic lesions, There was a significant difference between non-neoplastic and neoplastic lesions (bP < 0.01); C: Between adenoma/intramucosal cancer/SM superficial cancer, and SM deep cancer, there was a significant difference between the two groups (bP < 0.01). SM superficial cancer: Submucosal invasion was defined as less than 1000 μm from the muscularis mucosae; SM deep cancer: Submucosal invasion was defined as 1000 μm or more from the muscularis mucosae.
Figure 5.
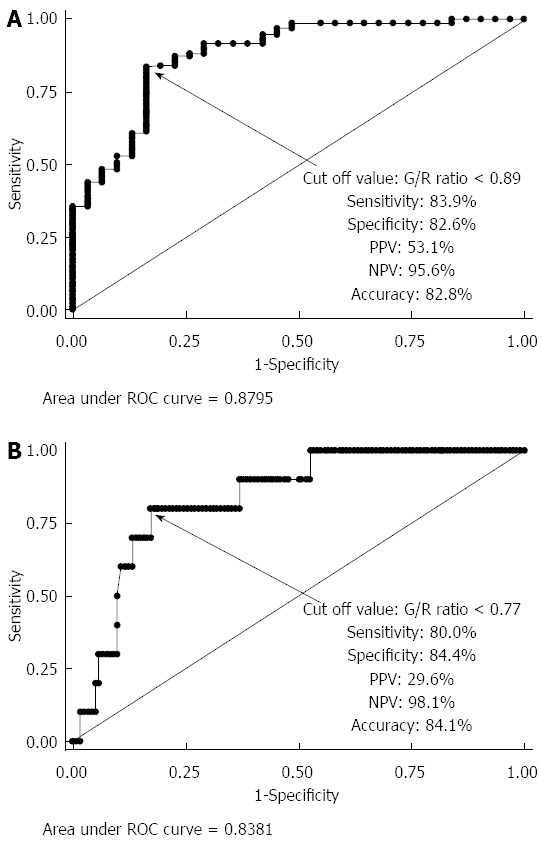
Receiver operating characteristic curve. A: For discriminating between hyperplastic polyps and neoplastic lesions, the area under the receiver operating characteristic curve (AUC) was 0.8795; B: For discriminating between adenoma/intramucosal cancer/SM superficial cancer and SM deep cancer, the area under AUC was 0.8381. G/R: Green/red.
Table 1.
Diagnostic value of colorectal lesions (for distinguishing neoplastic lesions from non-neoplastic lesions)
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
| Diagnosis based on the color analysis | 83.9% | 82.6% | 53.1% | 95.6% | 82.8% |
| Flat or depressed type | 93.1% | 79.0% | 94.4% | 75.0% | 90.1% |
| Protruded type | 66.7% | 91.7% | 97.6% | 35.5% | 70.8% |
| Diagnosis based on the endoscopic findings1 | 93.5% | 97.0% | 87.9% | 98.5% | 96.3% |
Using magnifying endoscopy with narrow band imaging and chromoendoscopy.
For neoplastic lesions, the mean G/R ratio was 0.834 ± 0.080 in adenoma/intramucosal cancer/SM superficial cancer and 0.746 ± 0.045 in SM deep cancer (Figure 4C). The G/R ratio of adenoma/intramucosal cancer/SM superficial cancer was significantly higher than that of SM deep cancer (P < 0.01), and the area under the ROC curve sensitivity and 1-specificity of the G/R ratio in discriminating between adenoma/intramucosal cancer/SM superficial cancer and SM deep cancer was 0.8381. When a G/R ratio cut-off value of < 0.77 was applied to distinguish SM deep cancers, the sensitivity, specificity, PPV, NPV, and accuracy were 80.0%, 84.4%, 29.6%, 98.1% and 84.1%, respectively (Figure 5B). Regarding the endoscopic diagnosis of depth of invasion by experienced endoscopists using magnifying endoscopy for SM deep cancers, the sensitivity, specificity, PPV, NPV, and accuracy were 60.0%, 100%, 100%, 96.8%, and 84.1%, respectively (Table 2).
Table 2.
Diagnostic value of colorectal lesions (For distinguishing intramucosal lesions/SM superficial cancer from SM deep cancer)
| Sensitivity | Specificity | Negative predictive value | Positive predictive value | Accuracy | |
| Diagnosis based on the color analysis | 80.00% | 84.40% | 29.60% | 98.10% | 84.10% |
| Diagnosis based on the endoscopic findings1 | 60.00% | 100% | 100% | 96.80% | 84.10% |
Using magnifying endoscopy with narrow band imaging and/or chromoendoscopy. Submucosal (SM) superficial cancer: Submucosal invasion was defined as less than 1000 μm from the muscularis mucosae; SM deep cancer: Submucosal invasion was defined as 1000 μm or more from the muscularis mucosae.
DISCUSSION
The NBI system, which enables precise observation of microvasculature and surface strictures of colorectal lesions[16-18], is considered an effective method for distinguishing non-neoplastic lesions from neoplastic lesions, and an international expert group has proposed the NBI International Colorectal Endoscopic (NICE) classification to distinguish adenomas from hyperplastic polyps[19]. However, because diagnosis of colorectal neoplasia using the NBI system is based on the subjective judgment of the endoscopist, the accuracy of diagnosis differs between expert endoscopists and community-based gastroenterologists[3]. Therefore, a method that permits objective and real-time diagnosis with respect to for distinguishing colorectal non-neoplastic lesions from neoplastic lesions is needed.
The results of the present study indicate that color analysis using the novel AFI system is an effective method for the objective diagnosis of colorectal lesions, although the sensitivity/specificity/PPV/NPV for determining neoplastic lesions obtained in this study were inferior to those previously reported[5] using an earlier AFI system. The mean G/R ratios of colorectal lesions (non-neoplastic lesions, 0.984; neoplastic lesions, 0.827) in the present study were lower than the ratios reported in a previous study (non-neoplastic, 1.12; neoplastic, 0.86)[5]. The improvements in the novel AFI system, including the noise-reduction algorithm, higher frame rate, and brighter light source, may affect color tone intensity. In addition, in a previous report, color tone sampling was performed in one area of the lesion that was judged to represent a particular AFI color tone[5]; however, in the present study, color tone sampling was performed on an ROI, that, was determined using chromoendoscopy under WLI. Moreover, the present study was limited to patients with polyps over 5 mm in size; however, there were no size limitations on polyps in the previous report[5]. The use of different methods of color tone sampling and different inclusion criteria are possible reasons for the differences in the results. However, the lower G/R ratios obtained in the present study might indicate that the novel AFI system permits clearer visualization of colorectal lesions than the former AFI system.
The results of the present study also indicate the potential of color tone analysis to predict the depth of invasion of colorectal neoplasia. Standard methods in use for predicting the depth of invasion of colorectal neoplasia for planning treatment strategies are pit pattern classification using magnifying endoscopy and/or endoscopic ultrasonography[20-26], both of which require long procedure times. A color tone analysis that involves a shorter procedure time and that permits objective diagnosis of the depth of invasion of colorectal neoplasia may provide significant benefits, especially to less endoscopists. However, because the number of SM deep lesions examined in this study was limited, larger studies are needed to further validate this method. The current study has several limitations. First, because it was difficult to estimate the effectiveness of color tone analysis in distinguishing neoplastic lesions from non-neoplastic lesions using the novel AFI system, the sample size was not calculated for this study. Second, the colonoscopies and ROI determinations performed in this study were conducted by experienced endoscopists; therefore, the results of this study might not be reproducible when patients are assessed by non-experienced clinicians. Finally, the lesions included in this study were limited to those over 5 mm in size, and the number of SM deep lesions was limited.
In conclusion, the results of the present study indicate that computer-assisted real-time color analysis using the novel AFI system was effective in distinguishing non-neoplastic lesions from neoplastic lesions. Color tone analysis, which is an objective and time-saving method, may have potential as a methods for predicting the depth of invasion of colorectal neoplasia, although the number of SM deep lesions examined in the present study was limited. Further studies are needed to determine the effectiveness of color tone analysis for predicting the depth of invasion of colorectal neoplasia.
COMMENTS
Background
Colonoscopy is the most effective method for the detection of colonic neoplastic lesions in their earlier and more curable stages. Accurate endoscopic determination of the histology of colorectal polyps could prevent unnecessary polypectomies and may allow the proposal of adequate surveillance recommendations. The authors have reported the effectiveness of autofluorescence imaging (AFI) systems in distinguishing colorectal non-neoplastic lesions from neoplastic lesions. Recently, a novel AFI system that employs second-generation light sources (EVIS LUCERA ELITE CLV-290SL; Olympus Medical Systems, Tokyo, Japan) and video processors (EVIS LUCERA ELITE CV-290; Olympus Medical Systems, Tokyo, Japan) was developed. In addition, software that enables real-time color analysis was developed.
Research frontiers
AFI systems can be used to visualize fluorophores (autofluorescent substances) in the gastrointestinal (GI) tract. Several studies have demonstrated that the autofluorescence emitted by neoplastic lesions in the colon has a relatively longer wavelength than the autofluorescence emitted by surrounding normal mucosa. The aim of this study was to prospectively evaluate the efficacy of computer-assisted color analysis of colorectal lesions using a novel AFI system to distinguish non-neoplastic lesions from neoplastic lesions. The authors also evaluated the effectiveness of the system in determining the depth of invasion of colorectal neoplasms.
Innovations and breakthroughs
The results of the present study indicate that color tone analysis using the novel AFI system is an effective method for the objective diagnosis of colorectal lesions. The improvements in the novel AFI system, including the noise reduction algorithm, higher frame rate, and brighter light source may affect color tone intensity. In addition, in the present study, color tone sampling was performed on the region of interest, which was determined using chromoendoscopy under white light imaging. The results of the present study also indicate the potential of color tone analysis for use in predicting the depth of invasion of colorectal neoplasia. A color tone analysis that involves a shorter procedure time and permits objective diagnosis of the depth of invasion of colorectal neoplasia may provide significant benefits to endoscopists, especially to those who are less experienced.
Applications
The study results suggest that computer-assisted real-time color analysis using the novel AFI system was effective in distinguishing non-neoplastic lesions from neoplastic lesions. In addition, color tone analysis, which allows objective and time-saving prediction of the depth of invasion of colorectal neoplasia, has potential for use in determining appropriate treatment strategies for colorectal neoplasia.
Terminology
AFI systems can be used to visualize fluorophores (autofluorescent substances) in the GI tract. In an AFI system, a rotation filter divides the light from the xenon light source and permits only blue and green light to pass. The returning reflection from the tissue, including fluorescent light, is captured by the CCD via a special barrier filter. Image processing then generates the AFI image. The novel AFI system has a brighter lamp that permits a higher frame rate, and produces, less flickering and color splitting, resultings in higher-resolution images with less noise interference.
Peer review
This study prospectively evaluated the efficacy of computer-assisted color analysis of colorectal lesions using a novel AFI system to distinguish non-neoplastic lesions from neoplastic lesions. In addition, the authors also evaluated the effectiveness of the system in diagnosing the depth of invasion of colorectal neoplasia. They found that the novel AFI system with color analysis was effective in distinguishing non-neoplastic lesions from neoplastic lesions and that it may allow determination of the depth of invasion. The study is interesting, and the manuscript is well-written.
Footnotes
P- Reviewers: Cho YS, Guan YS, Kobayashi N, Li Q, Ozkan OV, Sangkhathat S, Wang YH S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
References
- 1.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Kahi C, O’Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–422. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, Imperiale T, Gunaratnam N. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013;144:81–91. doi: 10.1053/j.gastro.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aihara H, Sumiyama K, Saito S, Tajiri H, Ikegami M. Numerical analysis of the autofluorescence intensity of neoplastic and non-neoplastic colorectal lesions by using a novel videoendoscopy system. Gastrointest Endosc. 2009;69:726–733. doi: 10.1016/j.gie.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Aihara H, Saito S, Inomata H, Ide D, Tamai N, Ohya TR, Kato T, Amitani S, Tajiri H. Computer-aided diagnosis of neoplastic colorectal lesions using ‘real-time’ numerical color analysis during autofluorescence endoscopy. Eur J Gastroenterol Hepatol. 2013;25:488–494. doi: 10.1097/MEG.0b013e32835c6d9a. [DOI] [PubMed] [Google Scholar]
- 6.Izuishi K, Tajiri H, Fujii T, Boku N, Ohtsu A, Ohnishi T, Ryu M, Kinoshita T, Yoshida S. The histological basis of detection of adenoma and cancer in the colon by autofluorescence endoscopic imaging. Endoscopy. 1999;31:511–516. doi: 10.1055/s-1999-57. [DOI] [PubMed] [Google Scholar]
- 7.Miura S, Kodaira S, Hosoda Y. Immunohistologic analysis of the extracellular matrix components of the fibrous stroma of human colon cancer. J Surg Oncol. 1993;53:36–42. doi: 10.1002/jso.2930530111. [DOI] [PubMed] [Google Scholar]
- 8.Schomacker KT, Frisoli JK, Compton CC, Flotte TJ, Richter JM, Deutsch TF, Nishioka NS. Ultraviolet laser-induced fluorescence of colonic polyps. Gastroenterology. 1992;102:1155–1160. [PubMed] [Google Scholar]
- 9.Nakao Y, Saito S, Ohya T, Aihara H, Arihiro S, Kato T, Ikegami M, Tajiri H. Endoscopic features of colorectal serrated lesions using image-enhanced endoscopy with pathological analysis. Eur J Gastroenterol Hepatol. 2013;25:981–988. doi: 10.1097/MEG.0b013e3283614b2b. [DOI] [PubMed] [Google Scholar]
- 10.Bretthauer M, Lynge AB, Thiis-Evensen E, Hoff G, Fausa O, Aabakken L. Carbon dioxide insufflation in colonoscopy: safe and effective in sedated patients. Endoscopy. 2005;37:706–709. doi: 10.1055/s-2005-870154. [DOI] [PubMed] [Google Scholar]
- 11.Saitoh Y, Obara T, Watari J, Nomura M, Taruishi M, Orii Y, Taniguchi M, Ayabe T, Ashida T, Kohgo Y. Invasion depth diagnosis of depressed type early colorectal cancers by combined use of videoendoscopy and chromoendoscopy. Gastrointest Endosc. 1998;48:362–370. doi: 10.1016/s0016-5107(98)70004-5. [DOI] [PubMed] [Google Scholar]
- 12.Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592–1597. doi: 10.1136/gut.2005.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii T, Hasegawa RT, Saitoh Y, Fleischer D, Saito Y, Sano Y, Kato S. Chromoscopy during colonoscopy. Endoscopy. 2001;33:1036–1041. doi: 10.1055/s-0036-1588009. [DOI] [PubMed] [Google Scholar]
- 14.Japanese Research Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus: histopathological classification. 6th ed. Tokyo: KaneharaSyuppan; 1998. pp. p60–90. [Google Scholar]
- 15.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito S, Tajiri H, Ohya T, Nikami T, Aihara H, Ikegami M. Imaging by Magnifying Endoscopy with NBI Implicates the Remnant Capillary Network As an Indication for Endoscopic Resection in Early Colon Cancer. Int J Surg Oncol. 2011;2011:242608. doi: 10.1155/2011/242608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Repici A, Hassan C, Radaelli F, Occhipinti P, De Angelis C, Romeo F, Paggi S, Saettone S, Cisarò F, Spaander M, et al. Accuracy of narrow-band imaging in predicting colonoscopy surveillance intervals and histology of distal diminutive polyps: results from a multicenter, prospective trial. Gastrointest Endosc. 2013;78:106–114. doi: 10.1016/j.gie.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, Kaneko K, Soetikno R, Yoshida S. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278–283. doi: 10.1016/j.gie.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 19.Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599–607.e1. doi: 10.1053/j.gastro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Togashi K, Konishi F, Ishizuka T, Sato T, Senba S, Kanazawa K. Efficacy of magnifying endoscopy in the differential diagnosis of neoplastic and non-neoplastic polyps of the large bowel. Dis Colon Rectum. 1999;42:1602–1608. doi: 10.1007/BF02236215. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda T, Fujii T, Saito Y, Nakajima T, Uraoka T, Kobayashi N, Ikehara H, Ikematsu H, Fu KI, Emura F, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700–2706. doi: 10.1111/j.1572-0241.2008.02190.x. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi Y, Kudo SE, Miyachi H, Hosoya T, Ikehara N, Ohtsuka K, Kashida H, Hamatani S, Hinotsu S, Kawakami K. Clinical usefulness of pit patterns for detecting colonic lesions requiring surgical treatment. Int J Colorectal Dis. 2011;26:1531–1540. doi: 10.1007/s00384-011-1246-0. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto T, Hizawa K, Esaki M, Kurahara K, Mizuno M, Hirakawa K, Yao T, Iida M. Comparison of EUS and magnifying colonoscopy for assessment of small colorectal cancers. Gastrointest Endosc. 2002;56:354–360. doi: 10.1016/s0016-5107(02)70038-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsuruta O, Kawano H, Fujita M, Tsuji Y, Miyazaki S, Fujisaki K, Watanabe M, Nakahara K, Tateishi H, Ban S, et al. Usefulness of the high-frequency ultrasound probe in pretherapeutic staging of superficial-type colorectal tumors. Int J Oncol. 1998;13:677–684. doi: 10.3892/ijo.13.4.677. [DOI] [PubMed] [Google Scholar]
- 26.Saitoh Y, Obara T, Einami K, Nomura M, Taruishi M, Ayabe T, Ashida T, Shibata Y, Kohgo Y. Efficacy of high-frequency ultrasound probes for the preoperative staging of invasion depth in flat and depressed colorectal tumors. Gastrointest Endosc. 1996;44:34–39. doi: 10.1016/s0016-5107(96)70226-2. [DOI] [PubMed] [Google Scholar]


