Abstract
AIM: To assess the clinicopathologic characteristics, risk factors, and prognosis for synchronous multiple early gastric cancer (SMGC).
METHODS: A total of 146 patients with SMGC and 1194 patients with single gastric cancer who had undergone gastrectomy between 1989 and 2008 were retrospectively analyzed to determine their clinicopathologic characteristics and postoperative survival. Tumors were classified into groups on the basis of location and histology. Smoking habits were evaluated using the Brinkman index. Clinical and pathological factors were compared using either Fisher’s exact test or Pearson’s χ2 test. Logistic regression analysis was performed to identify independent risk factors. Survival rate was calculated using the Kaplan-Meier method.
RESULTS: SMGCs accounted for 10.9% of gastric cancer cases and occurred predominantly in elderly male patients with a family history of gastric cancer who were both smokers and drinkers. These tumors were typically macroscopically elevated and histologically differentiated. There were no significant differences between SMGC and single gastric cancer patients with respect to tumor location, tumor size, lymph node metastasis, the number of metastatic lymph nodes, venous invasion, or tumor stage (P = 0.052, P = 0.347, P = 0.595, P = 0.805, P = 0.559, and P = 0.408, respectively). Further, there was no significant difference in postoperative survival between the patient groups (P = 0.200). Of the 146 SMGC patients, a single patient had remnant cancer.
CONCLUSION: A careful preoperative endoscopy is necessary for patients who are at high risk of SMGC, and minimally invasive treatment may be indicated in some cases.
Keywords: Gastric cancer, Synchronous, Multiple, Endoscopy, Prognosis
Core tip: This study compares the clinicopathologic characteristics of synchronous multiple gastric cancer (SMGC) and single gastric cancer, Further, we identified risk factors for SMGC and assessed whether they can be treated with a minimally invasive approach. We found that SMGC occurred predominantly in elderly male patients who had a family history of gastric cancer, and who were both smokers and drinkers. The tumors were macroscopically elevated and histologically differentiated. Lymph node metastasis and vascular invasion were equally prevalent, and there was no significant difference in postoperative survival between these patient groups. We suggest minimally invasive approach may be applicable.
INTRODUCTION
Due to the recent technical advances in endoscopic examinations, the number of patients with synchronous multiple gastric cancer (SMGC) has increased, and SMGC has been reported to account for 6%-14% of all gastric cancer cases[1-3]. A previous study reported that multiple gastric cancers have several clinicopathologic features, including incidence in old age, well differentiated tumors, and early stage tumors, and that the prognosis is similar to that of single gastric cancers[4]. Minimally invasive resection procedures, such as endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and laparoscopic surgery, are known to improve the quality of life of early gastric cancer patients[5,6]. However, the presence of SMGC may increase the risk of missing a remnant gastric lesion and may make it more difficult to determine the range of gastrectomy required. Furthermore, it is unclear whether the same indication criteria for endoscopic resection can be applied to SMGC because comparative studies on the incidence of recurrence and prognosis between multiple and single gastric cancers are limited. The aim of this study was to compare the clinicopathologic characteristics between SMGC and single gastric cancer, to identify risk factors for SMGC, and to assess whether SMGC can be safely treated with a minimally invasive approach.
MATERIALS AND METHODS
SMGC was defined in accordance with Moertel’s criteria[7] as follows: (1) each lesion must have a pathologically proven malignancy; (2) all lesions must be clearly separated by intervals of microscopically normal gastric wall; and (3) the possibility that one of the lesions represents a metastatic tumor must be ruled out beyond any reasonable doubt. Between 1989 and 2008, we identified a total of 1406 patients who underwent gastric resection surgery for early gastric cancer at the Department of Surgery at Kurume University School of Medicine. The patients were excluded from the study if they had undergone gastrectomy after neoadjuvant chemotherapy, if they had surgery after EMR or ESD, or if they had gastric cancer in the remnant stomach after a previous gastrectomy. Therefore, a total cohort of 1340 patients with gastric cancer was analyzed. Microscopic examination of surgically resected specimens revealed SMGC in 146 patients (group A) and a single gastric cancer in the remaining 1194 patients (group B). We retrospectively analyzed the patients’ clinicopathologic characteristics, including age, sex, family history, the presence of cancer in other organs (synchronous or metachronous), smoking and drinking habits, tumor location, tumor size, macroscopic type, histological type, depth of invasion, number of metastatic lymph nodes, lymphovascular invasion, and tumor stage as defined by the Japanese Classification of Gastric Carcinoma (3rd English edition)[8]. The design of this study and the procedures for obtaining informed consent were based on the principles of the Declaration of Helsinki. Our study was approved by The Ethical Committee of Kurume University (No. 13091).
Tumors were classified into groups based on whether they were located in the upper (U), middle (M), or lower (L) third of the stomach, and the macroscopic type of each cancer was classified as I (protruding), IIa (superficial, elevated), IIb (flat), IIc (superficial, depressed), III (excavated), or a combination of these (I + IIa, IIa + IIc, IIc + IIa, or IIc + III). All cases were regrouped into the elevated, flat, depressed, or mixed type. The histological type was classified as either differentiated (papillary adenocarcinoma, well-differentiated and moderately differentiated adenocarcinomas) or undifferentiated (poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma). If the depth of invasion of 2 or more lesions was equal, the largest was regarded as the primary lesion, with all other lesions regarded as accessory lesions.
Smoking habits were evaluated using the Brinkman index (BI)[9,10], which is defined as (number of cigarettes per day) × (number of years for which the patient smoked). Using this index, we divided the patients into 3 groups: nonsmokers, light smokers (BI < 400), and heavy smokers (BI ≥ 400). To assess drinking habits, all patients were asked about their frequency of drinking, the amount (1 go = 22.8 g ethanol) typically consumed on any one occasion, and the type of beverage usually consumed (sake, shochu, beer, whisky, wine, or others). From these data, we calculated the amount of ethanol (in grams) consumed per day, and we classified patients into 3 groups: nondrinkers, occasional drinkers, and daily drinkers (< 22.8, 22.8-45.5, and > 45.5 g of ethanol per day, respectively). For each factor assessed, we used the same questionnaire for all patients when they were admitted.
Clinical and pathological factors were compared using either Fisher’s exact test or Pearson’s χ2 test, as appropriate. Logistic regression analysis was performed to identify independent risk factors with odds ratios and 95%CI. The survival rate was calculated using the Kaplan-Meier method. P < 0.05 was considered statistically significant. Data analysis was performed using the statistical program JMP 8 (SAS Institute, Cary, NC).
RESULTS
A total of 1340 patients [146 patients with SMGC (group A) and 1194 patients with single gastric cancers (group B)] were included in this study. In group A, 120 patients had 2 lesions each, 22 had 3 lesions, 3 had 4 lesions, and 1 patient had 8 lesions, making a total of 326 lesions (Table 1).
Table 1.
Incidence of multiple lesions in synchronous multiple early gastric cancer patients
| No. of tumors | No. of patients | No. of lesions |
| 2 | 120 | 240 |
| 3 | 22 | 66 |
| 4 | 3 | 12 |
| 8 | 1 | 8 |
| Total | 146 | 326 |
The clinical differences between the groups are summarized in Table 2. The patients in group A were older (68.0 years vs 64.3 years, P < 0.001) and more likely to be male (81.5% vs 67.1%, P < 0.001) than those in group B. Furthermore, patients in group A had a significantly greater number of family members with gastric cancer compared with those in group B (P = 0.046), and SMGC patients also smoked (P = 0.020) and drank more (P = 0.005). However, there were no significant differences with respect to the presence of synchronous and metachronous cancers in other organs.
Table 2.
Clinical features of synchronous multiple early gastric cancer (group A) and single gastric cancer patients (group B) n (%)
| Factors | Group A (n = 146) | Group B (n = 1194) | P value |
| Tumor location | |||
| Upper | 30 (20.6) | 183 (15.3) | 0.052 |
| Middle | 40 (27.4) | 450 (37.7) | |
| Lower | 76 (52.1) | 554 (46.4) | |
| Entire | 0 (0.0) | 7 (0.5) | |
| Tumor size (mm) (mean ± SD) | 30.8 ± 15.9 | 32.5 ± 21.5 | 0.347 |
| Macroscopic type | |||
| Elevated | 39 (26.7) | 218 (18.3) | 0.019 |
| Flat | 2 (1.4) | 16 (1.3) | |
| Depressed | 81 (55.5) | 815 (68.3) | |
| Mixed | 24 (16.4) | 145 (12.1) | |
| Histological type | |||
| Differentiated | 118 (80.8) | 771 (64.6) | < 0.001 |
| Undifferentiated | 28 (19.2) | 423 (35.4) | |
| Depth of invasion | |||
| Mucosa | 66 (45.2) | 672 (56.1) | 0.011 |
| Submucosa | 80 (54.8) | 522 (43.9) | |
| LN metastasis | |||
| 0 | 134 (91.8) | 1110 (93.0) | 0.595 |
| 1 | 8 (5.5) | 63 (5.3) | |
| 2 | 4 (2.7) | 15 (1.3) | |
| 3 | 0 (0.0) | 6 (0.5) | |
| No. of metastatic LNs | 0.18 ± 0.72 | 0.21 ± 1.30 | 0.805 |
| Lymphatic invasion | 61 (41.8) | 394 (33.0) | 0.034 |
| Venous invasion | 13 (8.9) | 90 (7.5) | 0.559 |
| Stage | |||
| I | 142 (97.3) | 1173 (98.2) | 0.408 |
| II | 4 (2.7) | 21 (1.8) |
The pathological features of each group are listed in Table 3. Macroscopically, more than 50% of the tumors in both groups were of the depressed type, and this type was more common in group B than in group A. Elevated-type tumors were present more often in group A than in group B (68.3% vs 55.5% and 26.7% vs 18.3%, respectively). The differentiated type was present more often in group A than in group B (80.8% vs 64.6%, P < 0.001). Furthermore, the depth of invasion and the rate of lymphatic invasion in group A were both significantly higher than those in group B (P = 0.011, P = 0.034, respectively). There were no significant differences between the groups with respect to tumor location, tumor size, lymph node metastasis, the number of metastatic lymph nodes, venous invasion, or tumor stage (P = 0.052, P = 0.347, P = 0.595, P = 0.805, P = 0.559, and P = 0.408, respectively). Of the 9 factors that were statistically significant in the univariate analyses, multivariate logistic regression analysis showed that the important risk factors for SMGC were age (OR = 1.93, 95%CI: 1.27-2.99, P = 0.002) and sex (OR = 1.86, 95%CI: 1.07-3.32, P = 0.028, Table 4).
Table 3.
Pathological features of lesions observed in groups A and B n (%)
| Factors | Group A (n = 146) | Group B (n = 1194) | P value |
| Age (mean ± SD) (yr) | 68.0 ± 9.9 | 64.3 ± 11.1 | < 0.001 |
| Gender | |||
| Male | 119 (81.5) | 801 (67.1) | < 0.001 |
| Female | 27 (18.5) | 393 (32.9) | |
| No. of family members with gastric cancer | |||
| None | 103 (70.6) | 917 (76.8) | 0.046 |
| 1 | 30 (20.6) | 224 (18.7) | |
| ≥ 2 | 13 (8.9) | 53 (4.4) | |
| Presence of cancer in other organs | |||
| Synchronous | 4 (2.7) | 48 (4.0) | 0.450 |
| Metachronous | 20 (13.7) | 107 (9.0) | 0.065 |
| Smoking habits | |||
| Never | 49 (33.6) | 536 (44.9) | 0.020 |
| BI < 400 | 19 (13.0) | 157 (13.2) | |
| BI ≥ 400 | 78 (53.4) | 501 (42.0) | |
| Drinking habits | |||
| Non-drinker | 51 (34.9) | 587 (49.2) | 0.005 |
| Occasional-drinker | 55 (37.7) | 340 (28.5) | |
| Daily-drinker | 40 (27.4) | 267 (22.4) | |
Table 4.
Multivariate analysis of risk factors for synchronous multiple early gastric cancer
| Factor | Odds ratio | 95%CI1 | P value |
| Age (yr) | |||
| ≥ 65 vs < 65 | 1.93 | 1.27-2.99 | 0.002 |
| Sex | |||
| Male vs female | 1.86 | 1.07-3.32 | 0.028 |
| Family history | |||
| (+) vs (-) | 1.35 | 0.87-2.06 | 0.182 |
| Smoking habit | |||
| (+) vs (-) | 1.15 | 0.73-1.89 | 0.531 |
| Drinking habit | |||
| (+) vs (-) | 1.51 | 0.79-3.14 | 0.218 |
| Macroscopic type | |||
| Elevated vs depressed | 1.44 | 0.92-2.23 | 0.108 |
| Histological type | |||
| Differentiated vs undifferentiated | 1.53 | 0.94-2.61 | 0.088 |
| Depth of invasion | |||
| Submucosa vs mucosa | 1.61 | 0.90-2.81 | 0.109 |
| Lymphatic invasion | |||
| (+) vs (-) | 0.879 | 0.50-1.60 | 0.664 |
Determined using logistic regression analysis.
The overall median follow-up period was 78.1 months. Of the 146 patients in group A, a single patient had remnant cancer. This patient was diagnosed 1 year after distal gastrectomy, and the lesion was located in the cardia, necessitating a total gastrectomy. One patient died of the disease (hepatic metastasis), and there were 18 other deaths: 3 from an unknown cause, 14 from other diseases (including 5 deaths from cancer of another organ), and 1 from a traffic accident. The 3-year overall survival rates in group A and group B were 95.8% and 96.6%, respectively, and the 5-year overall survival rates in group A and group B were 90.3 and 93.2%, respectively (Figure 1). There was no significant difference in postoperative survival between the groups (P = 0.200).
Figure 1.
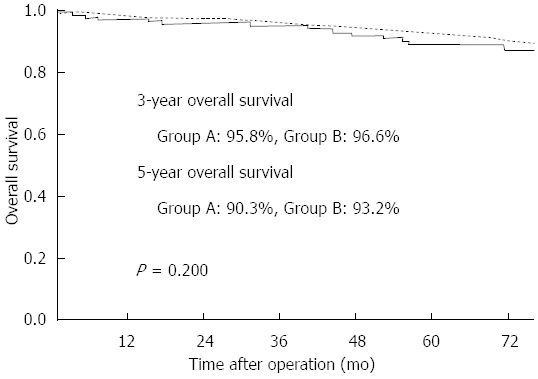
Survival curve for patients with synchronous multiple early gastric cancer (group A: solid line) and those with single gastric cancer (group B: dotted line). The 3-year survival rates of group A and group B were 95.8% and 96.6%, respectively, and the 5-year survival rates of group A and group B were 90.3% and 93.2%, respectively (P = 0.200).
DISCUSSION
Although the histogenesis of normal gastric cancer has been addressed in numerous studies, relatively little is known about the development of multiple gastric cancers. Recent advances in endoscopy have made it possible to detect early gastric cancer, for which EMR and ESD have become common treatment modalities[2,11]. Such therapies conserve function; however, after resection, a large quantity of the gastric mucosa remains, which can give rise to further cancers. Concurrent multiple cancers and the development of post-therapeutic asynchronous multiple cancers represent serious problems. Nasu et al[12] followed 143 patients with early gastric cancer who had undergone EMR and found that 16 (11%) patients developed SMGC within 1 year of the initial EMR. It is important to identify patients who are at a high risk of developing multiple gastric lesions due to the complexity involved in fully diagnosing and treating these patients.
Based on previous reports, SMGC accounts for 6%-14% of all early gastric cancers[1-4]. The incidence of SMGC among patients in this study was 10.9%, which is within this range. In the present study, we found that patients with SMGC were significantly older than those with single gastric cancer. The predominance of SMGC in elderly patients may be explained by the pathogenetic importance of intestinal metaplasia[13], as gastric glands generally show atrophic change with a concomitant increase in intestinal metaplasia in the stomachs of elderly people.
Patients with a family history of carcinoma were reported to have a high incidence of gastric cancer[14,15]. One report reviewed 15 case-control studies of family history and gastric cancer, all of which indicated a positive relationship between these parameters, with risk ratios that ranged from 1.5- to 3.5-fold[16]. The relationship between gastric cancer and genetics has been demonstrated by the non-random involvement of certain chromosomes and related oncogenes, especially Ras and p53[17,18]. Another study showed that gastric cancer was associated with intestinal metaplasia in 52% of the included patients[19]. In this study, compared with single gastric cancer patients, a significantly greater number of SMGC patients had a family history of gastric cancer based on univariate analysis. Furthermore, the number of family members affected also appears to be significant. This may be attributed to genetic factors, in addition to the environmental conditions presumably shared by members of same family, which result in familial clustering of gastric cancer. However, we cannot determine whether this result is due to environmental or genetic factors.
Smoking and alcohol consumption have been reported to be risk factors for gastric cancer[20-22], although a study by Morita et al. found that there was no significant association between the occurrence of multiple gastric cancer and either of these factors[3]. In this study, we also examined these factors in patients with SMGC and those with single gastric cancer. We found that there were significant differences in smoking and drinking habits between patients with SMGC and those with single gastric cancer based on univariate analysis. However, none of these habits were found to be independent risk factors in multivariate analysis. Therefore, the effects of smoking and alcohol on the occurrence of multiple gastric cancers remain controversial.
Tumor location is an important determinant of treatment, and a major concern for patients undergoing subtotal gastrectomy or endoscopic resection is the difficulty of detecting a lesion in the remnant portion of the stomach. In this study, 39.0% of patients with SMGC were found to have tumors located in different thirds of the stomach. In a report concerning the localization of SMGCs, Kitamura et al. stated that early multiple-tumor cases more frequently involved the upper region of the stomach than early single-tumor cases[23]. We also found that SMGC patients were more likely to have a tumor located in the upper third of the stomach compared with single gastric cancer patients. It is difficult to find lesions located in the upper third of the stomach because of the technical limitations of forward-viewing endoscopy. Therefore, this area should be observed very carefully, and caution should be exercised before performing a proximal gastrectomy for early gastric cancer in the cardia or high body.
Most SMGCs were shown to have a differentiated histological type in previous studies[2,3,12]. Correspondingly, we found that, among 326 lesions in the 146 patients with SMGC, 271 (83.1%) lesions were of the differentiated type. Lymphovascular involvement has also been shown to be significantly associated with lymph node metastasis in gastric cancer[24]. Although lymphatic invasion occurred more frequently in the SMGC cases in this study, the frequency of lymph node metastasis and venous invasion in these cases did not differ significantly from that in the single gastric cancer group.
The prognosis of early gastric cancer is generally favorable, and a 5-year relative postoperative survival of ≥ 90% has been reported[2]. In this study, there were no significant differences in the 3- and 5-year survival rates between patients with SMGC and those with single gastric cancer, and the 5-year survival rate was ≥ 90% in both groups.
A total gastrectomy has been recommended for the treatment of multiple gastric cancer because it is believed that the remnant stomach of patients who have undergone a partial gastrectomy is at increased risk for ongoing carcinogenesis[7]. However, prophylactic total gastrectomy reportedly did not improve outcome in patients with multiple gastric cancer[2]. The cumulative prevalence of gastric remnant cancer after ESD or surgical partial gastrectomy for early gastric cancer is reportedly 2.4%-14% at 5 years[12,25,26]. In the present study, 1 patient had remnant cancer, and 2 patients died of gastric cancer after gastrectomy. Early gastric cancer patients treated using EMR or ESD have been identified as a high-risk group for remnant gastric cancer. However, most metachronous lesions were intramucosal tumors with no lymphovascular involvement and no lymph node metastasis, indicating the potential for additional ESD or other local therapies rather than gastrectomy[5]. Endoscopists should recognize the characteristics of SMGCs, and special attention should be given to cases of EMR or ESD to avoid missing synchronous lesions, as SMGC patients are at a high risk of developing metachronous cancer in the remnant stomach after treatment[12,25-27]. Based on our findings, EMR or ESD may be applicable for SMGC cases if they meet the criteria for endoscopic resection.
The limitations of this study include the small number of patients enrolled, its retrospective design, and the fact that not every patient received total gastrectomy; therefore, whole stomach pathology could not be evaluated.
In conclusion, on the basis of the results from our study, we suggest that elderly male patients with a family history of gastric cancer who also have a history of smoking and alcohol consumption should be carefully examined preoperatively, especially if their tumor is macroscopically elevated and histologically differentiated. Additionally, a minimally invasive approach may be applicable for cases of SMGC if they meet the criteria.
COMMENTS
Background
Due to improved diagnostic procedures, more cases of gastric cancer have been diagnosed. Minimally invasive procedures, such as endoscopic resection and laparoscopic surgery, have also gained worldwide acceptance as they improve the quality of life of early gastric cancer patients. However, the benefits of such procedures have not been demonstrated for synchronous multiple early gastric cancer (SMGC).
Research frontiers
It is questionable whether the same indication criteria for minimally invasive procedures can be applied to SMGC because comparative studies on the incidence of LN metastasis and prognosis between multiple and single gastric cancers are limited. Here, we compare the clinicopathologic characteristics of SMGC and single gastric cancer to identify risk factors for SMGC and to assess whether SMGC patients can be safely treated with a minimally invasive approach.
Innovations and breakthroughs
There have been very few studies regarding the risk factors for SMGC. The clinicopathologic characteristics of SMGC and single gastric cancer were addressed in this retrospective study, using a long term follow-up of a large number of patients. Furthermore, this is the first demonstration that there are significant differences in the smoking habits, alcohol consumption, and family history of gastric cancer between patients with SMGC and those with single gastric cancer.
Applications
The findings of this study may lead to an improvement in the post-surgical prognosis of gastric cancer, especially in older men with a family history of smoking and alcohol consumption. Furthermore, if the tumor is macroscopically elevated and histologically differentiated, a minimally invasive approach may be applicable for SMGC cases, provided the criteria are met.
Terminology
SMGC was defined as follows: (1) each lesion must have a pathologically proven malignancy; (2) all lesions must be clearly separated by intervals of microscopically normal gastric wall; and (3) the possibility that one of the lesions represents a metastatic tumor must be ruled out beyond any reasonable doubt. Endoscopic mucosal resection is an endoscopic technique of resection of a lesion that requires the separation of the submucosa using normal saline solution. Endoscopic submucosal dissection is a new method of resection, allowing the dissection of the lesion within the thickness of the submucosa or the interface between the submucosa and the muscularis propria.
Peer review
This is a good retrospective study in which authors analyze the clinicopathologic characteristics and the risks factors of SMGC. The results are interesting and suggest that careful preoperative endoscopy and minimally invasive treatment must be used for patients with high risk of SMGC.
Footnotes
P- Reviewers: Luongo M, Lu XM, Romano C S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
References
- 1.Ribeiro U, Jorge UM, Safatle-Ribeiro AV, Yagi OK, Scapulatempo C, Perez RO, Corbett CE, Alves VA, Zilberstein B, Gama-Rodrigues J. Clinicopathologic and immunohistochemistry characterization of synchronous multiple primary gastric adenocarcinoma. J Gastrointest Surg. 2007;11:233–239. doi: 10.1007/s11605-007-0101-7. [DOI] [PubMed] [Google Scholar]
- 2.Otsuji E, Kuriu Y, Ichikawa D, Okamoto K, Hagiwara A, Yamagishi H. Clinicopathologic characteristics and prognosis of synchronous multifocal gastric carcinomas. Am J Surg. 2005;189:116–119. doi: 10.1016/j.amjsurg.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Morita M, Kuwano H, Baba H, Taketomi A, Kohnoe S, Tomoda H, Araki K, Saeki H, Kitamura K, Sugimachi K. Multifocal occurrence of gastric carcinoma in patients with a family history of gastric carcinoma. Cancer. 1998;83:1307–1311. [PubMed] [Google Scholar]
- 4.Borie F, Plaisant N, Millat B, Hay JM, Fagniez PL, De Saxce B. Treatment and prognosis of early multiple gastric cancer. Eur J Surg Oncol. 2003;29:511–514. doi: 10.1016/s0748-7983(03)00094-5. [DOI] [PubMed] [Google Scholar]
- 5.Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 6.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N; Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moertel CG, Bargen JA, Soule EH. Multiple gastric cancers; review of the literature and study of 42 cases. Gastroenterology. 1957;32:1095–1103. [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 9.Brinkman GL, Coates EO. The effect of bronchitis, smoking, and occupation on ventilation. Am Rev Respir Dis. 1963;87:684–693. doi: 10.1164/arrd.1963.87.5.684. [DOI] [PubMed] [Google Scholar]
- 10.Ichinoe M, Mikami T, Hara A, Tsuruta T, Okayasu I. Background submucosal cysts in early gastric cancer cases have unique clinicopathologic features suggestive of postgastritis and significant smoking association. Am J Clin Pathol. 2007;128:746–752. doi: 10.1309/WPBUNM51WQJWU4PC. [DOI] [PubMed] [Google Scholar]
- 11.Miyashita M, Tajiri T, Maruyama H, Makino H, Nomura T, Sasajima K, Yamashita K. Endoscopic mucosal resection scissors for the treatment of early gastric cancer. Endoscopy. 2003;35:611–612. doi: 10.1055/s-2003-40238. [DOI] [PubMed] [Google Scholar]
- 12.Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37:990–993. doi: 10.1055/s-2005-870198. [DOI] [PubMed] [Google Scholar]
- 13.Nitta T, Egashira Y, Akutagawa H, Edagawa G, Kurisu Y, Nomura E, Tanigawa N, Shibayama Y. Study of clinicopathological factors associated with the occurrence of synchronous multiple gastric carcinomas. Gastric Cancer. 2009;12:23–30. doi: 10.1007/s10120-008-0493-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen JD, Kearns S, Porter T, Richards FM, Maher ER, Teh BT. MET mutation and familial gastric cancer. J Med Genet. 2001;38:E26. doi: 10.1136/jmg.38.8.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato I, Tominaga S, Matsumoto K. A prospective study of stomach cancer among a rural Japanese population: a 6-year survey. Jpn J Cancer Res. 1992;83:568–575. doi: 10.1111/j.1349-7006.1992.tb00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaghoobi M, Bijarchi R, Narod SA. Family history and the risk of gastric cancer. Br J Cancer. 2010;102:237–242. doi: 10.1038/sj.bjc.6605380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh LL, Hsieh JT, Wang LY, Fang CY, Chang SH, Chen TC. p53 mutations in gastric cancers from Taiwan. Cancer Lett. 1996;100:107–113. doi: 10.1016/0304-3835(95)04077-3. [DOI] [PubMed] [Google Scholar]
- 18.Peddanna N, Holt S, Verma RS. Genetics of gastric cancer. Anticancer Res. 1995;15:2055–2064. [PubMed] [Google Scholar]
- 19.Ruol A, Parenti A, Zaninotto G, Merigliano S, Costantini M, Cagol M, Alfieri R, Bonavina L, Peracchia A, Ancona E. Intestinal metaplasia is the probable common precursor of adenocarcinoma in barrett esophagus and adenocarcinoma of the gastric cardia. Cancer. 2000;88:2520–2528. doi: 10.1002/1097-0142(20000601)88:11<2520::aid-cncr13>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Nomura A, Grove JS, Stemmermann GN, Severson RK. A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res. 1990;50:627–631. [PubMed] [Google Scholar]
- 21.Ji BT, Chow WH, Yang G, McLaughlin JK, Gao RN, Zheng W, Shu XO, Jin F, Fraumeni JF, Gao YT. The influence of cigarette smoking, alcohol, and green tea consumption on the risk of carcinoma of the cardia and distal stomach in Shanghai, China. Cancer. 1996;77:2449–2457. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2449::AID-CNCR6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70:50–55. doi: 10.1002/1097-0142(19920701)70:1<50::aid-cncr2820700109>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura K, Yamaguchi T, Okamoto K, Otsuji E, Taniguchi H, Hagiwara A, Sawai K, Takahashi T. Clinicopathologic features of synchronous multifocal early gastric cancers. Anticancer Res. 1997;17:643–646. [PubMed] [Google Scholar]
- 24.Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer. 2008;11:134–148. doi: 10.1007/s10120-008-0476-5. [DOI] [PubMed] [Google Scholar]
- 25.Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Yagi S, Kato J, et al. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:887–894. doi: 10.1016/j.gie.2008.03.1089. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Kim SG, Im JP, Kang SJ, Lee HJ, Yang HK, Kim JS, Kim WH, Jung HC, Song IS. Lymph node metastasis in multiple synchronous early gastric cancer. Gastrointest Endosc. 2011;74:276–284. doi: 10.1016/j.gie.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Fujita T, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kinoshita T. Clinical and histopathological features of remnant gastric cancers, after gastrectomy for synchronous multiple gastric cancers. J Surg Oncol. 2009;100:466–471. doi: 10.1002/jso.21352. [DOI] [PubMed] [Google Scholar]


