Abstract
AIM: To obtain reference values for pancreatic flow output rate (PFR) and peak time (PT) in healthy volunteers and chronic pancreatitis (CP); to correlate quantification of secretin enhanced magnetic resonance cholangiopancreatography (SMRCP) of pancreatic fluid output following secretin with fecal elastase-1 (FE-1) tests.
METHODS: The present study includes 53 subjects comprised of 17 healthy individuals and 36 patients with CP from April 2011 to January 2013. The 36 patients with CP were divided into three groups of mild CP (n = 14), moderate CP (n = 19) and advanced CP (n = 3) by M-ANNHEIM classification for CP.. Fifty-three cases underwent FE-1 test and magnetic resonance imaging using 3.0 T-device (Signa EXCITE, GE Healthcare). Coronal T2-weighted single-shot turbo spin-echo, spiratory triggered, covering the papillae, duodenum and small bowel. MRCP was performed with a heavily T2-weighted fat-suppressed long TE HASTE sequence (thick slab 2D MRCP sequence), repeated every 2 min up to 11 min after 0.1 mL/kg secretin injection (Secrelux, Sanochemia®, Germany). FE-1 test used sandwich enzyme-linked immunosorbent assay (ELISA) test (ScheBo. Tech®, Germany).
RESULTS: A good linear correlation showed between the calculated volume and the actual volume by Phantom experiments. Fifty-three paired Quantification of secretin enhanced magnetic resonance cholangiopancreatography (MRCPQ) and FE-1 data sets were analyzed. The mean FE-1 of 53 cases was 525.41 ± 94.44 μg/g for 17 healthy volunteers, 464.95 ± 136.13 μg/g for mild CP, 301.55 ± 181.55 μg/g for moderate CP, 229.30 ± 146.60 μg/g for advanced CP. Also, there was statistically significant difference in FE-1 (P = 0.0001) between health and CP. The mean values of PFR and PT were 8.18 ± 1.11 mL/min, 5.76 ± 1.71 min for normal; 7.27 ± 2.04 mL/min, 7.71 ± 2.55 min for mild CP; 4.98 ± 2.57 mL/min, 9.10 ± 3.00 min for moderate CP; 4.13 ± 1.83 mL/min, 12.33 ± 1.55 min for advanced CP. Further, statistically significant difference in PFR (P = 0.0001) and PT (P = 0.0001) was observed between health and CP. Besides, there was correlation (r = 0.79) and consistency (K = 0.6) between MRCPQ and ELISA Test. It was related between M-ANNHEIM classification and PFR (r = 0.55), FE-1 (r = 0.57).
CONCLUSION: SMRCP can provide a safe, non-invasive and efficient method to evaluate the exocrine function of the pancreas.
Keywords: Secretin, Magnetic resonance cholangiopancreatography, Pancreatic exocrine function, Chronic pancreatitis, Magnetic resonance imaging
Core tip: After all subjects were injected secretin, the results of secretin enhanced magnetic resonance cholangiopancreatography (SMRCP) were that the mean values of PFR and PT were 8.18 ± 1.11 mL/min, 5.76 ± 1.71 min for normal; 7.27 ± 2.04 mL/min, 7.71 ± 2.55 min for mild chronic pancreatitis (CP); 4.98 ± 2.57 mL/min, 9.10 ± 3.00 min for moderate CP; 4.13 ± 1.83 mL/min, 12.33 ± 1.55 min for advanced CP. Statistically significant difference in PFR and PT was observed between health and CP. Also, there was correlation and consistency between Quantification of secretin enhanced magnetic resonance cholangiopancreatography and enzyme-linked immunosorbent assay test. SMRCP can provide a safe, non-invasive and efficient method to evaluate the exocrine function of the pancreas.
INTRODUCTION
Pancreatic exocrine function test plays an important role of in the diagnosis of chronic pancreatitis. It can guide the clinical diagnosis and therapy. At present, the “tubed” secretin test is the standard reference method for functional investigations. Unfortunately, this test is hampered by lack of test standardization and is uncomfortable for the patient due to prolonged duodenal intubation[1,2]. Indirect pancreatic function test is non-invasive, safe and cost-effective, but it is positive only in a serious shortage of pancreatic exocrine function[3,4]. FE-1 (fecal elastase-1) test is one of the indirect pancreatic exocrine tests, and widely used in the clinical. Its role in diagnosing chronic pancreatitis is controversial[5-7]. However, previous studies have proven a positive correlation between the secretin test and FE-1, suggesting that it has a possible role in evaluating exocrine pancreatic function[8-10].
Magnetic resonance cholangiopancreatography (MRCP) has been a reliable noninvasive method for the diagnosis of both pancreatic and biliary disease[11,12]. Magnetic resonance imaging is a noninvasive diagnostic imaging method without ionizing radiation and provides details of pancreatic parenchymal and ductal morphology with potential relevance for pancreatic exocrine function[13].
Secretin enhanced magnetic resonance cholangiopancreatography (SMRCP) is routinely performed as part of the work up of patients with known or suspected pancreatic disease in many centers. Recently, SMRCP has gained increasing interest as a noninvasive imaging method enabling visualization and quantitative assessment of various aspects of pancreatic exocrine function[14-19].
In this study we propose a similar quantification method using S-MRCP to evaluate pancreatic exocrine function during secretin stimulation by measuring pancreatic flow rate (PFR). Our study has both primary and secondary purposes: (1) to obtain reference values for PFR in healthy volunteers and CP; and (2) to correlate quantification of secretin enhanced magnetic resonance cholangiopancreatography (MRCPQ) of pancreatic fluid output following secretin with FE-1 tests.
MATERIALS AND METHODS
Ethics
This work has been supported by Natural Science Foundation of China. This study was approved ethically by the Second Military Medical University. All patients provided informed written consent.
Patients
From April 2011 to January 2013, the study included 53 subjects (18 females and 35 males) composed of 17 healthy volunteers and 36 patients with CP. Twelve health were male and five health were female, with a mean age of 43.71 ± 14.48 years (range, 64-24 years) and a mean body mass index (BMI) of 24.60 ± 3.45 kg/m2 (range, 31.12-24.60 kg/m2). Twenty-three patients were male and thirteen patients were female, with a mean age of 41.73 ± 14.13 years (range, 15-78 years) and a mean BMI of 23.60 ± 4.42 kg/m2 (range, 15.39-34.65 kg/m2). Thirty six patients with CP were divided into three groups of mild CP (n = 14), moderate CP (n = 19) and advanced CP (n = 3) by M-ANNHEIM classification for CP[20-24].
Phantom experiments
Measurement phantoms (MP) had a cylindrical shape and were filled with water. MPs were moved to isocenter. All images were acquired with the same the positions ensuring all imaging volume included matching phantoms. Volumes from 0 mL up to 200 mL water were applied. Put 200 mL of water into MP with different increments (0-50 mL in 5 mL increments, 50-100 mL in 10 mL increments, 100-200 mL in 50 mL increments). Then, the correlation between actual volume and calculated volume was examined.
Voxels containing 100% water were identified from the middle slice of the last volume step, when MP underwent maximal filling[17]. Commercially available software provided to measure the mean signal intensity/voxel.
FE-1 Test
For the FE-1 test, a single random stool sample was required with no need to stop enzyme supplements. Pancreatic exocrine reserve was in all patients using the FE-1 test determined. Results were determined using the sandwich enzyme-linked immunosorbent assay (ELISA) test, with two monoclonal antibodies that only bind to human elastase-1. A commercial kit was used (ScheBo Tech®, Germany) containing a human elastase-specific antibody-sensitized ELISA plate. Elastase concentration was then determined by a photometric method. The FE-1 values were separately scored as 1 for FE-1≥ 200 μg/g, and 0 for FE-1 < 200 μg/g.
MR technique
All examinations were performed using a 3.0-T MR (Signa EXCITE, GE Healthcare) with a phased array body coil. All examinations were fasted for at least 4 h before examination. Immediately, before imaging patients drank 100 mL of water ensuring the presence of a voxel containing 100% water within the imaging volume.
The pancreatic MR examination protocol consisted of: (1) coronal T2-weighted single-shot turbo spin-echo, respiratory-triggered, covering the papillae, duodenum and small bowel. The parameters for coronal T2-weighted sequences were the following: TR/TE 1375/119 ms, slice thickness 64 mm, no gap, echo time 119 ms, FA 90°, field of view (FOV) 400 × 400, matrix 224 × 288; and (2) MRCP was performed with a heavily T2-weighted fat-suppressed long-TE HASTE sequence (thick slab 2D MRCP sequence). Imaging parameters were TR/TE 7000 ms/1270 ms, flip angle 90°, 64 mm slice thickness, FOV 320 mm × 320 mm - 420 mm × 420 mm, matrix 288 × 288, echo time 1274 ms. After the first dynamic acquisition, a bolus of secretin was injected intravenously at a dose of 1 CU/kg body weight. The secretin (Secrelux Sanochemia®, Germany) dose 0.1 mL/kg was administered and the exact same sequence repeated at 1, 3, 5, 7, 9 and 11 min. All positions before and after injection remained unchanged.
Imaging analysis
Two single radiologists assessed pancreatic imaging without knowledge of clinical and FE-1 data.
A semi-quantitative evaluation of duodenal filling (DF), a function of pancreatic exocrine secretion, was performed on MRCP images obtained 10 min after secretin injection and compared with MRCP images acquired before secretin administration. MRCP images were assessed as follows described by Matos et al[25], grade 0 for absence of DF; grade 1 for fluid limited into the duodenal bulb; grade 2 for DF up to the genu inferius; grade 3 for filling beyond to the genu inferius. In agreement within the literature, pancreatic exocrine function reduced when DF was less than grade 3.
Subsequently, a quantitative analysis of DF was performed by drawing an appropriate region of interest (ROI). Changes in signal intensity in the imaging volume were plotted against time, and the flow rate derived from the gradient. PFR were calculated using the method previously described in[17,19,25]. Pancreatic exocrine secretions were quantified by PFR and PT. Then the PFR values were separately scored as 1 for PFR ≥ 5 mL/min, and 0 for PFR < 5 mL/min. The volume of water was calculated with the following formula: Volume = (Mean signal intensity/Voxel × Size of the region of interest)/Signal intensity (100% water).
Statistical analysis
Statistical analyses were performed in SPSS 20.0 for Windows software. Data are reported as the median or the mean ± one SD. Mean flow rates and FE-1 were compared using the one-way ANOVA LSD test and Student’s t test. It was statistically significant different at the 0.05 level. Linear regression analysis was used to compare the values between PFR and FE-1. Correlations between DF grades, M-ANNHEIM classifications and PFR, FE-1 were tested using the Spearman’s rank correlation coefficients respectively. Correlation was significant at the 0.01 level. The consistency of the two methods was compared with the test where MRCPQ < 5 mL/min, FE-1 < 200 μg/g as abnormal.
RESULTS
The result of phantom experiments
Phantom experiments showed a good correlation between known and calculated volumes of water (Figure 1).
Figure 1.
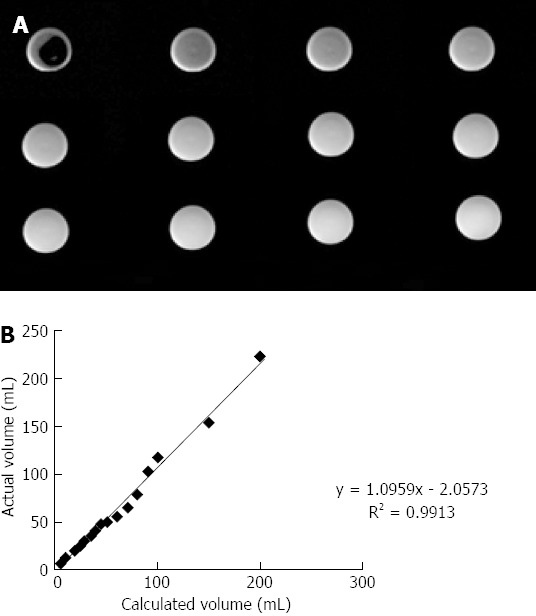
Images of Phantom experiments. A: Measurement phantoms (MP) images with 5-70 mL of water in the MP; B: Correlation between the calculated and actual volumes of water in vitro study.
Semi-quantitative image analysis
Ten minutes after secretin administration, 17 volunteers and 23/33 patients showed a DF beyond the genu inferius (grade 3); whereas 8/36 showed a DF up to the genu inferius (grade 2); 5/36 showed a DF limited to the duodenal bulb (grade 1); None of the patients showed absent filling (grade 0).
Quantitative image analysis
Table 1 demonstrated results of the mean FE-1, PFR and PT of volunteers and patients based on M-ANNHEIM classification for CP. Table 2 demonstrated results of the mean FE-1, PFR and PT of volunteers and patients based on DF volume for CP. There was correlation (r = 0.79) and consistency (K = 0.6) between MRCPQ and FE-1. Also, it was related between M-ANNHEIM classification and PFR (r = 0.55), FE-1 (r = 0.57). There were correlations between DF grades and PFR (r = 0.36), but no FE-1 (r = 0.29, Figures 2 and 3, Tables 3 and 4).
Table 1.
Results in normal and chronic pancreatitis
| n | FE-1 (μg/g) | PFR (mL/min) | PT (min) | |
| Normal | 17 | 525.41 ± 94.44 | 8.18 ± 1.11 | 5.76 ± 1.71 |
| Mild | 14 | 464.95 ± 136.13 | 7.27 ± 2.04 | 7.71 ± 2.55 |
| Moderate | 19 | 301.55 ± 181.55 | 4.98 ± 2.57 | 9.10 ± 3.00 |
| Advanced | 3 | 229.30 ± 146.60 | 4.13 ± 1.83 | 12.33 ± 1.15 |
| F value | 9.45 | 9.59 | 9.06 | |
| P value | 0.000 | 0.000 | 0.000 |
FE-1: Fecal elastase-1; PFR: Pancreatic flow output rate; PT: Peak time.
Table 2.
Results in normal and reduced duodenal filling
| n | FE-1 (μg/g) | PFR (mL/min) | PT (min) | |
| Normal DF ( grade 3) | 40 | 453.64 ± 162.74 | 7.16 ± 2.34 | 7.28 ± 2.56 |
| Reduced DF ( grade 1, 2) | 13 | 285.59 ± 158.03 | 4.71 ± 1.92 | 9.61 ± 3.57 |
| P value | 0.002 | 0.001 | 0.012 |
FE-1: Fecal elastase-1; PFR: Pancreatic flow output rate; PT: Peak time; DF: Duodenal filling.
Figure 2.
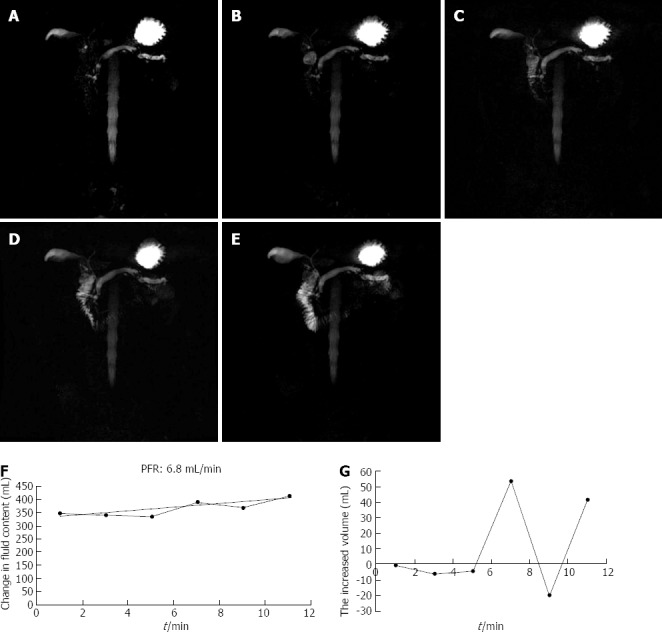
An illustration of the secretin effect with the calculated pancreatic flow rate in a 54-year-old man with chronic pancreatitis. A-E: There was a visible increase in DF at 1, 3, 5, 7, 9, 11 min after secretin, grade 3 DF was present on SMRCP at 10 min; F, G: A plot of change in volume over time from patient data, normal response with a flow rate of 6.8 mL/s and PT of 7 min.
Figure 3.
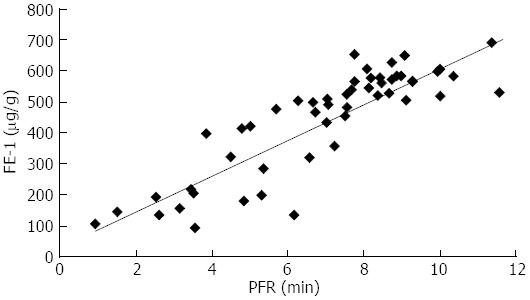
Scatter plot of pancreatic flow output rate and fecal elastase-1.
Table 3.
Quantification of secretin enhanced magnetic resonance cholangiopancreatography vs Fecal elastase-1 n (%)
| Normal FE-1 | Abnormal FE-1 | |
| Normal MRCPQ | 18 (51.4) | 3 (8.6) |
| Abnormal MRCPQ | 4 (11.4) | 10 (28.6) |
MRCPQ (quantification of secretin enhanced magnetic resonance cholangiopancreatography) < 5 mL/min and FE-1 < 200 μg/g are abnormal K = 0.6. FE-1: Fecal elastase-1.
Table 4.
Results of correlation
|
M-ANNHEIM |
DF |
|||
| r value | P value | r value | P value | |
| PFR | 0.55 | 0.000 | 0.36 | 0.009 |
| FE-1 | 0.57 | 0.000 | 0.29 | 0.038 |
Correlation is significant at the 0.01 level (2-tailed). DF: duodenal filling.
DISCUSSION
In this study, we quantified pancreatic exocrine function of CP. Some technical details are worth emphasis. The coil we used was the body coil not torso phased-array coil, because the body receiver coil provided a uniform sensitivity[26,27]. Second, Voxels containing 100% water was chosen in order to reduce the impaction of surrounding. If not, it would cause a high PFR[19,20]. Third, all cases fasted for at least four hours before examination. Immediately, patients were given 100 mL of water to drink before imaging, to ensure the presence of a voxel containing 100% water within the imaging volume. Keeping positions constant is also critical before and after the injection of secretin. Fourth, the scanning images should be along the long axis of the pancreas[28-31]. Lastly, we chose 64 mm slice thickness and large ROI including the papillae, duodenum and small bowel to prevent fluid loss.
There are some limitations in this study. The study sample is relatively small, in particular, the subgroup. Besides, it is distinguishing between water filled in measurement phantoms and pancreatic juice. Additionally, phantom experiments are only valid in vitro. The other data[15,16,18,29] show that a thin multilayer coronal T2WI images were obtained after the injection of secretin; based on change of signal, the volume of pancreatic secretion by the equation is calculated. This method needed patients to drink water many times. Finally, what we calculated is PFR, not pancreatic flow volume.
In summary, we have shown a good linear correlation between the calculated volume and the actual volume by Phantom experiments, which also has been reported in the literature[32-34]. The data gathered by the phantom studies strongly support the feasibility of this technique. Using the same technique, we have been able to measure pancreatic flow rates in health and CP. Moreover, there are correlation and consistency between MRCPQ and FE-1. Our study confirmed the capability of MRCP images to quantitatively evaluate the pancreatic exocrine reserve. Nevertheless, considering the limitations of this study, we need to confirm our results with further trials.
COMMENTS
Background
Pancreatic exocrine function test plays an important role of in the diagnosis of chronic pancreatitis. It can guide the clinical diagnosis and therapy. At present, the “tubed” secretin test as the standard reference method is hampered by lack of test standardization and is uncomfortable for the patient due to prolonged duodenal intubation. Indirect pancreatic function test is non-invasive, safe and cost-effective, but it is positive only in a serious shortage of pancreatic exocrine function. Recently, secretin enhanced magnetic resonance cholangiopancreatography (SMRCP) has gained increasing interest as a non-invasive imaging method enabling visualization and quantitative assessment of various aspects of pancreatic exocrine function.
Research frontiers
The authors examined the published literatures on quantification of pancreatic exocrine function and there were no there were no other related or similar studies in China.
Innovations and breakthroughs
In this study, the authors chose 64 mm slice thickness and large region of interest to quantify pancreatic exocrine function of chronic pancreatitis. This way is non-invasive, safe and effective and above all, directly measure pancreatic fluid output.
Applications
The results of the present study indicate that SMRCP has been a reliable noninvasive method for the diagnosis of pancreatic exocrine function, whereas it can displace the “tubed” secretin test and other indirect tests.
Terminology
Quantification of secretin enhanced-MRCPQ was acquired as follows: The secretin dose 0.1 mL/kg was administered and the exact same sequence repeated at 1, 3, 5, 7, 9 and 11 min. All positions before and after injection remained unchanged.
Peer review
Pancreatic exocrine function test plays an important role of in the diagnosis of chronic pancreatitis. It can guide the clinical diagnosis and therapy. Recent developments in SMRCP techniques have shown the feasibility of a quantitative assessment of the pancreatic exocrine function.
Footnotes
P- Reviewers: Gholamrezanezhad A, Tsai HH S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Stevens T, Parsi MA. Update on endoscopic pancreatic function testing. World J Gastroenterol. 2011;17:3957–3961. doi: 10.3748/wjg.v17.i35.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace MB. Imaging the pancreas: into the deep. Gastroenterology. 2007;132:484–487. doi: 10.1053/j.gastro.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Chari ST, Singer MV. The problem of classification and staging of chronic pancreatitis. Proposals based on current knowledge of its natural history. Scand J Gastroenterol. 1994;29:949–960. doi: 10.3109/00365529409094869. [DOI] [PubMed] [Google Scholar]
- 4.Siegmund E, Löhr JM, Schuff-Werner P. The diagnostic validity of non-invasive pancreatic function tests--a meta-analysis. Z Gastroenterol. 2004;42:1117–1128. doi: 10.1055/s-2004-813604. [DOI] [PubMed] [Google Scholar]
- 5.Wali PD, Loveridge-Lenza B, He Z, Horvath K. Comparison of fecal elastase-1 and pancreatic function testing in children. J Pediatr Gastroenterol Nutr. 2012;54:277–280. doi: 10.1097/MPG.0b013e31820b0227. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury RS, Forsmark CE. Review article: Pancreatic function testing. Aliment Pharmacol Ther. 2003;17:733–750. doi: 10.1046/j.1365-2036.2003.01495.x. [DOI] [PubMed] [Google Scholar]
- 7.Glasbrenner B, Kahl S, Malfertheiner P. Modern diagnostics of chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14:935–941. doi: 10.1097/00042737-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Adamek HE, Albert J, Breer H, Weitz M, Schilling D, Riemann JF. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356:190–193. doi: 10.1016/S0140-6736(00)02479-X. [DOI] [PubMed] [Google Scholar]
- 9.Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39:580–586. doi: 10.1136/gut.39.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walkowiak J, Cichy WK, Herzig KH. Comparison of fecal elastase-1 determination with the secretin-cholecystokinin test in patients with cystic fibrosis. Scand J Gastroenterol. 1999;34:202–207. doi: 10.1080/00365529950173104. [DOI] [PubMed] [Google Scholar]
- 11.Benini L, Amodio A, Campagnola P, Agugiaro F, Cristofori C, Micciolo R, Magro A, Gabbrielli A, Cabrini G, Moser L, et al. Fecal elastase-1 is useful in the detection of steatorrhea in patients with pancreatic diseases but not after pancreatic resection. Pancreatology. 2013;13:38–42. doi: 10.1016/j.pan.2012.11.307. [DOI] [PubMed] [Google Scholar]
- 12.Calvo MM, Bujanda L, Calderón A, Heras I, Cabriada JL, Bernal A, Orive V, Astigarraga E. Comparison between magnetic resonance cholangiopancreatography and ERCP for evaluation of the pancreatic duct. Am J Gastroenterol. 2002;97:347–353. doi: 10.1111/j.1572-0241.2002.05468.x. [DOI] [PubMed] [Google Scholar]
- 13.Wathle GK, Tjora E, Ersland L, Dimcevski G, Salvesen OO, Molven A, Njølstad PR, Haldorsen IS. Assessment of exocrine pancreatic function by secretin-stimulated magnetic resonance cholangiopancreaticography and diffusion-weighted imaging in healthy controls. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24167. [DOI] [PubMed] [Google Scholar]
- 14.Bali MA, Sztantics A, Metens T, Arvanitakis M, Delhaye M, Devière J, Matos C. Quantification of pancreatic exocrine function with secretin-enhanced magnetic resonance cholangiopancreatography: normal values and short-term effects of pancreatic duct drainage procedures in chronic pancreatitis. Initial results. Eur Radiol. 2005;15:2110–2121. doi: 10.1007/s00330-005-2819-5. [DOI] [PubMed] [Google Scholar]
- 15.Schneider AR, Hammerstingl R, Heller M, Povse N, Murzynski L, Vogl TJ, Caspary WF, Stein J. Does secretin-stimulated MRCP predict exocrine pancreatic insufficiency?: A comparison with noninvasive exocrine pancreatic function tests. J Clin Gastroenterol. 2006;40:851–855. doi: 10.1097/01.mcg.0000225652.00308.a2. [DOI] [PubMed] [Google Scholar]
- 16.Burbridge JA, Barch DM. Emotional valence and reference disturbance in schizophrenia. J Abnorm Psychol. 2002;111:186–191. [PubMed] [Google Scholar]
- 17.Punwani S, Gillams AR, Lees WR. Non-invasive quantification of pancreatic exocrine function using secretin-stimulated MRCP. Eur Radiol. 2003;13:273–276. doi: 10.1007/s00330-002-1605-x. [DOI] [PubMed] [Google Scholar]
- 18.Manfredi R, Perandini S, Mantovani W, Frulloni L, Faccioli N, Pozzi Mucelli R. Quantitative MRCP assessment of pancreatic exocrine reserve and its correlation with faecal elastase-1 in patients with chronic pancreatitis. Radiol Med. 2012;117:282–292. doi: 10.1007/s11547-011-0774-6. [DOI] [PubMed] [Google Scholar]
- 19.Gillams A, Pereira S, Webster G, Lees W. Correlation of MRCP quantification (MRCPQ) with conventional non-invasive pancreatic exocrine function tests. Abdom Imaging. 2008;33:469–473. doi: 10.1007/s00261-007-9286-1. [DOI] [PubMed] [Google Scholar]
- 20.Ammann RW. A clinically based classification system for alcoholic chronic pancreatitis: summary of an international workshop on chronic pancreatitis. Pancreas. 1997;14:215–221. doi: 10.1097/00006676-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss Med Wkly. 2006;136:166–174. doi: 10.4414/smw.2006.11182. [DOI] [PubMed] [Google Scholar]
- 22.Schneider A, Löhr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101–119. doi: 10.1007/s00535-006-1945-4. [DOI] [PubMed] [Google Scholar]
- 23.Tsimmerman IaS. New International Classification of Chronic Pancreatitis (M-ANNHEIM multifactor classification system, 2007): principles, merits, and demerits. Klin Med (Mosk) 2008;86:7–13. [PubMed] [Google Scholar]
- 24.Schneider A, Lohr J, Singer MV. New international classification of chronic pancreatitis (2007) M-ANNHEIM. Eksp Klin Gastroenterol. 2010;(8):3–16. [PubMed] [Google Scholar]
- 25.Matos C, Metens T, Devière J, Nicaise N, Braudé P, Van Yperen G, Cremer M, Struyven J. Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology. 1997;203:435–441. doi: 10.1148/radiology.203.2.9114101. [DOI] [PubMed] [Google Scholar]
- 26.Heverhagen JT, Boehm D, Klose KJ. Calibrated magnetic resonance hydrometry: an in vitro study. J Magn Reson Imaging. 2003;17:472–477. doi: 10.1002/jmri.10267. [DOI] [PubMed] [Google Scholar]
- 27.Heverhagen JT, Müller D, Battmann A, Ishaque N, Boehm D, Katschinski M, Wagner HJ, Klose KJ. MR hydrometry to assess exocrine function of the pancreas: initial results of noninvasive quantification of secretion. Radiology. 2001;218:61–67. doi: 10.1148/radiology.218.1.r01ja2061. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed SA, Wray C, Rilo HL, Choe KA, Gelrud A, Howington JA, Lowy AM, Matthews JB. Chronic pancreatitis: recent advances and ongoing challenges. Curr Probl Surg. 2006;43:127–238. doi: 10.1067/j.cpsurg.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Zuccaro P, Stevens T, Repas K, Diamond R, Lopez R, Wu B, Conwell DL. Magnetic resonance cholangiopancreatography reports in the evaluation of chronic pancreatitis: a need for quality improvement. Pancreatology. 2009;9:764–769. doi: 10.1159/000201304. [DOI] [PubMed] [Google Scholar]
- 30.Tirkes T, Menias CO, Sandrasegaran K. MR imaging techniques for pancreas. Radiol Clin North Am. 2012;50:379–393. doi: 10.1016/j.rcl.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Balci NC, Bieneman BK, Bilgin M, Akduman IE, Fattahi R, Burton FR. Magnetic resonance imaging in pancreatitis. Top Magn Reson Imaging. 2009;20:25–30. doi: 10.1097/RMR.0b013e3181b483c2. [DOI] [PubMed] [Google Scholar]
- 32.Jin EH, Ichikawa T, Erturk SM, Motosugi U, Hirano M, Araki T. Calibrated magnetic resonance hydrometry to quantify pancreatic juice: a preliminary study. J Magn Reson Imaging. 2009;29:217–220. doi: 10.1002/jmri.21614. [DOI] [PubMed] [Google Scholar]
- 33.Heverhagen JT, Battmann A, Kirsch M, Boehm D, Eissele R, Klose KJ, Wagner HJ. Magnetic resonance hydrometry: non-invasive quantification of the exocrine pancreatic function. Rofo. 2002;174:291–296. doi: 10.1055/s-2002-20606. [DOI] [PubMed] [Google Scholar]
- 34.Heverhagen JT, Hartlieb T, Boehm D, Klose KJ, Wagner HJ. Magnetic resonance cystometry: accurate assessment of bladder volume with magnetic resonance imaging. Urology. 2002;60:309–314. doi: 10.1016/s0090-4295(02)01726-0. [DOI] [PubMed] [Google Scholar]


