Abstract
The concept of fecal microbiota transplantation (FMT) has been used in traditional Chinese medicine at least since the 4th century. Evidence from recent human studies strongly supports the link between intestinal bacteria and inflammatory bowel disease. We proposed that standardized FMT might be a promising rescue therapy for refractory inflammatory bowel disease. However, there were no reports of FMT used in patients with severe Crohn’s disease (CD). Here, we report the successful treatment of standardized FMT as a rescue therapy for a case of refractory CD complicated with fistula, residual Barium sulfate and formation of intraperitoneal large inflammatory mass. As far as we know, this is the first case of severe CD treated using FMT through mid-gut.
Keywords: Fecal microbiota transplantation, Crohn’s disease, Rescue therapy, Inflammatory bowel disease, Fistula
Core tip: We proposed that standardized fecal microbiota transplantation (FMT) might be a promising rescue therapy for refractory inflammatory bowel disease. This case report provided the first description of severe Crohn’s disease in sustained clinical remission after FMT, and the brief protocol of patient preparation before and during FMT. Although there was only one case, the present result in our pilot clinical trial strongly supported our initial hypothesis and highlighted the attractive role of the remodeling of gut flora in host diseases.
INTRODUCTION
The gut microbiota is considered to constitute a “microbial organ” which plays a pivotal role in the intestinal diseases[1]. The gut metagenome sequencing showed that over 99% of the genes are bacterial[2]. Although fecal microbiota transplantation (FMT) has only recently gained popularity with its success in treating Clostridium difficile infection[3], the concept of FMT for treatment of human intestinal diseases has been recorded at least for 1700 years in traditional Chinese medicine[4]. Evidence from human studies strongly supports the link between intestinal bacteria and inflammatory bowel diseases (IBD)[5-7]. IBD includes ulcerative colitis (UC) and Crohn’s disease (CD). However, there have been only four publications on FMT for the treatment of IBD in 18 cases of UC[8-10] and one case of newly diagnosed CD[10]. To date, there has been no report of FMT used as a rescue therapy in patients with severe CD. We proposed that standardized FMT might be a useful rescue therapeutic option for refractory inflammatory bowel disease.
CASE REPORT
A 32-year-old Chinese man with known severe enterocolonic CD presented to our hospital in November 2012 because of progressive abdominal pain, bloody and purulent diarrhea and high fever of 38 °C-39.5 °C for 8 wk. He was diagnosed with CD in May 2010 when he was found three stricturing lesions and a penetrating lesion in ileum. In fact, he had gastrointestinal symptoms such as abdominal pain since 2005 and received enterectomy for presumptive appendicitis in 2007.
He was initially treated with intravenous prednisolone and Pentasa (mesalazine) 4.0 g daily for 6 years with frequent relapses. Colonoscopy in May 2011 revealed severe Crohn colitis with extensive ulcers throughout the entire colon. Therefore, he was treated with intravenous prednisolone and Mycophenloate Mofetil 1.0 g by mouth daily. In September 2012, many large ulcers were found in colon. He refused anti-tumour necrosis factor antibody infusion due to lack of medical insurance. He then received Mycophenloate Mofetil capsules 1.25 g daily and mesalazine 3.0 g daily. He was transferred to our center in November 2012.
On physical examination on admission, the patient showed poor general conditions. A large mass could be seen in the hypogastrium with tenderness. Laboratory tests showed leukocyte count of 17.5 × 109/L with 89.4% of neutrophils, erythrocyte sedimentation rate of 97 mm per hour and C-reactive protein of 141 mg/L. He had normal liver function test except album of 27.4 g/L (normal range, 35-55 g/L) and total cholesterol 2.6 mmol/L (normal range, 3.0-6.0 mmol/L).
CT scan showed an abdominal mass measuring 14 cm × 8 cm × 10 cm, enterocolic fistula and residual Barium sulfate in the mass (Figure 1A). The patient underwent barium meal examination in another hospital three months ago. Colonoscope could not pass through the severely inflamed sigmoid colon. He was given antibiotics intravenously for 10 d. However, symptoms of frequent fever, abdominal pain and abdominal mass were still the therapeutic dilemma.
Figure 1.
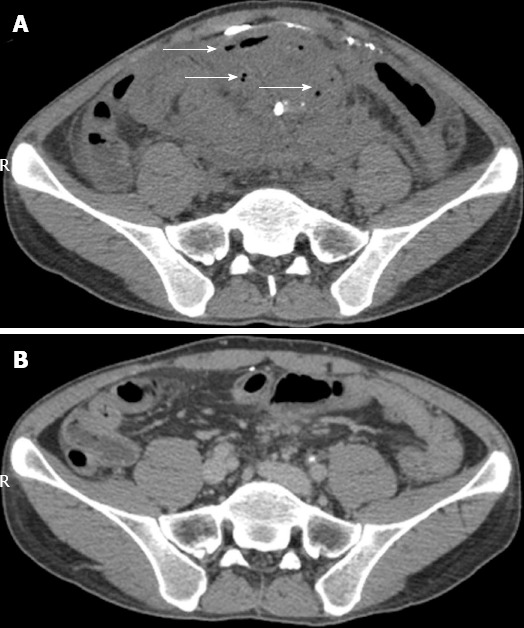
Abdominal computerized tomography scan. A: Before FMT, formation of an intraperitoneal large inflammatory mass measuring 14 cm × 8 cm × 10 cm, arrows showing gas indicating fistula, and the scattered bright image indicating Barium sulfate in fistula; B: Four months after FMT, no mass and gas was present and only a little Barium sulfate in the mass was shown. FMT: Fecal microbiota transplantation.
He agreed to participate in a clinical trial with FMT for moderate to severe refractory CD (NCT01793831), approved by the ethics committee of our center. One week prior to FMT, Mycophenloate Mofetil capsules and Etiasa were stopped, and Salofalk (mesalazine) 3.0 g was given daily. His CD Activity Index (CDAI) score was 537.
Based on the patient’s desire and the protocol of scanning tests and questionnaires made by our group (not shown in the present article), the donor was his 10-year-old healthy daughter. According to the protocol of standardized preparation, Esomeprazole Magnesium 40 mg by intravenous push and metoclopramide 10 mg by intramuscular injection were given one hour before endoscopic procedure. The highly purified gut flora at lab was prepared as 150 mL liquid suspension and was transplanted into mid-gut below Vater papilla[11] by a tube within the channel of gastroscope under anesthesia. The time from collection of stool to FMT procedure with endoscopy was 50 min. A week after FMT, his symptoms, such as fever, bloody purulent stool and abdominal pain, were dramatically alleviated, the size of intraperitoneal inflammatory mass became much smaller than that before FMT, and the CDAI score was reduced to 228. He had a severe cold in the whole third week after he was discharged with clinical improvement. At one month of follow-up after FMT, his CDAI score was further reduced to 143, which met the criteria of clinical remission. Three months after FMT, CDAI score was further reduced to 62, suggesting sustained clinical remission. CT scan (Figure 1B) showed resolved mass without exudation and the disappearance of the previous Barium sulfate intraperitoneally. Then, Salofalk 2.0 g daily was given. The patient was followed up for 9 mo and his CDAI score remained at 62, suggesting sustained clinical remission. Of note, he has gained his body weight by 11 kg, compared with the 50 kg as his baseline body weight before FMT. His nutrition status has also improved, evidenced by normalized album and total cholesterol 47.4 g/L (normal range, 35-55) and 4.5 mmol/L (normal range, 3.0-6.0), respectively. The key clinical parameter changes are shown in Table 1.
Table 1.
Clinical parameter changes of the patient during follow-up
| Parameter (normal range) | Before FMT |
After FMT |
|||
| 1 wk | 1 mo | 3 mo | 9 mo | ||
| Body weight (kg) | 50 | 51 | 52 | 56 | 61 |
| CDAI score | 537 | 228 | 143 | 62 | 62 |
| Haemoglobin (110-160, g/L) | 97 | 113 | 120 | 142 | 144 |
| CRP (0-10, mg/mL) | 141 | 8 | 9 | 1.7 | 7 |
| ESR (0-20, mm/h) | 97 | 10 | 13 | 10 | 10 |
| Album (g/L) | 27.4 | - | 35 | 49.9 | 47.4 |
| Total cholesterol (3.0-5.7 mmol/L) | 2.6 | - | 2.7 | 4.2 | 4.5 |
| Triglycerides (0.4-1.7 mmol/L) | 0.5 | - | 0.6 | 2.4 | 2.5 |
| HDL-C (1-2 3.1 mmol/L) | 0.6 | - | 0.7 | 1.3 | 1.3 |
| LDL-C (< 3.1 mmol/L) | 1.8 | - | 1.9 | 2.5 | 2.6 |
| IgM (6.8-14.5, g/L) | 0.5 | - | 0.7 | 1.0 | 0.8 |
CDAI: Crohn's disease activity index; CRP: C reactive protein; ESR: Erythrocyte sedimentation rate; HDL-C: High density lipoprotein-cholesterol; LDL-C: Low density lipoprotein-cholesterol: FMT: Fecal microbiota transplantation.
DISCUSSION
CD usually affects the intestine, but may occur anywhere from the mouth to the end of the rectum (anus). CD with fistula and formation of intraperitoneal large inflammatory mass has considerable morbidity associated with this complication and remains an unresolved challenge[12]. This case presented CD with fistula, residual Barium sulfate and refractory inflammation in a large mass after enterectomy and long-term use of masalazine, prednisolone and mycophenloate mofetil. Further medications (aminosalicylic acid preparations, steroids, immunomodulators, antibiotics and biologics such as anti-tumor necrosis factor antibody) not only yielded significant side effects, but also unpredictable outcome. Long-term use of immunomodulators increased the risk of refractory inflammation. Surgery for this case does pose a challenge for a surgeon in the skill and expertise according to the recent consensus[13].
The etiology of CD is unknown, but one dominant hypothesis is that the inflammation might result from altered or pathogenic microbiota in a genetically susceptible host[14]. We proposed that FMT might be a promising rescue therapy for refractory CD. Based on the attractive therapeutic effect[15] and less concerns in safety[16], as well as the long history of recognization in traditional Chinese medicine[4], standardized FMT is acceptable in treating refractory CD. As a rescue therapy in this severe CD, it is intriguing that standardized FMT was safe. The patient’s sustained remission indicated that a single application of standardized FMT via mid-gut should be effective.
In order to prove the therapic role of FMT, one week before FMT, immunosuppressive agent was stopped and only Salofalk 3.0 g was continued daily. CDAI decreased dramaticly during the first week after FMT. The large mass and inflammation within the fistula subsided in 4-6 wk. The expulsion of residual Barium sulfate within 4 mo actually not only decreased the risk of refractory infection within fistula, but also played a role in improving the internal fistula.
Gut microbial communities represent one source of human genetic and metabolic diversity. Previous studies have shown that relatives of patients with CD bear the risk of this disease and have altered gut microbiome[17]. The recent reports also have shown that the gut microbiota composition in healthy persons has the age-associated changes[18]. These experimental evidences indicated that the family members of CD patients might not be the best donor of stool for FMT. However, in the present case, the clinical results demonstrated that the patient’s 10-year-old daughter under healthy state was his right choice as a donor. Further studies are needed to clarify whether the ideal donor of stool for FMT can be from CD patient’s relatives or family members.
We reported here the first case of severe CD using FMT through mid-gut. The single standardized FMT resulted in sustained clinical remission for more than 9 mo and the follow-up is going on. Although there was only one case, the present result in our clinical trial strongly supported our initial hypothesis and highlighted the attractive role of the remodeling of gut flora in host diseases. Our ongoing study will report more evidences of standardized FMT through mid-gut on refractory intestinal diseases in a difficult therapeutic dilemma.
Footnotes
Supported by (in part) The Public Donated Grant “Intestine Initiative”
P- Reviewers: El-Tawil AM, Kuo SM S- Editor: Qi Y L- Editor: Ma JY E- Editor: Ma S
References
- 1.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- 2.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–98; quiz 499. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 4.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755; author reply p.1755-p.1756. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 5.Sha S, Xu B, Wang X, Zhang Y, Wang H, Kong X, Zhu H, Wu K. The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn Microbiol Infect Dis. 2013;75:245–251. doi: 10.1016/j.diagmicrobio.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Ng SC, Benjamin JL, McCarthy NE, Hedin CR, Koutsoumpas A, Plamondon S, Price CL, Hart AL, Kamm MA, Forbes A, et al. Relationship between human intestinal dendritic cells, gut microbiota, and disease activity in Crohn’s disease. Inflamm Bowel Dis. 2011;17:2027–2037. doi: 10.1002/ibd.21590. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503–516. doi: 10.1111/j.1365-2036.2012.05220.x. [DOI] [PubMed] [Google Scholar]
- 8.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164. doi: 10.1016/s0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 10.Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, Amateau SK, Khashab MA, Okolo PI. Mid-gut stents. Curr Opin Gastroenterol. 2012;28:451–460. doi: 10.1097/MOG.0b013e3283561f3b. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen OH, Rogler G, Hahnloser D, Thomsen OØ. Diagnosis and management of fistulizing Crohn’s disease. Nat Clin Pract Gastroenterol Hepatol. 2009;6:92–106. doi: 10.1038/ncpgasthep1340. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–83; quiz 464, 484. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 14.Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1452–1459. doi: 10.1038/ajg.2012.93. [DOI] [PubMed] [Google Scholar]
- 15.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 16.De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1036–1038. doi: 10.1016/j.cgh.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 17.Hedin CR, Stagg AJ, Whelan K, Lindsay JO. Family studies in Crohn’s disease: new horizons in understanding disease pathogenesis, risk and prevention. Gut. 2012;61:311–318. doi: 10.1136/gut.2011.238568. [DOI] [PubMed] [Google Scholar]
- 18.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]


