Abstract
Aims
Despite the known benefits of regular exercise, the reasons why many coronary heart disease (CHD) patients engage in little physical activity are not well understood. This study identifies factors associated with low activity levels in individuals with chronic CHD participating in the STABILITY study, a global clinical outcomes trial evaluating the lipoprotein phospholipaseA2 inhibitor darapladib.
Methods and results
Prior to randomization, 15 486 (97.8%) participants from 39 countries completed a lifestyle questionnaire. Total physical activity was estimated from individual subject self-reports of hours spend each week on mild, moderate, and vigorous exercise, corresponding approximately to 2, 4, and 8 METS, respectively. Multivariate logistic regression evaluated clinical and demographic variables for the lowest compared with higher overall exercise levels, and for individuals who decreased rather than maintained or increased activity since diagnosis of CHD. The least active 5280 subjects (34%) reported exercise of ≤24MET.h/week. A total of 7191 subjects (46%) reported less exercise compared with before diagnosis of CHD. The majority of participants were either ‘not limited’ or ‘limited a little’ walking 100 m (84%), climbing one flight of stairs (82%), or walking 1 km/½ mile (68%), and <10% were limited ‘a lot’ by dyspnoea or angina. Variables independently associated with both low physical activity and decreasing exercise after diagnosis of CHD included more co-morbid conditions, poorer general health, fewer years of education, race, and country (P < 0.001 for all).
Conclusion
In this international study, low physical activity was only partly explained by cardiovascular symptoms. Potentially modifiable societal and health system factors are important determinants of physical inactivity in patients with chronic CHD.
Keywords: Physical activity, Exercise, Coronary artery disease, Cardiac rehabilitation
See page 3245 for the editorial comment on this article (doi:10.1093/eurheartj/eht363)
Introduction
Regular exercise and greater physical fitness are associated with lower cardiovascular and total mortality, both in healthy populations,1–3 and in patients with coronary heart disease (CHD)4 and heart failure.5 In meta-analyses of randomized clinical trials, exercise training after myocardial infarction improves quality of life and reduces the risk of recurrent myocardial infarction and death.6 For most CHD patients, the benefits of increasing physical activity are likely to outweigh the small risk of exercise triggering myocardial infarction or sudden death.7,8 Clinical practice guidelines therefore recommend that patients with CHD engage in regular moderate intensity exercise, which is usually defined as 30 min on most days of the week.9–11
Currently there are limited data on the reasons why many CHD patients are sedentary.12 Coronary heart disease patients with symptoms such as angina, dyspnoea, and fatigue during exercise would be expected to engage in less exercise. Change in usual levels of physical activity could also be influenced by the psychological consequences of CHD, and by professional and other advice given to the patient. Sedentary behaviour may also partly reflect longstanding exercise patterns which predate the diagnosis of CHD. Social, economic, and cultural factors may also be important. A better understanding of the relative importance of these various factors may help inform the development of more effective interventions.
The aim of this study was to determine the degree to which sedentary behaviours are related to an individual's general health, to symptoms that develop during exercise, and to broader societal factors associated with education level, race, and country of residence. The study population is the large international cohort of patients with stable CHD participating in the Stabilisation of Atherosclerotic Plaque By Initiation of Darapladib Therapy (STABILITY) trial.13
Methods
Study population
The STABILITY trial is a global outcomes trial designed to determine whether darapladib, a specific inhibitor of lipoprotein associated phospholipase A2 (Lp-PLA2) will reduce the risk of cardiovascular death, myocardial infarction, and stroke in patients with chronic CHD.13 In total, 15 828 subjects from 39 countries were randomized. All patients had chronic stable CHD, defined as prior myocardial infarction, prior coronary revascularization, or multi-vessel CHD confirmed by coronary angiography. In addition, patients had to meet at least one of the following cardiovascular risk criteria: age ≥60 years; diabetes mellitus requiring pharmacotherapy; HDL-cholesterol <1.03 mmol/; current or previous smoker defined as ≥5 cigarettes per day on average; significant renal dysfunction (estimated glomerular filtration rate ≥30 and <60 mL/min per 1.73 m2 or urine albumin:creatinine ratio ≥30 mg albumin/g creatinine); or polyvascular disease (CHD and cerebrovascular disease or CHD and peripheral arterial disease). More detailed descriptions of the study design and population have been published previously.13,14
Data collection
At baseline, in addition to a detailed medical history, physical examination, and fasting blood samples, participants were invited to complete a lifestyle questionnaire. The questions related to physical activity, based on the International Physical Activity Questionnaire,15 were completed by 15 486 (97.8%) subjects.
Evaluation of physical activity
Each subject was asked to estimate the number of hours spent undertaking ‘mild,’ ‘moderate,’ and ‘vigorous’ physical activity during a typical week. Examples of mild activity were easy walking, yoga, Tai Chi, and mild house work. Moderate exercise included fast walking, jogging, aerobics, gardening, bicycling, dancing, swimming, or house cleaning. Examples of vigorous physical activity were running, lifting heavy objects, playing strenuous sports or strenuous work. The total amount of physical activity was estimated in MET (metabolic equivalent) hours/week from the self-reported time undertaking mild (2 METS), moderate (4 METS), and vigorous intensity (8 METS) physical activity during an average week.15 In separate questions, subjects were asked to indicate whether they undertook any ‘moderate intensity’ physical activity during work and separately during leisure time. To evaluate a change in exercise pattern, each participant was asked ‘Comparing your current lifestyle to your lifestyle before your first heart problem do you exercise “less now,” “about the same,” or “more now”’.
Evaluation of symptoms during exercise
Subjects were asked whether they were limited ‘a little’ or ‘a lot’ in the following activities; walking 100 m; climbing one flight of stairs; and walking 1 km. To estimate the overall degree of limitation, a score was calculated from the summed responses to five activities (no limitation, limited a little ‘1’ and limited a lot ‘2,’ or ‘I do not do this activity’ ‘I’). Subjects also indicated whether physical activity was ‘not limited,’ limited ‘a little’ or ‘a lot’ by chest discomfort or tightness, shortness of breath, fatigue or tiredness, muscle weakness, dizziness, or arthritis.
General health, co-morbidities, and mood
Subjects rated their general health as poor, fair, good, very good, or excellent. Current and past co-morbidities were recorded on the baseline case report form. The number of the following co-morbidities, each associated with reduced physical activity, was determined; multi-vessel CHD, prior heart failure, renal dysfunction, diabetes type 1 or 2, peripheral vascular disease, chronic obstructive pulmonary disease or asthma, obstructive sleep apnoea, and prior stroke. To evaluate depression, participants were asked ‘Have you felt sad, low in your spirits or depressed?’ and ‘Have you lost interest in hobbies, work or activities that previously gave you pleasure?’ Those who responded to either question ‘always’ or ‘often’ were classified as being ‘depressed.’16
Race groups, country, and education
In a pre-specified grouping, individuals were classified to one of the following race or ethnic groups: (i) White (White/Caucasian/European Heritage/Arabic/North African Heritage and not Hispanic/Latino ethnicity; (ii) White and Hispanic/Latino; (iii) Black (African American/African Heritage); (iv) Central/South/South East Asian; (v) East Asian/Japanese; and (iv) other including indigenous peoples (American Indian/Alaskan Native/Native Hawaiian/Other Pacific Islander), and 113 patients who checked more than one category.
Countries were classified as high income, upper middle income, or lower middle income according to the World Bank classification.17 No low income countries were included in the study. Because the number of subjects from lower middle income countries was small, lower and upper middle income countries were combined for the analysis.
Years of formal education completed were defined as ‘none or 1–8 years,’ ‘9–12 years,’ and either ‘trade school’ or ‘college/university.’
Statistical analysis
Data collected at baseline assessment and before randomization are included. To summarize relations between physical activity and other variables, total physical activity in MET.h/week was divided into tertiles. χ2 tests were used to assess associations between physical activity tertiles and change in exercise since CHD diagnosis, level of limitation by symptoms, and demographic factors including age, sex, and time since CHD diagnosis. χ2 tests were also used to assess associations between change in exercise since CHD diagnosis and level of limitation by symptoms. Two-sided P-values were generated for descriptive purposes only.
Logistic regression models were used to assess differences in the lowest tertile vs. the highest two tertiles in physical activity and for subjects who decreased compared with those who did not change or increased their level of exercise after a diagnosis of CHD. Factors included were age, sex, level of limitation during exercise, general health, number of co-morbidities, mood, obesity, country, race group, country income level, and years of education. Backwards selection was used to remove factors not statistically significant at the 0.10 level to provide the final multivariate models.
All statistical analyses were conducted in SAS version 9.1. The bubble plot was produced in R version 2.15.2.
Results
Exercise levels for subjects in the lowest, middle, and highest tertile of overall physical activity are given in Table 1. Almost half of the study participants were exercising less compared with before the diagnosis of CHD. Overall, more subjects reported moderate or greater intensity exercise during leisure than at work, but only 33% of subjects were currently working. Differences in physical activity levels by age and sex were small, and there was no association with time since CHD diagnosis.
Table 1.
Physical activity levels in the STABILITY study population
| Least active <24 MET.h/week | Intermediate activity 26 to 56 MET.h/week | Most active >58 MET.h/week | |
|---|---|---|---|
| Number of subjects | 5280 | 5055 | 5151 |
| Age, mean ± standard deviation, years | 64.6 ± 9.6 | 64.8 ± 9.2 | 63.7 ± 9.1 |
| Male, % | 83 | 80 | 81 |
| Time since CHD diagnosis, mean ± SD, months | 52 ± 56 | 54 ± 57 | 53 ± 54 |
| Exercise | |||
| Total exercise, MET.h/week | 14 (6, 18) | 40 (32, 46) | 90 (70, 122) |
| Mild-intensity activity (2 METS), h/week | 8 (4, 14) | 18 (12, 28) | 28 (14, 42) |
| Moderate-intensity activity (4 METS), h/week | 0 (0, 8) | 16 (8, 28) | 44 (32, 80) |
| Vigorous-intensity activity (8 METS), h/week | 0 (0, 0) | 0 (0, 0) | 8 (0, 40) |
| ≥10MET.h/week moderate or vigorous activity (%) | 16 | 74 | 100 |
| Some moderate-intensity exercise during leisure time (%) | 10 | 23 | 38 |
| Some moderate-intensity exercise at work (%) | 7 | 8 | 19 |
| Change in exercise since CHD diagnosis (%) | |||
| Less now | 55 | 47 | 38 |
| About the same | 30 | 31 | 40 |
| More now | 15 | 22 | 22 |
Results are mean ± standard deviation (SD), median (inter-quartile range), or % of group. Differences between groups have P < 0.001, except for gender and time since CHD diagnosis. Statistical tests were not performed for the number of exercise h/week.
CHD, coronary heart disease.
Importance of symptoms
Subjects reporting low physical activity were more likely to be limited ‘a lot’ by common daily activities such as walking 100 m or 1 km, and climbing one flight of stairs than those who were more active (Table 2). However, most sedentary subjects did not report ‘a lot’ of limitation with these activities. The least active subjects were more likely to be limited ‘a lot’ by symptoms, the most common of which were fatigue, shortness of breath, and arthritis. However, for the least active tertile, only 12% of subjects were limited ‘a lot’ by shortness of breath compared with 7% for the most active tertile (P < 0.001). Seven percent of the least active tertile reported they were limited ‘a lot’ by chest discomfort or tightness, compared with 5% of the most active tertile of subjects (Table 2).
Table 2.
Relationships between physical activity and symptoms during exercise
| Low physical activity | Intermediate physical activity | Most physically active | Decreased exercise since CHD diagnosis | Same exercise since CHD diagnosis | Greater exercise since CHD diagnosis | |
|---|---|---|---|---|---|---|
| Number of subjects | 5280 | 5055 | 5151 | 7191 | 5244 | 3014 |
| Limited during activities (%) | ||||||
| Walking 100 m | 30 | 22 | 18 | 32 | 17 | 13 |
| Climbing one flight of stairs | 42 | 34 | 28 | 44 | 28 | 23 |
| Walking 1 km or half mile | 46 | 41 | 38 | 53 | 34 | 26 |
| Limited by symptoms (%) | ||||||
| Dypsnoea | 53 | 50 | 47 | 60 | 42 | 38 |
| Chest pain or tightness | 39 | 38 | 35 | 49 | 28 | 27 |
| Fatigue | 62 | 61 | 61 | 72 | 53 | 51 |
| Arthritis | 38 | 38 | 44 | 46 | 34 | 32 |
| Muscle weakness | 44 | 40 | 41 | 51 | 35 | 32 |
| Dizziness | 27 | 26 | 23 | 33 | 18 | 19 |
The proportion of subjects who report being limited either ‘a little’ or ‘a lot’ during the activity or by the symptoms is given.
All differences between all groups have P < 0.001 by χ2 test.
CHD, coronary heart disease.
Variables associated with low physical activity
Increasing age and being male were associated with low physical activity in both the unadjusted and fully adjusted models (Table 3). Low physical activity was also associated with more frequently reported limitation by symptoms during exercise, poorer self-reported general health, and a larger number of co-morbidities. The strength of these associations was weaker in the fully adjusted model with the exception of sex. There was a modest association between depressed mood and low physical activity, but not in the fully adjusted model. Obesity was associated with lower physical activity, in both the unadjusted and adjusted models.
Table 3.
Predictors of low physical activity and of taking less exercise compared with before diagnosis of coronary heart disease
| Proportion of study participants (%) | OR for low PA (95% CI) | OR for low PA in fully adjusted modela (95%CI) | OR for decreasing PA after CHD diagnosis (95% CI) | OR for decreasing PA after CHD diagnosis in fully adjusted model (95% CI) | |
|---|---|---|---|---|---|
| Age (+10 years) | — | 1.05 (1.01, 1.08) | 1.05 (1.00, 1.09) | 1.09 (1.05, 1.13) | 1.16 (1.11, 1.22) |
| Male vs. Female | 81 | 1.13 (1.03, 1.23) | 1.46 (1.32, 1.62) | 0.78 (0.72, 0.84) | 1.24 (1.12, 1.38) |
| Individual health measures | |||||
| Good vs. very good general health | 44 | 1.34 (1.20, 1.49) | 1.23 (1.10, 1.39) | 2.45 (2.19, 2.73) | 1.78 (1.58, 2.01) |
| Poor/fair vs. very good health | 41 | 1.83 (1.65, 2.04) | 1.41 (1.24, 1.61) | 5.88 (5.26, 6.57) | 3.17 (2.78, 3.63) |
| Amount of limitation during exercise (score +1) | — | 1.12 (1.11, 1.13) | 1.11 (1.09, 1.13) | 1.27 (1.25, 1.28) | 1.20 (1.18, 1.22) |
| Number of co-morbidities (+1) | — | 1.17 (1.14, 1.21) | 1.10 (1.07, 1.14) | 1.32 (1.28,1.35) | 1.11 (1.07, 1.15) |
| Depressed mood (yes vs. no) | 12 | 1.19 (1.07, 1.31) | 0.91 (0.81, 1.03) | 1.79 (1.62, 1.98) | 1.12 (0.99, 1.27) |
| Obesity (BMI >30 vs. ≤30) | 36 | 1.16 (1.08, 1.24) | 1.28 (1.17, 1.39) | 1.25 (1.17, 1.34) | 1.14 (1.05, 1.24) |
| Cardiac rehabilitation | |||||
| No vs. Yes | 65 | 1.35 (1.26, 1.45) | 1.11 (1.01, 1.20) | 1.47 (1.37, 1.57) | 1.14 (1.05, 1.24) |
| Country | |||||
| Low/Middle vs. high income | 30 | 1.56 (1.45, 1.67) | 0.63 (0.49, 0.83) | 2.16 (2.01, 2.31) | 3.54 (2.67, 4.70) |
| Race/ethnic group | |||||
| Latino/Hispanic vs. white | 9 | 2.85 (2.55, 3.18) | 1.37 (1.03, 1.83) | 1.44 (1.29, 1.61) | 1.06 (0.78, 1.43) |
| Central + South +SE Asian vs. white | 7 | 2.38 (2.10, 2.69) | 1.54 (1.04, 2.29) | 1.27 (1.13, 1.44) | 1.79 (1.18, 2.70) |
| East Asian/Japanese vs. white | 10 | 1.82 (1.63, 2.03) | 3.04 (1.83, 5.67) | 0.82 (0.74, 0.92) | 1.28 (0.68, 2.40) |
| Black vs. white | 2 | 2.24 (1.79, 2.82) | 1.46 (1.10, 1.94) | 1.46 (1.17, 1.83) | 1.39 (1.04, 1.87) |
| Indigenous/other vs. white | 0.7 | 1.20 (0.81, 1.79) | 0.91 (0.59, 1.41) | 0.98 (0.68, 1.43) | 1.24 (0.80, 1.91) |
| Education | |||||
| 9–12 years vs. trade/college | 31 | 1.17 (1.08, 1.26) | 1.00 (0.91, 1.09) | 1.11 (1.03, 1.20) | 1.02 (0.94, 1.12) |
| < = 8 years vs. trade/college | 23 | 1.63 (1.50, 1.77) | 1.10 (0.99, 1.23) | 1.27 (1.17, 1.38) | 1.15 (1.03, 1.27) |
OR, odds ratio; PA, physical activity; CHD, coronary heart disease.
aThe fully adjusted model includes age, gender, obesity, exercise limitation, co-morbidities, general health, mood, country income level, race, education, and country.
The majority of subjects (65%) had never participated in a cardiac rehabilitation programme, and these subjects were more likely to report taking less exercise. Living in a lower or upper middle income country compared with a high income country was associated with higher odds ratio for low physical activity in the unadjusted but not in the adjusted model. This may be the result of collinearity between this parameter and country. East Asian/Japanese races had more than twice the odds ratio for low physical activity compared with Whites.
Change in activity since diagnosis of coronary heart disease
Overall, 46% of subjects had reduced their level of exercise compared with before the diagnosis of CHD, while 34% had increased their physical activity (Table 3). Factors associated with a greater likelihood of decreasing exercise since diagnosis of CHD were similar to those for low physical activity. The strongest associations with reduced activity were for poorer self-reported health and symptoms during exercise. Living in a middle compared with a high income country was also strongly associated with a greater likelihood of decreasing exercise. There were also modest independent associations for Asian and Black race groups.
International differences
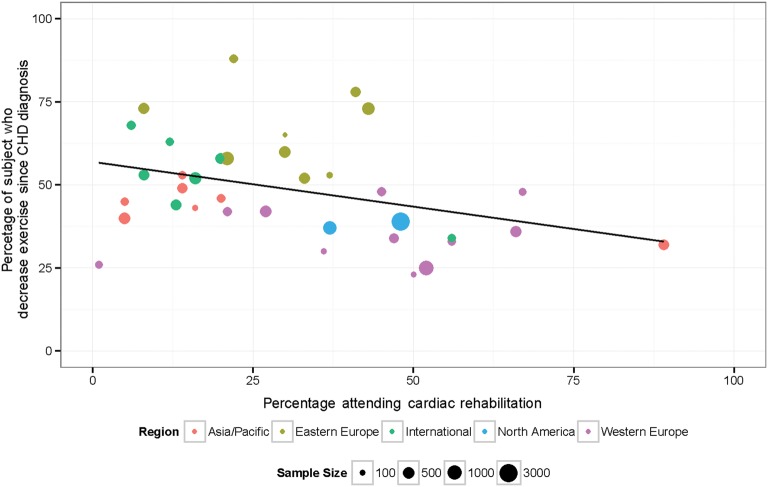
There were large international differences in proportion of subjects reporting low physical activity, with subjects living in Asia and Latin America reporting the lowest levels of physical activity. (Figure 1, Table 4) There were also large international differences in the proportion of subjects who decreased physical activity since CHD diagnosis, and in attendance at a cardiac rehabilitation programme (Figure 2, Table 4). Subjects living in Russia and several Eastern European countries reported the greatest decreases in physical activity after CHD diagnosis. Fewer subjects living in Latin America, Asian, or Eastern European Countries had attended a cardiac rehabilitation programme compared with those living in North America, Western Europe, Australia, and New Zealand.
Figure 1.
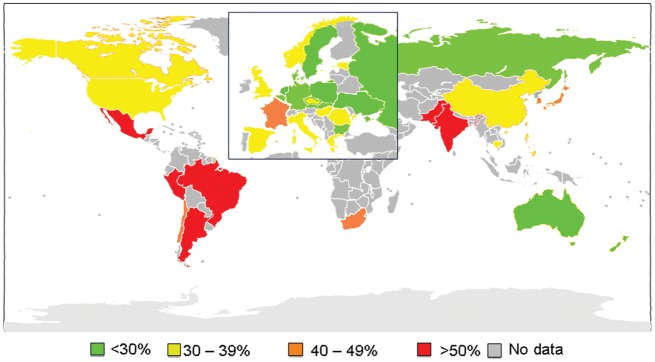
Proportion of study subjects who reported low levels of physical activity (<24MET.h/week) by country.
Table 4.
International differences in reported physical activity, decrease in exercise after coronary heart disease diagnosis, and cardiac rehabilitation attendance for STABILITY study participants assessed at baseline
| Number of subjects | World bank income classification | Attended cardiac rehabilitation (%) | Low physical activity (%) | Decreased exercise since CHD diagnosis (%) | |
|---|---|---|---|---|---|
| Argentina | 542 | Upper middle | 16 | 56 | 52 |
| Australia | 306 | High | 48 | 25 | 38 |
| Belgium | 202 | High | 56 | 32 | 33 |
| Brazil | 384 | Upper middle | 20 | 60 | 58 |
| Bulgaria | 222 | Upper middle | 22 | 21 | 88 |
| Canada | 780 | High | 37 | 38 | 37 |
| Chile | 195 | Upper middle | 12 | 40 | 63 |
| China | 369 | Upper middle | 89 | 39 | 32 |
| Czech Republic | 774 | High | 21 | 12 | 58 |
| Denmark | 102 | High | 50 | 21 | 23 |
| Estonia | 77 | High | 30 | 24 | 65 |
| France | 250 | High | 45 | 40 | 48 |
| Germany | 1089 | High | 52 | 20 | 25 |
| Greece | 187 | High | 1 | 35 | 26 |
| Hong Kong | 117 | High | 16 | 50 | 43 |
| Hungary | 410 | High | 33 | 30 | 52 |
| India | 398 | Lower middle | 13 | 59 | 44 |
| Italy | 256 | High | 21 | 38 | 42 |
| Japan | 318 | High | 14 | 45 | 49 |
| Korea | 503 | High | 5 | 42 | 40 |
| Mexico | 141 | Upper middle | 20 | 56 | 58 |
| Netherlands | 444 | High | 66 | 26 | 36 |
| New Zealand | 202 | High | 56 | 28 | 34 |
| Norway | 113 | High | 36 | 35 | 30 |
| Pakistan | 250 | Lower middle | 6 | 53 | 68 |
| Peru | 78 | Upper middle | 37 | 56 | 53 |
| Philippines | 219 | Lower middle | 20 | 39 | 46 |
| Poland | 510 | High | 30 | 30 | 60 |
| Romania | 411 | Upper middle | 8 | 31 | 73 |
| Russia | 654 | Upper middle | 43 | 22 | 73 |
| Slovakia | 120 | High | 37 | 25 | 53 |
| South Africa | 386 | Upper middle | 8 | 41 | 53 |
| Spain | 474 | High | 27 | 39 | 42 |
| Sweden | 299 | High | 47 | 29 | 34 |
| Taiwan | 200 | High | 5 | 35 | 45 |
| Thailand | 207 | Upper middle | 14 | 38 | 53 |
| UK | 184 | High | 67 | 31 | 48 |
| Ukraine | 353 | Lower middle | 41 | 28 | 78 |
| USA | 3102 | High | 48 | 33 | 39 |
CHD, coronary heart disease.
Figure 2.
Proportion of study subjects in each country who attended a cardiac rehabilitation program and who reported decreasing their physical activity since diagnosis of coronary heart disease.
Discussion
In this large international study, about one-third of patients with chronic CHD reported substantially less physical activity than recommended in current guidelines.10,11 Factors associated with being sedentary fell into two broad groups, those related to individual health, and those related to race group, country, and level of education. The observations are consistent with socioeconomic, general population, and/or health system-related factors being important determinants of the amount of exercise taken by patients with CHD.18
Physical activity in patients with CHD is likely to reflect long-standing patterns of exercise as well as change in physical activity since the diagnosis of CHD. In this study, about two-thirds of subjects did not attend cardiac rehabilitation, which was independently associated with both lower physical activity and a greater risk of decreasing exercise after CHD diagnosis. Cardiac rehabilitation and other interventions which increase early return to normal activities and help to maintain regular exercise after an acute coronary event are likely to reduce the proportion of CHD patients who become sedentary.19 The observation of higher rates of non-attendance at cardiac rehabilitation in countries where more subjects reported decreasing physical activity after diagnosis of CHD, is further evidence supporting the importance of cultural and/or health system-related factors in influencing physical activity in CHD patients.
Most previous large studies on physical activity have evaluated general populations,1,3 but CHD patients differ in several important respects. The risk of adverse events, especially during vigorous exercise, is greater for CHD patients. Thus while guidelines recommend 30 min of moderate or vigorous exercise each day,10,11 many patients and their health advisors may be cautious about more vigorous exercise. Coronary heart disease patients are also more likely to have symptoms which limit exercise compared with a healthy general population. In the current study, poorer general health, cardiac, and non-cardiac co-morbidities and exertional symptoms were all associated with less physical activity. Despite this, the majority of those reporting low physical activity were ‘not limited’ or only ‘limited a little’ by activities which involved moderate intensity exercise. Also physical activity was most often limited by non-specific symptoms such as shortness of breath, fatigue, and weakness, and limitation by chest tightness or discomfort was less common. In clinical trials, exercise training reduces dyspnoea and fatigue, increases muscle strength, and improves angina.6,20,21 It is therefore possible that lack of fitness in part explains the association between symptoms and less physical activity. Depression was associated with low physical activity, but not in the fully adjusted model. This may in part be explained by the association between depressed mood and poorer general health.22
In previous studies, leisure time physical activity has been more clearly related to lower CHD mortality than activity at work.23 The majority (67%) of STABILITY study participants were not working, and a higher proportion of subjects reported moderate or greater physical activity during ‘leisure time’ than ‘at work’. However, not all exercise was ‘at work’ or ‘during leisure’, probably reflecting exercise during usual activities of daily life. It is possible women reported greater physical exercise than men, because on average they spend more time on household chores.
Limitations
Physical activity may differ in a clinical trial population compared with usual CHD patients, and these differences could vary by country. STABILITY study participants had high levels of adherence to evidence-based pharmacological therapies, suggesting they were well-motivated and received good medical care.14 Almost all subjects completed the lifestyle questionnaire at the baseline visit, so bias related to non-participation within the STABILITY study would be small. Self-reporting of activity level is likely to be relatively imprecise, and for some subjects may overestimate the exercise taken. The use of standard questionnaires based on the widely used and validated International Physical Activity Questionnaire15 allows comparison of diverse geographic and ethnic groups, but cultural differences in the interpretation of some questions is possible. Independent validation of the lifestyle questionnaire used in this study, including questions related to change in physical activity was not undertaken. A threshold of 30 min of moderate or vigorous exercise on at least 5 days each week has been used in many guidelines.11 In this study, mild exercise was also included because there is a graded association between exercise and mortality, with benefits from mild compared with no exercise.24 Also some CHD patients are advised to avoid vigorous exercise. This analysis was undertaken prior to completion of the STABILITY trial and therefore includes only baseline data. The relationship between self-reported physical activity and morbidity and mortality in the STABILITY study population will be assessed in future.
Conclusions
In this study, the majority of sedentary CHD patients were not limited ‘a lot’ by symptoms, suggesting most could be more active. The observation of large international differences in activity levels, in the proportion of subjects reporting a decrease in exercise since CHD diagnosis, and in rates of attendance at cardiac rehabilitation, suggest that modifiable societal and health system-related factors are important determinants of physical inactivity in patients with CHD.
Cultural and regional geographic factors need to be considered when planning strategies to increase physical activity in CHD patients. Particularly in middle income countries, quality of life and prognosis of patients with CHD may be improved by health professionals focusing more on cardiac rehabilitation and other interventions which help to maintain or increase exercise after CHD diagnosis. Further research is needed to improve understanding of ways to overcome barriers to exercise such as the patient's, family's, or physician's concerns about risk, and to evaluate novel approaches such as with information technology to engage inactive patients and reinforce changes with regular feedback.
Funding
The STABILITY trial and the lifestyle sub-study were funded by GSK. Study design and drafting and approval of the manuscript were undertaken by the study authors. The charge for Open Access will be paid by GlaxoSmithKline, who were the sponsor for the Stability trial.
Conflict of interest: Rebekkah Brown, Richard Davies and Joseph Sofer are employees of GlaxoSmithKline and Ralph Stewart, Claes Held, Ola Vedin, Emil Hagstrom, Eva Lonn, Paul Armstrong, Christopher B. Granger, Judith Hochman, Lars Wallentin and Harvey White are Stability Study Investigators.
Acknowledgements
We thank all patients who participated in the STABILITY trial, as well as study nurses and investigators at 639 participating sites.
References
- 1.Shiroma EJ, Lee IM. Physical activity and cardiovascular health. Circulation. 2010;122:743–752. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 2.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Phashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 4.Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shepherd P. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106:666–671. doi: 10.1161/01.cir.0000024413.15949.ed. [DOI] [PubMed] [Google Scholar]
- 5.Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T, McMurray J, Pieske B, Piotrowicz E, Schmid JP, Anker SD, Solal AC, Filippatos GS, Hoes AW, Gielen S, Giannuzzi P, Ponikowski PP. Exercise training in heart failure: from theory to practice. A concensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitaion. Eur J Heart Fail. 2011;13:347–357. doi: 10.1093/eurjhf/hfr017. [DOI] [PubMed] [Google Scholar]
- 6.Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database SystRev. 2011:CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahabreh IJ, Paulus JK. Association of episodic physical and sexual activity with triggering of acute cardiac events: systematic review and meta-analysis. JAMA. 2011;305:1225–1233. doi: 10.1001/jama.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA, III, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron MA, Pelliccia A, Wenger NK, Willich SN, Costa F. American Heart Association Council on Nutrition, Physical Activity and Metabolism; American Heart Association Council on Clinical Cardiology; American College of Sports Medicine. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115:2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 9.Corra U, Piepoli MF, Carre F, Heuschmann P, Hoffmann U, Verschuren M, Halcox J. Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training: key components of the position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur Heart J. 2010;31:1967–1974. doi: 10.1093/eurheartj/ehq236. [DOI] [PubMed] [Google Scholar]
- 10.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 11.Perk J, de Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Kaadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur J Prev Cardiol. 2012;19:585–667. [Google Scholar]
- 12.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet. 2009;373:929–940. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- 13.White HD, Held C, Stewart RA, Watson D, Harrington R, Budaj A, Steg PG, Cannon CP, Krug-Gourley S, Wittes J, Trivedi T, Tarka E, Wallentin L. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilisation of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with clinical coronary heart disease) Am Heart J. 2010;160:655–661. doi: 10.1016/j.ahj.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Vedin O, Hagstrom E, Stewart R, Brown R, Krug-Gourley S, Davies R, Wallentin L, White H, Held C. Secondary prevention and risk factor target achievement in a global, high-risk population with established coronary heart disease: baseline results from the STABILITY study. Eur J Prev Cardiol. 2012 doi: 10.1177/2047487312444995. doi:10.1177/2047487312444995. Published online ahead of print 10 April 2012. [DOI] [PubMed] [Google Scholar]
- 15.Craig CL, Marshall AJ, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt BE, Ekeland U, Yngve A, Sallis JF, Oja P. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 16.Depression: management of depression in primary and secondary care– NICE guidance. National Institute for Health and Clinical Excellence (NICE) London: 2004. p. 23. [Internet] Available from http://www.nice.org.uk/guidance/index.jsp?Action=byID&o=10958. (last accessed April 30, 2013). [Google Scholar]
- 17.2012. How we classify countries The World Bank [Internet] Available from http://data.worldbank.org/about/country-classifications. (last accessed April 30, 2013).
- 18.Heath GW, Parra DC, Sarmiento OL, Andersen LB, Owen N, Goenka S, Montes F, Brownson RC. Evidence-based intervention in physical activity: lessons from around the world. Lancet. 2012;380:272–281. doi: 10.1016/S0140-6736(12)60816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roffi M, Wenaweser P, Windecker S, Mehta H, Eberli FR, Seiler C, Fleisch M, Garachemani A, Pedrazzini GB, Hess OM, Meier B. Early exercise after coronary stenting is safe. J Am Coll Cardiol. 2003;42:1569–1573. doi: 10.1016/j.jacc.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Heaps CL, Parker JL. Effects of exercise training on coronary collateralization and control of collateral resistance. J Appl Physiol. 2011;111:587–598. doi: 10.1152/japplphysiol.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meer S, Zwerink M, van Brussel M, van der V, Wajon E, van der PJ. Effect of outpatient exercise training programmes in patients with chronic heart failure: a systematic review. Eur J Prev Cardiol. 2012;19:795–803. doi: 10.1177/1741826711410516. [DOI] [PubMed] [Google Scholar]
- 22.Stewart RA, North FM, West TM, Sharples KJ, Simes RJ, Colquhoun DM, White HD, Tonkin AM. Depression and cardiovascular morbidity and mortality: cause or consequence? Eur Heart J. 2003;24:2027–2037. doi: 10.1016/j.ehj.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Held C, Iqbal R, Lear SA, Rosengren A, Islam S, Mathew J, Yusuf S. Physical activity levels, ownership of goods promoting sedentary behaviour and risk of myocardial infarction: results of the INTERHEART study. Eur Heart J. 2012;33:452–466. doi: 10.1093/eurheartj/ehr432. [DOI] [PubMed] [Google Scholar]
- 24.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]