Abstract
The epidermal growth factor receptor (EGFR) is a central regulator of tumor progression in a variety of human cancers. Cetuximab is an anti-EGFR monoclonal antibody that has been approved for head and neck and colorectal cancer treatment, but many patients treated with cetuximab don't respond or eventually acquire resistance. To determine how tumor cells acquire resistance to cetuximab, we previously developed a model of acquired resistance using the non-small cell lung cancer line NCI-H226. These cetuximab-resistant (CtxR) cells exhibit increased steady-state EGFR expression secondary to alterations in EGFR trafficking and degradation and, further, retained dependence on EGFR signaling for enhanced growth potential. Here, we examined Sym004, a novel mixture of antibodies directed against distinct epitopes on the extracellular domain of EGFR, as an alternative therapy for CtxR tumor cells. Sym004 treatment of CtxR clones resulted in rapid EGFR degradation, followed by robust inhibition of cell proliferation and down-regulation of several mitogen-activated protein kinase pathways. To determine whether Sym004 could have therapeutic benefit in vivo, we established de novo CtxR NCI-H226 mouse xenografts and subsequently treated CtxR tumors with Sym004. Sym004 treatment of mice harboring CtxR tumors resulted in growth delay compared to mice continued on cetuximab. Levels of total and phospho-EGFR were robustly decreased in CtxR tumors treated with Sym004. Immunohistochemical analysis of these Sym004-treated xenograft tumors further demonstrated decreased expression of Ki67, and phospho-rpS6, as well as a modest increase in cleaved caspase-3. These results indicate that Sym004 may be an effective targeted therapy for CtxR tumors.
Introduction
The epidermal growth factor receptor (EGFR) is a member of the human epidermal growth factor receptor (HER) family of receptor tyrosine kinases that consists of four members: EGFR (ErbB1/HER1), HER2/neu (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). Stimulation of EGFR through ligand binding promotes receptor homodimerization or heterodimerization with other HER family receptors, leading to activation of its intrinsic tyrosine kinase [1,2]. Activation of EGFR initiates various signaling cascades, one of which is the mitogen-activated protein kinase (MAPK) network. MAPKs belong to a group of intracellular serine/threonine protein kinases that consist of various members, including the extracellular signal-regulated kinases 1 and 2 (ERK1/2) and p38 MAPKs (alpha, beta, gamma, delta). Upon activation of EGFR, the MAPK pathway is initiated through the promotion of Ras binding to guanosine triphosphate (GTP), which in turn activates RAF kinases MAPK/extracellular signal-regulated kinases (MEK), and MAPKs. MAPKs can subsequently activate numerous transcription factors such as c-Fos, c-Myc, and Elk-1 and numerous protein kinases such as ribosomal S6 kinase 1 (RSK1) [3]. Collectively, the stimulation of the MAPK pathway by EGFR has been shown to greatly enhance cell proliferation, angiogenesis, invasion, and metastasis in cancer cells.
Aberrant expression or activity of EGFR has been identified in many human epithelial cancers, including non-small cell lung cancer (NSCLC). Therefore, targeting EGFR has been intensely pursued over the last three decades as a cancer treatment strategy. One approach to inhibit the activation of EGFR is through the use of monoclonal antibodies (mAbs) that bind the extracellular domain of EGFR to block natural ligand binding. Cetuximab (IMC-225, Erbitux) is a human/murine chimeric mAb that was developed to bind to the extracellular domain III of EGFR. This interaction partially blocks the ligand-binding domain and sterically hinders the correct extended conformation of the dimerization arm on domain II [4]. The Food and Drug Administration has approved cetuximab treatment for patients with metastatic colorectal cancer (mCRC) and head and neck squamous cell carcinoma (HNSCC). However, while cetuximab has shown beneficial antitumor effects in these cancers, the majority of patients who initially respond eventually acquire resistance [1,5,6].
Numerous efforts have been undertaken to define the molecular mechanisms of acquired resistance to cetuximab [7–14]. Our laboratory has established panels of cetuximab-resistant (CtxR) clones from the NSCLC cell line NCI-H226 by exposing these cells in vitro to increasing concentrations of cetuximab. Using this model, we have shown that impaired EGFR internalization and degradation lead to increased EGFR surface level expression, increased EGFR kinase activity, and dependence on EGFR induced signaling pathways [7]. These data suggest that EGFR remains a molecular target in this setting and that new therapeutics that can initiate the rapid internalization and robust degradation of EGFR may be a potent anticancer strategy.
Sym004 is a novel 1:1 mixture of a pair of mAbs (992 and 1024) directed against nonoverlapping epitopes on EGFR [15,16]. Sym004 inhibits cancer cell growth and survival by blocking ligand binding, receptor activation, and downstream signaling. Unlike cetuximab, Sym004 induces rapid and efficient removal of the receptor from the cell surface by triggering EGFR internalization and degradation. Additionally, Sym004 has been shown to elicit antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in vivo [16], while cetuximab has been shown to elicit antibody-dependent cellular cytotoxicity only [16–19].
In this study, we hypothesized that cells with acquired resistance to cetuximab retain growth dependency on the EGFR and would therefore benefit from therapeutic agents that promote EGFR degradation. We found that Sym004 treatment delays the emergence of acquired resistance to cetuximab in NCI-H226 cells both in vitro and in vivo. Down-regulation of several MAPK pathways was also observed in response to Sym004 treatment. The results presented herein suggest that tumors with acquired resistance to cetuximab can thus be successfully targeted with the novel anti-EGFR therapeutic Sym004.
Materials and Methods
Cell Lines
The human NSCLC cell line NCI-H226 was provided by Drs J. Minna and A. Gazdar (University of Texas Southwestern Medical School, Dallas, TX). The cells were maintained in 10% FBS in RPMI-1640 (Mediatech, Inc, Manassas, VA) with 1% penicillin and streptomycin. The development of CtxR cells has been described previously [10].
Small Interfering RNA and Transfection
For small interfering RNAs (siRNAs), CtxR cells (HC1, HC4, and HC8) were transiently transfected with siEGFR (ON-TARGETplus, SMART pool #L-003114-00; Dharmacon, Lafayette, CO) using Lipofectamine RNAiMAX according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). The nontargeting siRNA (ON-TARGETplus Non-targeting Pool, #D-001810-10) was obtained from Dharmacon as a control. Cells were then lysed for analysis of protein knockdown by immunoblot analysis after siRNA transfection.
Materials
Cetuximab (IMC-225, Erbitux) was purchased from the University of Wisconsin Pharmacy. Sym004 was generously provided by Symphogen A/S (Lyngby, Denmark).
Antibodies
All antibodies were purchased from commercial sources as indicated below: EGFR, pEGFR (Y1173), and HRP-conjugated goat anti-rabbit IgG and goat anti-mouse IgG were obtained from Santa Cruz Biotechnology, Inc (Dallas, TX). pEGFR (Y1045), pEGFR (Y1068), pERK1/2 (T202/Y204), p44/42 ERK1/2, p-rpS6 (S235/236), rpS6, p-c-RAF (S289/296/301), c-RAF, pRSK1 (S380), RSK1, p-p38 (T180/Y182), and p38 were obtained from Cell Signaling Technology (Danvers, MA). pERK1/2 (T202/Y204/T185/Y187) was purchased from Abcam (Cambridge, MA). α-Tubulin was purchased from Calbiochem (Billerica, MA).
Cell Proliferation Assay
This was performed as previously described [10]. Cells were seeded in six-well plates. Following treatment, monolayers were analyzed by crystal violet assay. All treatments were performed in triplicate. Cells were seeded at 2000 cells per well in 100 µl of media on a 96-well plate, grown for 24 hours, and then treated with drug for 72 hours before analysis using the Cell Counting Kit 8 (Dojindo Molecular Technologies, Rockville, MD) according to the manufacturer's instructions. All treatments were performed in quadruplicate.
Cell Growth Assay In Vivo
Athymic nude mice (4- to 6-week-old males) were obtained from Harlan Laboratories (Indianapolis, IN). All animal procedures and maintenance were conducted in accordance with the institutional guidelines of the University of Wisconsin. Mice were randomized into treatment or control groups. Parental NCI-H226 (HP), HC1, or HC4 cells were injected in the dorsal flank of the mouse at respective day 0 (1 x 106 cells). Once tumors reached 250 mm3, mice were started on 0.5 mg of cetuximab treatments twice a week by intraperitoneal (i.p.) injection. Tumor volume measurements were evaluated by digital calipers and calculated by the formula (p)/6 x (large diameter) x (small diameter)2.
Immunoblot Analysis
Whole-cell protein lysate was obtained using Tween-20 lysis buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 0.1% Tween-20, 10% glycerol, 2.5 mM EGTA, 1 mM EDTA, 1 mM DTT, 1 mM Na3VO4, 1 mM PMSF, 1 mM beta-glycerophosphate (BGP), and 10 µg/ml leupeptin and aprotinin). Immunoblot analysis was conducted as previously described [20].
Bromodeoxyuridine Cell Cycle Distribution Analysis
Cells were plated at a density of 800,000 per 100 mm2 plate and allowed to adhere overnight. The cells were treated with vehicle, 20 µg/ml cetuximab, or 20 µg/ml Sym004 for 24 hours. On the following day, the cells were pulsed with 10 µM bromodeoxyuridine for 1 hour. The cells were harvested by trypsinization, washed with cold phosphate-buffered saline (PBS), and fixed with 70% ethanol for 20 minutes. The cells were then labeled with a fluorescein isothiocyanate-conjugated mouse anti-bromodeoxyuridine antibody and processed according to the manufacturer's recommendations (BD Pharmingen, San Jose, CA). The cells were analyzed by flow cytometry (BD FACScan). ModFit Software (Verity Software House, Topsham, ME) was used to analyze the data.
Phospho-MAPK Array
Cell lines were analyzed in the panel of phosphorylation profiles of MAPK and other serine/threonine kinases after treatment with Sym004 (Human Phospho-MAPK, ARY002B; R&D Systems, Minneapolis, MN). This array specifically screens for relative levels of phosphorylation of 26 individual proteins including 9 MAPKs and other intracellular proteins involved in cellular proliferation. After treatment with or without Sym004 (50 µg/ml), cell lysates were incubated with the membrane. Thereafter, a cocktail of biotinylated detection antibodies, streptavidin-HRP, and chemiluminescent detection reagents was used to detect the phosphorylated protein. The relative expression of specific phosphorylated protein was determined following quantification of scanned images by ImageJ compared with Sym004 and vehicle.
Mouse Xenograft Model
Athymic nude mice (4- to 6-week-old males) were obtained from Harlan Laboratories. All animal procedures and maintenance were conducted in accordance with the institutional guidelines of the University of Wisconsin. Mice were randomized into treatment or control groups. Mice were injected in the dorsal flank of the mouse at respective day 0 (2 x 106 cells). Once tumors reached 200 mm3, mice were started on their respective treatments (IgG, cetuximab, Sym004). The dose of cetuximab and Sym004 for the experiment was 1, 5, 20, and 50 mg/kg and twice a week by i.p. injection. Tumor volume measurements were evaluated by digital calipers and calculated by the formula (p)/6 x (large diameter) x (small diameter)2.
Mouse CtxR Human Tumor Xenografts
Mice were injected with NCI-H226 (2 x 106 cells), and tumors were allowed to grow to 100 mm3. All mice were randomized to treatment or control groups and treated with 1 mg/mouse (40 mg/kg) of either cetuximab or IgG i.p. twice weekly. Tumors were monitored for cetuximab resistance that was defined as marked tumor growth in the presence of continued cetuximab therapy. Once CtxR tumors reached a volume of ∼1000 mm3, mice were grouped according to similar time points of resistance. At this point, each mouse was treated with either cetuximab (1 mg) or Sym004 (20 mg/kg) i.p. twice weekly. Tumor volume measurements were evaluated by digital calipers and calculated by the formula (p)/6 x (large diameter) x (small diameter)2.
Mouse Tumor Collection and Protein Isolation
Tumors were collected 3 hours after the last cetuximab or Sym004 treatment. Mice were sedated using isoflurane mixed with oxygen until unconscious. Mice were killed by cervical dislocation, and tumors were promptly collected, washed in PBS, and frozen with isopentane on dry ice. Whole-cell protein lysates from tumor samples were obtained with NP-40lysis buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 10% glycerol, 2.5 mM EGTA, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, and 10 µg/ml leupeptin and aprotinin), homogenized by 10 strokes in a tightly fitting Dounce homogenizer, and quantified. Protein quantitation and immunoblot analysis were performed as stated above.
Immunohistochemistry
Tumor tissue samples were collected from xenograft tumors. Tumor samples were fixed in 4% formaldehyde/PBS paraffin-embedded and section. Sections were heated in 10 mM citrate buffer (pH 6.0) for EGFR, Ki67, and phospho-rpS6 or in boric acid buffer (pH 8.0) for cleaved caspase-3 by Decloaking Chamber. Samples were incubated with rabbit anti-EGFR (Abcam; ab52894, 1:200), rabbit anti-Ki67 (Abcam; ab66155, 1:1000), rabbit cleaved caspase-3 (Biocare, Concord, CA; CP229b, 1:100), and rabbit phospho-rpS6 (S235/236; Cell Signaling Technology; 1:400). Sections were stained by BrightVision rabbit/HRP (Immunologic, Duiven, The Netherlands; DPVR110HRP) or LSAB kit/HRP (Dako, Carpinteria, CA). Antibody binding was revealed by addition of 3,3′-diaminobenzidine substrate. Tissues were counterstained with Mayer's hematoxylin (Thermo Fisher Scientific, Waltham, MA) and were examined using an Olympus BX51 microscope. The immunohistochemical staining was judged as follows; Ki67: staining was analyzed with a PC-based image analysis system (Leica Q500, Cambridge, United Kingdom). With this equipment the positively stained DAB reaction was measured and expressed as percentage of positive stained area in relation to total analyzed viable tumor area. Cleaved Caspase-3: no positive cells, low numbers of positive cells (1+) or moderate numbers of positive cells (2+).
Results
CtxR Clones Have Increased EGFR Activity
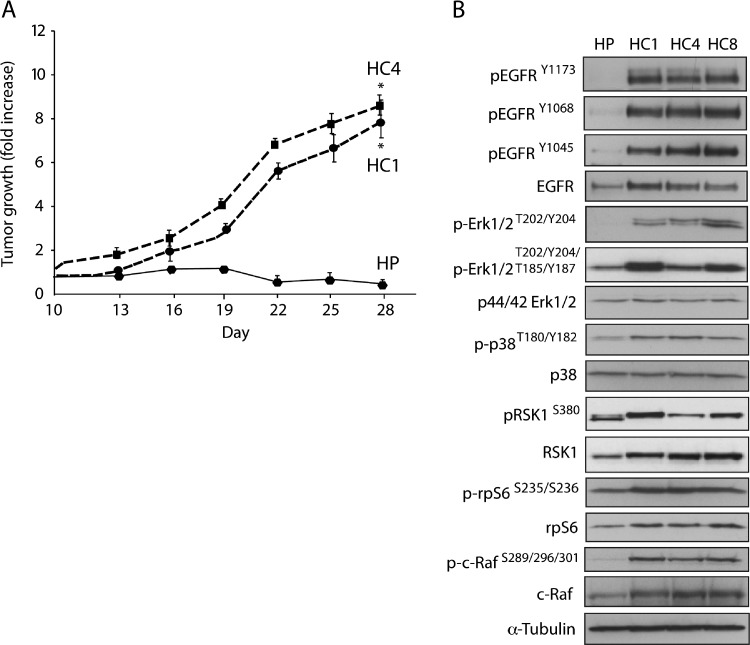
We have previously reported that three CtxR clones (HC1, HC4, and HC8) display a robust CtxR phenotype when challenged with increasing doses of cetuximab as compared to HP parental control cells at 72 hours [10]. To examine the stability of the resistant phenotype in vivo, we inoculated 1 x 106 cells of both HP and CtxR clones HC1 and HC4 into the dorsal flank of athymic nude mice. Tumors were allowed to grow to 250 mm3 and then treated with 0.5 mg of cetuximab/mouse i.p. twice weekly for the time indicated. As shown in Figure 1A, CtxR clones established in culture maintained their resistant phenotype in the xenograft model system. CtxR clones displayed increased steady-state expression of EGFR and EGFR phosphorylation (Y1173, Y1068, and Y1045). Increased phosphorylation levels of downstream signaling molecules, including ERK1/2 (T202/Y204/T185/Y187), p38 (T180/Y182), RSK1 (S380), c-RAF (S289/296/301), and rpS6 (S235/236), were detected by immunoblot analysis in the CtxR clones (HC1, HC4, HC8) relative to HP control cells (Figure 1B). Taken together, these results confirm the establishment of stable CtxR clones that exhibit robust activation of the EGFR signaling cascade.
Figure 1.
CtxR clones have increased EGFR activity. (A) CtxR clones are resistant to cetuximab in vivo. Male athymic nude mice were injected subcutaneously with 1 x 106 CtxS HP or CtxR cells (HC1 or HC4) into the dorsal flank. Once tumors reached a volume of 250 mm3, mice were treated with 0.5 mg of cetuximab twice weekly. Tumor diameters were measured serially with calipers. Tumor volumes were calculated and graphed as fold change in tumor volume + S.E. for each cell line. (B) CtxR cells have increased EGFR activity and phosphorylation levels of downstream signaling molecules. HP and CtxR clones (HC1, HC4, HC8) were harvested, and protein lysates were fractionated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblot analysis for the indicated proteins. α-Tubulin was examined as a loading control for each immunoblot analysis.
CtxR Clones Are Dependent on EGFR for Proliferative Potential
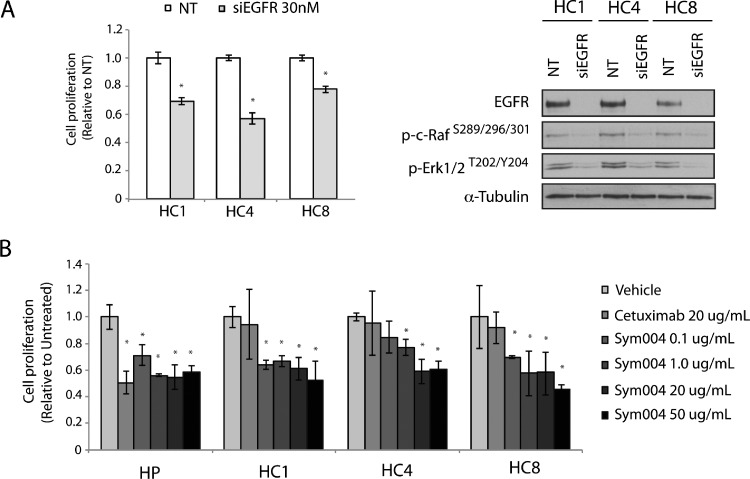
To determine if CtxR clones remained dependent on EGFR for enhanced growth potential, we performed proliferation assays using siRNAs targeting EGFR. All three CtxR clones displayed proliferation inhibitory effects at 30 nM siEGFR (Figure 2A). Loss of EGFR expression resulted in loss of activated c-RAF (S289/296/301) and ERK1/2 (T202/Y204) kinases, demonstrating that MAPK pathway activation is dependent on EGFR activity. Next, we treated CtxR clones with 20 µg/ml cetuximab or increasing doses of Sym004 for 72 hours. All CtxR clones demonstrated statistically significant, dose-dependent inhibition of proliferation in response to Sym004 treatment compared to cetuximab or vehicle treatment (Figure 2B).
Figure 2.
CtxR clones are dependent on EGFR for proliferative potential. (A) siEGFR inhibits the proliferation of CtxR clones. CtxR clones were plated and treated with 30 nM of EGFR siRNA or 30 nM nontargeting (NT) siRNA. Proliferation was measured at 72 hours after treatment using the cell counting kit-8 (CCK-8) proliferation assay described in the experimental procedures. Data points are represented as means ± SEM (n = 4). *P ≤ .05. CtxR clones (HC1, HC4, HC8) were harvested, and protein lysates were fractionated on SDS-PAGE, followed by immunoblot analysis for the indicated proteins. α-Tubulin was used as a loading control. (B) Sym004 inhibits the proliferation of CtxR clones. HP and CtxR cell lines (HC1, HC4, HC8) were plated and allowed to adhere for 24 hours before vehicle, cetuximab (20 µg/ml), or Sym004 treatment: 0.1, 1, 20, or 50 µg/ml. Proliferation was measured at 72 hours after drug treatment using the crystal violet assay and plotted as a percentage of growth relative to untreated control cells. Data points are represented as means ± SEM (n = 3).
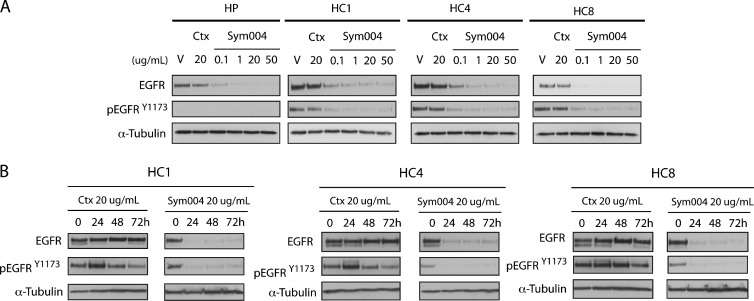
Sym004 Effectively Degrades EGFR in CtxR Clones, whereas Cetuximab Has No Effect on Total and Phosphorylated EGFR Levels
To investigate if inhibition of cell proliferation by Sym004 is due to EGFR degradation, we examined the expression levels of EGFR in CtxR clones treated with 20 µg/ml cetuximab or Sym004 (0.1, 1, 20, 50 µg/ml) for 24 hours (Figure 3A). Since our previous study showed that small molecule tyrosine kinase inhibitors (TKIs) decreased EGFR phosphorylation at tyrosine 1173 (Y1173), we chose this site for evaluation of Sym004 [10]. Both activated and total levels of EGFR were decreased at all doses of Sym004 examined in CtxR clones tested; increasing dosing of Sym004 led to more potent decreases in EGFR levels. In contrast, cetuximab had no impact on total EGFR levels. In a time course experiment, CtxR clones were treated with a single dose of 20 µg/ml Sym004 or cetuximab for 24, 48, or 72 hours (Figure 3B). The total and phosphorylated EGFR levels were inhibited by Sym004 at all three time points, whereas cetuximab had no effect on total EGFR and slightly activated EGFR in HC1 and HC4 clones at 24 hours post treatment. These results demonstrate that Sym004 can effectively downregulate EGFR in all CtxR clones for prolonged periods of time post treatment.
Figure 3.
Sym004 effectively degrades EGFR in CtxR clones, whereas cetuximab has no effect on total and phosphorylated EGFR levels. (A) Sym004 downregulates total and phosphorylated EGFR in CtxR clones. CtxR clones (HC1, HC4, HC8) were treated with vehicle, cetuximab (20 µg/ml), or Sym004 (0.1, 1, 20, or 50 µg/ml) for 24 hours. Whole-cell protein lysates were fractionated on SDS-PAGE followed by immunoblot analysis for the indicated proteins. α-Tubulin was used as a loading control. (B) Total and phosphorylated forms of EGFR remain downregulated 72 hours post Sym004 treatment in CtxR clones. Cells were treated with a single dose of 20 µg/ml cetuximab or 20 µg/ml Sym004 for 24, 48, or 72 hours. Cell lysates were prepared, and EGFR and phospho-EGFR were determined by immunoblot analysis.
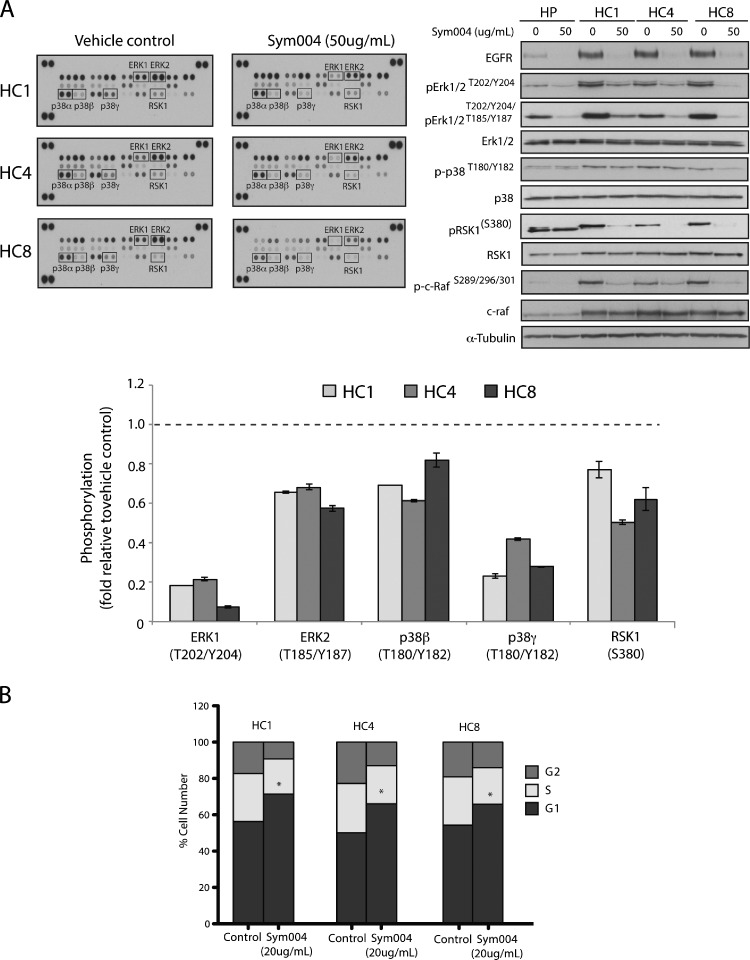
Multiple MAPK Pathway Proteins Are Inhibited in CtxR Cells by Sym004 Treatment
CtxR cells exhibited an increase in EGFR expression and activation, as well as activated MAPK pathway proteins (Figure 1B). To investigate potential mechanisms by which Sym004 elicits anti-proliferative effects in CtxR clones, we analyzed the effect of Sym004 treatment on MAPK signaling in each clone using a Human Phospho-MAPK Array. CtxR clones were treated with vehicle or Sym004 (50 µg/ml) for 24 hours. This Human Phospho-MAPK array includes 26 kinases, 9 of these are MAPKs. As illustrated in Figure 4A, Sym004 decreased phosphorylation in three CtxR clones by averages of 80% for ERK1, 70% for p38γ, 40% for ERK2, 40% for RSK1, and 30% for P38β, compared with the vehicle control. Consistent with the array results, Western blot analysis indicated that phosphorylation of c-RAF (S289/296/301), ERK1/2 (T202/Y204/T185/Y187), p38 (T180/Y182), and RSK1 (Ser380) were robustly inhibited after Sym004 treatment in all CtxR cells.
Figure 4.
Multiple MAPK effector molecules are inhibited in CtxR clones by Sym004 treatment. (A) Sym004 inhibited multiple downstream MAPK effector molecules detected through phospho-kinase array. After treatment with Sym004 (50 µg/ml), cells were collected and cell extracts were incubated with membranes containing antibodies to 26 individual proteins. The membranes were washed and incubated with a cocktail of biotinylated detection antibodies, streptavidin-HRP, and chemiluminescent detection reagents to measure the levels of phosphorylated protein. Quantitation of phosphorylated proteins was completed using scanned images from ImageJ software. Data points are represented as the mean of duplicate spots. Cell extracts were also fractionated on SDS-PAGE, followed by immunoblot analysis for the indicated proteins. α-Tubulin was examined as a loading control for each immunoblot analysis. (B) Sym004 can induce a G1-phase cell cycle arrest in CtxR clones. Cells were treated with 20 µg/ml Sym004 for 24 hours, and cell cycle phase distribution was analyzed as described in the Materials and Methods section. Data points are represented as means ± SEM (n =3). *P < .05.
Next, cell cycle phase distribution of CtxR clones was analyzed post Sym004 treatment. Flow cytometric analysis demonstrated that Sym004 induced a strong G1 arrest in CtxR cell lines (Figure 4B). Taken together, these data demonstrate that Sym004 can inhibit EGFR signaling through the MAPK pathway, ultimately impacting cell cycle progression in CtxR clones.
Both Cetuximab and Sym004 Delay Tumor Growth in Cetuximab-Sensitive H226 Cells
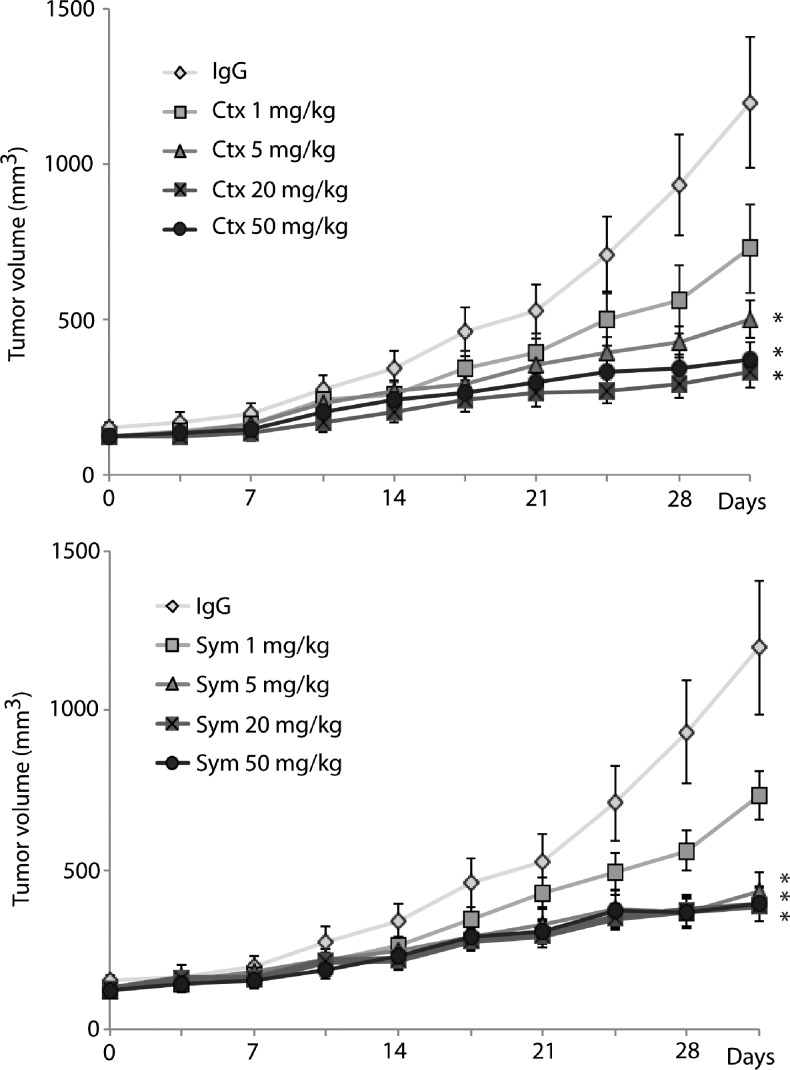
To investigate the effects of Sym004 in vivo, mice bearing established NCI-H226 [cetuximab-sensitive (CtxS) parental cell line] xenografts were treated with vehicle control, cetuximab, or Sym004. Mice were injected in the dorsal flank on day 0 (2 x 106 NCI-H226 cells), and once tumors reached an average volume of 200 mm3 (∼18 days), mice were randomly grouped. Cetuximab or Sym004 was administered through i.p. injection at a dose of 1, 5, 20, or 50 mg/kg twice weekly for four consecutive weeks. Mouse weight was measured weekly, and no discernible toxicity was observed in either the cetuximab or Sym004 treatment group. Treatment with either cetuximab or Sym004 showed clear dose-dependent antitumor activity, and both agents significantly delay tumor growth of CtxS H226 xenografts at doses of 5 mg/kg or higher compared to the IgG control group (Figure 5).
Figure 5.
Both cetuximab and Sym004 can effectively control the growth of CtxS mouse xenograft tumors. NCI-H226 cells were injected into mice, and tumors were allowed to grow to 200 mm3. All mice were randomized to treatment or control groups and treated with cetuximab (1, 5, 20, or 50 mg/kg), Sym004 (1, 5, 20, or 50 mg/kg), or IgG (50 mg/kg) i.p. twice weekly.
Sym004 Treatment of CtxR Tumors Leads to Tumor Growth Delay In Vivo
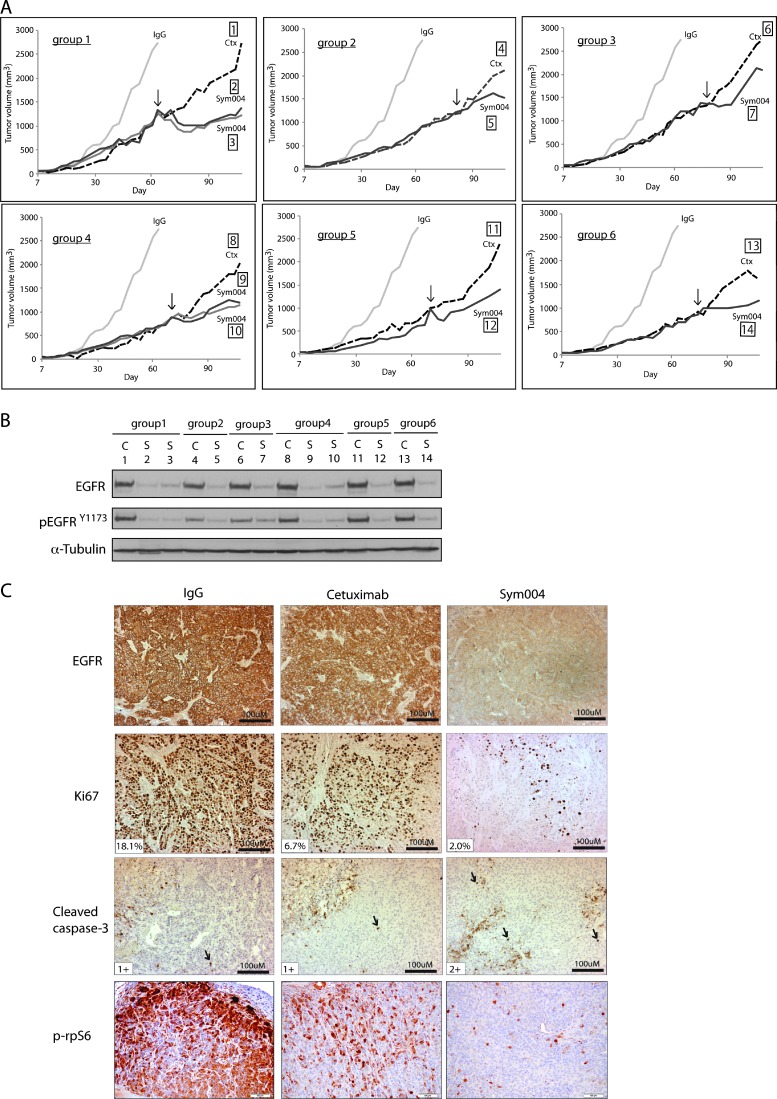
Next, we performed a series of mouse xenograft studies of a previously developed model of de novo acquired resistance to cetuximab [7]. To develop acquired resistance to cetuximab in vivo, we inoculated 40 mice with the NSCLC NCI-H226 cell line unilaterally with 2 x 106 tumor cells in the dorsal flank. Tumors were allowed to grow to 100 mm3, at which time 30 mice were treated with cetuximab (1 mg/mouse) and 10 mice were treated with IgG control (1 mg/mouse) twice weekly by i.p. injection. IgG-treated tumors grew rapidly uninhibited, whereas cetuximab-treated animals demonstrated tumor control and delayed growth. Tumors were monitored for the development of cetuximab resistance, defined as marked tumor growth in the presence of continued cetuximab therapy. Once CtxR tumors reached a volume of ∼1000 mm3, mice were grouped according to similar size at the time of resistance detection. CtxR was observed in 21 of 30 tumor xenografts (70%) treated with cetuximab, similar to previous studies from our laboratory [7]. Thus, a total of nine CtxR mouse xenograft groups was selected for further study (21 mice in total). Upon establishment of CtxR mouse groups, one mouse was maintained on cetuximab (1 mg) therapy, while the other mouse (or mice) in the group was removed from cetuximab and started on 20 mg/kg Sym004 (i.p. twice weekly). The average tumor volume of mice treated with IgG alone is included in all groups for comparison purposes. Eight of 12 (67%) CtxR tumors treated with Sym004 demonstrated a tumor response compared to the cetuximab-treated mouse in each group, while four (33%) tumors failed to respond. In Figure 6A, we illustrate six of the nine groups where Sym004 delayed tumor growth more than 30 days. The black arrow designates the starting time point of Sym004 treatment.
Figure 6.
Sym004 treatment delays the growth of CtxR xenograft tumors. (A) Growth-inhibitory effects of Sym004 in CtxR tumors in vivo. Mice were injected with NCI-H226, and tumors were allowed grow to 100 mm3. All mice were randomized to treatment or control groups and treated with either 1 mg/mouse (40 mg/kg) of cetuximab or IgG i.p. twice weekly. Tumors were monitored for cetuximab resistance, defined as marked tumor growth in the presence of continued cetuximab therapy. Once CtxR tumors reached a volume > 1000 mm3, mice were grouped according to similar time points of resistance. At this point, each mouse was treated with either cetuximab or Sym004 i.p. twice weekly. The black arrow designates the starting time point of Sym004 treatment. The average tumor volume of mice treated with IgG is included in all groups for comparison purposes. (B) Sym004-induced EGFR degradation in vivo. Immunoblot analysis of total and activated EGFR in CtxR xenograft tumors after cetuximab or Sym004 treatment. C, cetuximab; S, Sym004. (C) The degradation of EGFR in CtxR tumors corresponds with loss of proliferation and enhancement of apoptosis. CtxR tumor samples after cetuximab or Sym004 treatment in vivo were prepared and analyzed for EGFR, proliferation (Ki67), apoptosis (cleaved caspase-3), and phospho-rpS6 immunohistochemistry. Black arrows denote cells positive for cleaved caspase-3. The percentage of Ki67-positive cells and the number of positive cells for cleaved caspase-3 expression are shown.
To further investigate the ability of Sym004 to effectively target EGFR in large tumors in vivo, we examined total and phospho-EGFR levels in individual tumors by immunoblot analysis (Figure 6B). Sym004-treated CtxR tumors had essentially undetectable levels of EGFR, whereas cetuximab-treated CtxR tumors retained significant levels of EGFR. These findings were very similar to the results presented in Figure 3A. Next, we verified these findings in tissue sections of the tumors by immunohistochemical analysis of EGFR, as well as markers for cell proliferation (Ki67) and apoptosis (cleaved caspase-3; Figure 6C). Strong membrane and intracellular EGFR staining were observed in IgG- and cetuximab-treated CtxR tumors. Sym004-treated CtxR tumors showed moderate intracellular EGFR staining, and no or very limited membrane staining was seen in the majority of tumors examined. The highest numbers of Ki67-positive cells (18%) are seen in IgG followed by cetuximab-treated group (7%), and the lowest numbers are seen in Sym004-treated group (2%). Cleaved caspase-3 staining (black arrows) revealed that the most extensive expression was seen in samples treated with Sym004 followed by cetuximab-treated samples, and the most restricted expression was seen in IgG-treated samples. As a survival marker, phospho-rpS6 (S235/S236) was also stained in these CtxR tumors. CtxR tumors treated with Sym004 demonstrated prominent inhibition of p-rpS6 compared to IgG or cetuximab-treated CtxR tumors (Figure 6C). These xenograft studies suggest that Sym004 can effectively inhibit the growth of large CtxR tumors, providing strong evidence for the use of Sym004 in the setting of acquired resistance to cetuximab.
Discussion
EGFR is one of the most highly targeted receptors in oncology due to its frequent overexpression and aberrant activation in numerous cancers. The anti-EGFR mAb therapy cetuximab is a Food and Drug Administration-approved treatment for mCRC [21] and HNSCC [22] and has shown efficacy in other tumor types such as NSCLC [23,24]. While cetuximab has demonstrated clinical success, both intrinsic and acquired resistance is commonly observed. To characterize the mechanisms of resistance to cetuximab in NSCLC, the cell line NCI-H226 was treated with increasing doses of cetuximab until resistant cell clones developed. In this model, we found that CtxR clones had increased EGFR expression and dependency on EGFR for enhanced proliferative potential [10,11]. Therefore, we hypothesized that Sym004, a novel anti-EGFR mAb mixture that leads to rapid internalization and degradation of the EGFR [15], may overcome resistance to cetuximab. The present study demonstrates that Sym004 can elicit potent antiproliferative effects in CtxR clones, which corresponded to the degradation of total EGFR. The loss of EGFR inhibited the activity of multiple MAPK signaling proteins corresponding to G1 phase cell cycle arrest. De novo xenograft models of NSCLC (NCI-H226) acquired CtxR further indicated that Sym004 could significantly delay the growth of large CtxR tumors. Collectively, these data suggest that Sym004 may be an invaluable treatment for EGFR-overexpressing CtxR tumors.
Cetuximab is a human/murine chimeric mAb that binds to the extracellular ligand-binding domain III of EGFR [4,25]. Cetuximab prevents EGFR ligand binding and sterically hinders dimerization with other HER receptors; thus, cetuximab has been shown to inhibit signaling cascades emanating from activated EGFR [4,26]. Sym004 is a mixture of two EGFR-directed antibodies that bind to two distinct epitopes on domain III [15,16]. Sym004 can inhibit EGFR activation and downstream signaling pathways but in contrast to cetuximab, it can induce robust EGFR cross-links on the cell surface leading to effective internalization and degradation of the receptor [15,16]. In the present study, increased doses of Sym004 induced potent antiproliferative effects in CtxR cell lines (Figure 2B), which corresponded to more dramatic losses in total EGFR levels (Figure 3A); this finding suggests that loss of EGFR is a central mechanism by which proliferation is inhibited. Treatment of CtxR cells with cetuximab had no effect on EGFR levels and slightly enhanced EGFR activity (Figure 3B). Mandic et al. reported a similar phenomenon in a variety of different HNSCC cell lines treated with cetuximab [27], indicating that cetuximab may function as a weak ligand in some cases. While the current CtxR model has increased dependency on the EGFR, other models of CtxR have undergone oncogenic shift to other receptor tyrosine kinases (RTKs) [28] or constitutive activation of EGFR downstream effector molecules [29]; thus, Sym004 may not be an effective treatment option for all CtxR tumors. Overall, EGFR-dependent CtxR clones can be effectively targeted with Sym004 through the robust degradation of EGFR and loss of downstream signaling emanating from this receptor.
Previous studies in our laboratory have indicated that the CtxR clones used in this study have increased expression of EGFR ligands [8]. Further experimentation demonstrated that addition of EGFR ligands to the heparin binding (HP) CtxS cell line could enhance resistance to cetuximab. This phenomenon was observed by Hatakeyama et al., where increased expression of HB-EGF was detected in intrinsic CtxR HNSCC cell lines and in tumor samples from patients with recurrent disease [30]. Pedersen et al. supported these findings by reporting that EGF could enhance resistance to cetuximab in numerous cancer cell lines; interestingly, researchers further showed that Sym004 treatment of the same cell lines yielded less ligand-induced resistance [15]. Therefore, it seems plausible that Sym004-directed degradation of EGFR in the current CtxR model might overcome compensatory up-regulation of ligand.
Another mechanism by which Sym004-directed degradation of EGFR may overcome CtxR is through the inhibition of EGFR nuclear translocation. Numerous studies have demonstrated that nuclear EGFR can enhance resistance to cetuximab [8], gefitinib [31], radiation [32,33], and chemotherapy [34,35]. Previous research with the current model demonstrated that inhibition of nuclear EGFR could resensitize resistant clones to cetuximab [8]. Sym004-directed degradation of EGFR appears to deplete both the cell membrane component of EGFR, as well as nuclear EGFR, leading to effective knockdown of the EGFR signaling network. Thus, Sym004 may play a vital role in overcoming resistance to gefitinib, radiation, and chemotherapy as well.
The MAPK signaling pathway is one of the main pathways emanating from activated EGFR, and thus, CtxR clones express activated forms of MAPK pathway proteins (Figure 1B). Sym004 treatment inhibited two of the four major groups of conventional MAPKs, including ERK1/2 and p38 MAPK, in addition to RSK1 and c-RAF (Figure 4A). RSK1 has specifically been shown to play an important role in promoting cell cycle progression past the G1 checkpoint through the phosphorylation of p27Kip1 and serum response factor, ultimately enhancing cyclin D expression [36]. Therefore, inhibition of RSK1 may play a role in the G1 phase cell cycle arrest observed upon Sym004 treatment (Figure 4B). While the status of MAPK signaling proteins were analyzed in this study, the functions of other proteins were likely modulated as well, and therefore, the antiproliferative effects of Sym004 observed here may not be solely due to MAPK inhibition. Additionally, while Sym004 yielded potent antiproliferative effects in vitro, apoptosis was only minimally increased (data not shown).
The antiproliferative effects observed in vitro were also observed in de novo models of acquired resistance to cetuximab in vitro. De novo acquired resistant models were chosen for study because they more accurately portray the cellular events that result in cetuximab resistance in the clinic. In this model, CtxR xenograft tumors were significantly growth delayed upon treatment with Sym004 (Figure 6A) compared to CtxR tumors that were continued on cetuximab therapy. The fact that 67% of CtxR tumors put on Sym004 yielded tumor growth delay demonstrates a unique property of Sym004 inhibition rather than just spontaneous reduction in tumor size. While tumor shrinkage was observed in some mice upon Sym004 treatment, most experienced a delay in growth; growth delay in tumors of this size (>1000 mm3) demonstrates that Sym004 can robustly inhibit proliferation, which may translate into potent antitumor effects in human patients. Additionally, Sym004 treatment led to robust losses of EGFR and Ki67 staining, while there were minimal changes in cleaved caspase-3 levels (Figure 6C). Collectively, the antitumor effects of Sym004 observed in this model of CtxR may be exerted through modulations in proliferation pathways rather than apoptosis pathways.
Determining why patients with cancer become resistant to anti-EGFR therapeutics is a major clinical challenge. Data from the present study suggest that CtxR tumors may still be addicted to EGFR signaling, and therefore, using Sym004 may be of great clinical promise. Degradation of the EGFR, rather than inactivation, is a powerful anticancer strategy because both kinase-dependent and independent functions of EGFR will be abolished. This therapeutic may also have antitumor effects in the setting of EGFR-activating mutations (such as L858R and subsequent gatekeeper mutations such as T790M), EGFR S492R mutations (found in patients with mCRC yielding resistance to cetuximab [37]), wild-type and mutant KRAS colorectal tumors (where some KRAS mutant mCRCs may still contain sensitivity to cetuximab [38,39]), and in the case of EGFR-overexpressing breast cancers. Sym004 has now undergone phase I clinical trials in patients with advanced solid tumors, in which patients treated with multiple doses of Sym004 had manageable adverse effects [40]. Sym004 is currently undergoing phase II testing in CRC and HNSCC (http://clinicaltrials.gov). Overall, Sym004 is a promising new molecular targeting agent that may provide more beneficial antitumor effects compared to cetuximab in EGFR-overexpressing cancers.
Abbreviations
- CtxR
cetuximab-resistant
- CtxS
cetuximab-sensitive
- EGFR
epidermal growth factor receptor
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- HP
parental NCI-H226 cells
- HNSCC
head and neck squamous cell carcinoma
- i.p.
intraperitoneal
- MAPK
mitogen-activated protein kinase
- mAb
monoclonal antibody
- mCRC
metastatic colorectal cancer
- NSCLC
non-small cell lung cancer
- siRNA
small interfering RNA
Footnotes
The project described was supported, in part, by grant UL1TR000427 from the Clinical and Translational Science Award program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences, grant RSG-10-193-01-TBG from the American Cancer Society (D.L.W.), grant 888157 from Symphogen A/S (D.L.W.), and NIH grant T32 GM08.1061-01A2 from Graduate Training in Cellular and Molecular Pathogenesis of Human Diseases (T.M.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Conflict of interest: D.L.W. holds a laboratory research agreement with Symphogen A/S. M.W.P., I.D.H., and M.K. are employed by Symphogen A/S. No potential conflicts of interest were disclosed by other authors.
References
- 1.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors—impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 3.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1–9. doi: 10.1111/j.1365-2249.2009.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand TM, Iida M, Wheeler DL. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol Ther. 2011;11:777–792. doi: 10.4161/cbt.11.9.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand TM, Dunn EF, Iida M, Myers RA, Kostopoulos KT, Li C, Peet CR, Wheeler DL. Erlotinib is a viable treatment for tumors with acquired resistance to cetuximab. Cancer Biol Ther. 2011;12:436–446. doi: 10.4161/cbt.12.5.16394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene. 2009;28:3801–3813. doi: 10.1038/onc.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, Iida M, Dunn EF, Wheeler DL. Dasatinib blocks cetuximaband radiation-induced nuclear translocation of the epidermal growth factor receptor in head and neck squamous cell carcinoma. Radiother Oncol. 2010;97:330–337. doi: 10.1016/j.radonc.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler DL, Iida M, Kruser TJ, Nechrebecki MM, Dunn EF, Armstrong EA, Huang S, Harari PM. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol Ther. 2009;8:696–703. doi: 10.4161/cbt.8.8.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Veeken J, Oliveira S, Schiffelers RM, Storm G, van Bergen En Henegouwen PM, Roovers RC. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets. 2009;9:748–760. doi: 10.2174/156800909789271495. [DOI] [PubMed] [Google Scholar]
- 13.Desbois-Mouthon C, Baron A, Blivet-Van Eggelpoël MJ, Fartoux L, Venot C, Bladt F, Housset C, Rosmorduc O. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin Cancer Res. 2009;15:5445–5456. doi: 10.1158/1078-0432.CCR-08-2980. [DOI] [PubMed] [Google Scholar]
- 14.Benavente S, Huang S, Armstrong EA, Chi A, Hsu KT, Wheeler DL, Harari PM. Establishment and characterization of a model of acquired resistance to epidermal growth factor receptor targeting agents in human cancer cells. Clin Cancer Res. 2009;15:1585–1592. doi: 10.1158/1078-0432.CCR-08-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS, Kragh M. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70:588–597. doi: 10.1158/0008-5472.CAN-09-1417. [DOI] [PubMed] [Google Scholar]
- 16.Koefoed K, Steinaa L, Søderberg JN, Kjæer I, Jacobsen HJ, Meijer PJ, Haurum JS, Jensen A, Kragh M, Andersen PS, et al. Rational identification of an optimal antibody mixture for targeting the epidermal growth factor receptor. mAbs. 2011;3:584–595. doi: 10.4161/mabs.3.6.17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, Touge H, Makino H, Takata M, Miyata M, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 18.Derer S, Berger S, Schlaeth M, Schneider-Merck T, Klausz K, Lohse S, Overdijk MB, Dechant M, Kellner C, Nagelmeier I, et al. Oncogenic KRAS impairs EGFR antibodies' efficiency by C/EBPβ-dependent suppression of EGFR expression. Neoplasia. 2012;14:190–205. doi: 10.1593/neo.111636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larbouret C, Gaborit N, Chardès T, Coelho M, Campigna E, Bascoul-Mollevi C, Mach JP, Azria D, Robert B, Pèlegrin A. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors' down-regulation and dimers' disruption. Neoplasia. 2012;14:121–130. doi: 10.1593/neo.111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iida M, Brand TM, Campbell DA, Li C, Wheeler DL. Yes and Lyn play a role in nuclear translocation of the epidermal growth factor receptor. Oncogene. 2013;32:759–767. doi: 10.1038/onc.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 22.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A, Baselga J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 23.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, Park K, Yu CT, Ganul V, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 24.Carillio G, Montanino A, Costanzo R, Sandomenico C, Piccirillo MC, Di Maio M, Daniele G, Giordano P, Bryce J, Normanno N, et al. Cetuximab in non-small-cell lung cancer. Expert Rev Anticancer Ther. 2012;12:163–175. doi: 10.1586/era.11.178. [DOI] [PubMed] [Google Scholar]
- 25.Voigt M, Braig F, Göthel M, Schulte A, Lamszus K, Bokemeyer C, Binder M. Functional dissection of the epidermal growth factor receptor epitopes targeted by panitumumab and cetuximab. Neoplasia. 2012;14:1023–1031. doi: 10.1593/neo.121242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham J, Muhsin M, Kirkpatrick P. Cetuximab. Nat Rev Drug Discov. 2004;3:549–550. doi: 10.1038/nrd1445. [DOI] [PubMed] [Google Scholar]
- 27.Mandic R, Rodgarkia-Dara CJ, Zhu L, Folz BJ, Bette M, Weihe E, Neubauer A, Werner JA. Treatment of HNSCC cell lines with the EGFR-specific inhibitor cetuximab (Erbitux) results in paradox phosphorylation of tyrosine 1173 in the receptor. FEBS Lett. 2006;580:4793–4800. doi: 10.1016/j.febslet.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 28.Bianco R, Rosa R, Damiano V, Daniele G, Gelardi T, Garofalo S, Tarallo V, De Falco S, Melisi D, Benelli R, et al. Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin Cancer Res. 2008;14:5069–5080. doi: 10.1158/1078-0432.CCR-07-4905. [DOI] [PubMed] [Google Scholar]
- 29.Kim SM, Kim JS, Kim JH, Yun CO, Kim EM, Kim HK, Solca F, Choi SY, Cho BC. Acquired resistance to cetuximab is mediated by increased PTEN instability and leads cross-resistance to gefitinib in HCC827 NSCLC cells. Cancer Lett. 2010;296:150–159. doi: 10.1016/j.canlet.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Hatakeyama H, Cheng H, Wirth P, Counsell A, Marcrom SR, Wood CB, Pohlmann PR, Gilbert J, Murphy B, Yarbrough WG, et al. Regulation of heparin-binding EGF-like growth factor by miR-212 and acquired cetuximab-resistance in head and neck squamous cell carcinoma. PLoS One. 2010;5:e12702. doi: 10.1371/journal.pone.0012702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang WC, Chen YJ, Li LY, Wei YL, Hsu SC, Tsai SL, Chiu PC, Huang WP, Wang YN, Chen CH, et al. Nuclear translocation of epidermal growth factor receptor by Akt-dependent phosphorylation enhances breast cancer-resistant protein expression in gefitinib-resistant cells. J Biol Chem. 2011;286:20558–20568. doi: 10.1074/jbc.M111.240796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 33.Steinle M, Palme D, Misovic M, Rudner J, Dittmann K, Lukowski R, Ruth P, Huber SM. Ionizing radiation induces migration of glioblastoma cells by activating BK K+ channels. Radiother Oncol. 2011;101:122–126. doi: 10.1016/j.radonc.2011.05.069. [DOI] [PubMed] [Google Scholar]
- 34.Hsu SC, Miller SA, Wang Y, Hung MC. Nuclear EGFR is required for cisplatin resistance and DNA repair. Am J Transl Res. 2009;1:249–258. [PMC free article] [PubMed] [Google Scholar]
- 35.Liccardi G, Hartley JA, Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 2011;71:1103–1114. doi: 10.1158/0008-5472.CAN-10-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 37.Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP, et al. Identification of a mutation in the extracellular domain of the epidermal growth factor receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221–223. doi: 10.1038/nm.2609. [DOI] [PubMed] [Google Scholar]
- 38.Dunn EF, Iida M, Myers RA, Campbell DA, Hintz KA, Armstrong EA, Li C, Wheeler DL. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene. 2011;30:561–574. doi: 10.1038/onc.2010.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 40.Dienstmann R. Phase I trial of the first-in-class EGFR mAb mixture, Sym004, in patients with refractory advanced solid tumors. JCO, 2011 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2011;29(15 suppl):3089. [Google Scholar]