Abstract
Purpose
Viral etiology is common in cases of children with acute diarrhea, and antibiotic therapy is usually not required. Therefore, it is important to determine the distribution of common viruses among children hospitalized with acute diarrhea.
Methods
We included 186 children who suffered from acute diarrhea and were hospitalized at the Wonkwang University Hospital Pediatric ward from December 1, 2010 to June 30, 2011 in this study. Stool samples were collected and multiplex reverse transcriptase polymerase chain reaction (multiplex RT-PCR) was used to simultaneously determine the viral etiology such as rotavirus, norovirus, astrovirus, or adenovirus.
Results
Causative viruses were detected in 72 of the 186 cases (38.7%). The mean age of the virus-positive cases was 1 year and 9 months (range, 1 month to 11 years). Rotavirus was detected in 50/186 (26.9%); norovirus, in 18/186 (9.7%); and astrovirus, in 3/186 cases (1.6%). Adenovirus was not detected in any of the cases. Proportions of norovirus genogroups I and II were 21.1% and 78.9%, respectively. Four of the 51 rotavirus-positive cases (7.8%) had received rotavirus vaccination at least once. The mean duration of diarrhea was 2.8 days (range, 1 to 10 days) and vomiting occurred in 39 of the 72 cases (54.2%).
Conclusion
Viral etiology was confirmed in about one-third of the children with acute diarrhea, and the most common viral agent was rotavirus, followed by norovirus.
Keywords: Rotavirus, Norovirus, Reverse transcriptase polymerase chain reaction
Introduction
Acute diarrhea is a common disease among infants and children due to age-specific differences in host defense mechanisms1). Current laboratory methods allow relatively easy and simplified detection of viruses in diarrhea stool using multiplex reverse transcription polymerase chain reaction (multiplex RT-PCR), which is sensitive and specific in its detection of rotavirus, norovirus (groups I and II), astrovirus, and group F adenovirus (serotypes 40 and 41)2,3). Recently, the viral etiology of acute diarrhea in infants and children has been actively demonstrated in our country. Rotavirus was identified as the most common viral agent, and norovirus infection was the second most common virus in children with acute diarrhea during the past several years4). Moreover, vaccination for the prevention of rotavirus infection was introduced for clinical usage in our country5). Therefore, we expected some changes in the incidence of viral gastroenteritis due to infection with rotavirus, norovirus, adenovirus, and astrovirus in the pediatric age group suffering from acute diarrhea.
Epidemics of acute viral gastroenteritis occur every year, and rise in the incidence of this diseases, especially that caused by rotavirus infection, is observed from late fall to spring6). Recently, the temporal trend in peak rotaviral activity has gradually shifted from winter to early spring in Japan7). A similar trend was expected in our country but the data were limited5,8). The number of hospitalized patients in our hospital abruptly increased in December of 2010 and declined in June 2011. Therefore, in this study, we wanted to detect viral etiology in stool using a multiplex RT-PCR and delineate clinical characteristics.
Material and methods
1. Patients
This study was performed in patients of 186 children with symptoms of acute diarrhea hospitalized in the Wonkwang University Hospital in Iksan, between December 2010 and June 2011. Acute diarrhea was defined as over 3 times sudden onset watery diarrhea in a period of 24 hours. The 186 enrolled study population was determined afterwards by exclusion of bacterial gastroenteritis that was based on results of routine stool cultures. Multiplex RT-PCR was performed using the collected stool samples to detect rotavirus, norovirus, astrovirus, and adenovirus simultaneously. The stool samples in this study were collected from patients with an age distribution of 1 month to 11 years. Out of the 186 patients, 119 (64%) were male.
2. Viral nucleic acid extraction and multiplex RT-PCR
For pretreatment of stool, we placed 100 µL of collected diarrhea stool into the microcentrifuge tube with 900 µL of phosphate buffered saline and vortexed for more than 10 seconds. The stool sample was then left for 10 minutes at 100 ℃, followed by 5 minutes at room temperature. The sample was then centrifuged for 10 minutes at 14,000 rpm, and the supernatant was collected. Viral nucleic acid extraction from the supernatant was performed using a GeneAll Ribospin vRD Viral RNA/DNA Extraction Kit (GeneAll Biotechnology Co., Seoul, Korea), and cDNA synthesis was performed using a Revert Aid First Standard cDNA Synthesis Kit (Fermentas, Burlington, ON, Canada), according to the manufacturer's instructions. For multiplex RT-PCR, we mixed 8 µL of extracted nucleic acid with primers and buffers using a Seeplex Diarrhea-V ACE detection kit (Seegene Inc., Seoul, Korea), according to the manufacturer's instructions. PCR amplification was performed using a GeneAmp PCR system 2700 (Applied Biosystem, Foster City, CA, USA). The amplified viral nucleic acid product was detected by 2% agarose gel electrophoresis.
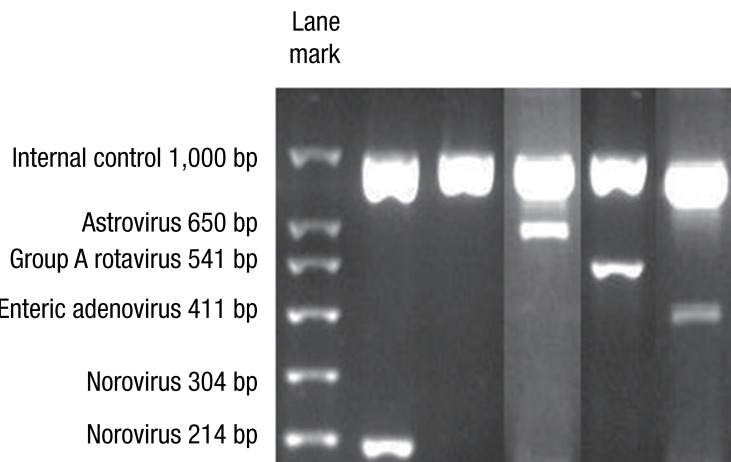
Agarose gel electrophoresis results showed expected PCR product sizes for viruses in the control samples analyzed through the novel multiplex RT-PCR method, as observed in the electrophoresis images (Fig. 1). Using the DNA ladder marker as reference, PCR product sizes corresponding to enteric adenovirus (411 bp), astrovirus (650 bp), rotavirus (541 bp), and norovirus GII (214 bp) were observed.
Fig. 1.
Agarose gel electrophoresis results show expected polymerase chain reaction (PCR) product sizes for viruses, in the control samples analyzed through the novel multiplex reverse transcriptase PCR method. Using the DNA ladder marker as reference, DNA bands corresponding to norovirus G2 (214 bp), astrovirus (650 bp), rotavirus (541 bp), and enteric adenovirus (411 bp) are observed.
3. Clinical and laboratory manifestations
We retrospectively analyzed the clinical and laboratory characteristics of patients who were positive for rotavirus or norovirus according to multiplex RT-PCR results. We compared these groups based on duration and frequency of diarrhea, vomiting, and fever. The patients were discharged when the symptoms, such as fever, vomiting, diarrhea were improved. We also compared the laboratory findings between these groups, such as white blood cell (WBC) count with differential count, as well as hemoglobin, hematocrit, blood urea nitrogen, creatinine, aspartate transaminase (AST), and alanine transaminase levels in peripheral blood.
4. Statistical analysis
IBM SPSS ver.19.0 (IBM Co., Armonk, NY, USA) was used for frequency and t test statistical analysis.
Results
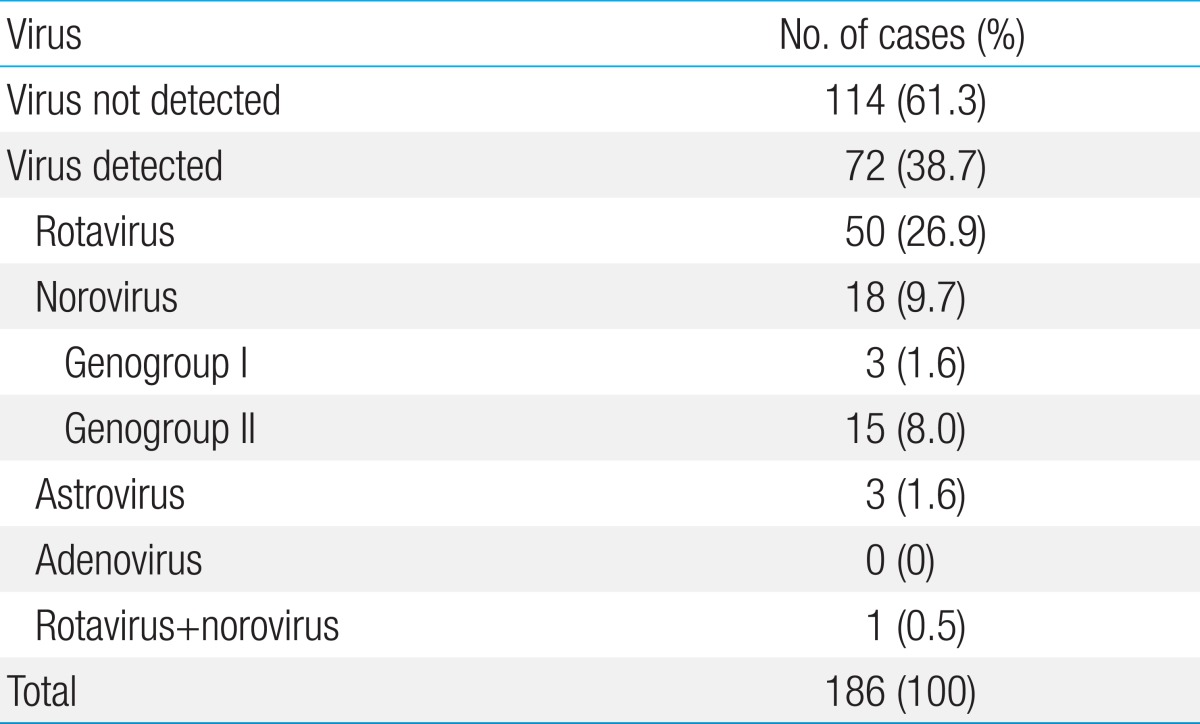
Viruses were detected in 72 of the 186 cases (38.7%) (Table 1). The most common virus was rotavirus, detected in 50 cases (26.8%), followed by norovirus, in 18 of the 186 cases (9.7%). Astrovirus was detected in 3 cases, and adenovirus was not detected in any of the cases. Rotavirus and norovirus were detected in one case (Table 1). Rotavirus vaccination had been received in 4 of the 50 cases (8%) of rotavirus infection.
Table 1.
Incidence of causative viruses in children with acute diarrhea analyzed by multiplex reverse transcriptase-polymerase chain reaction
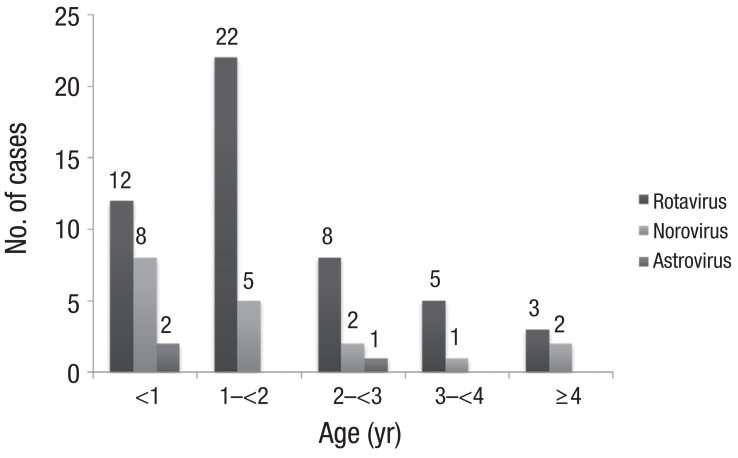
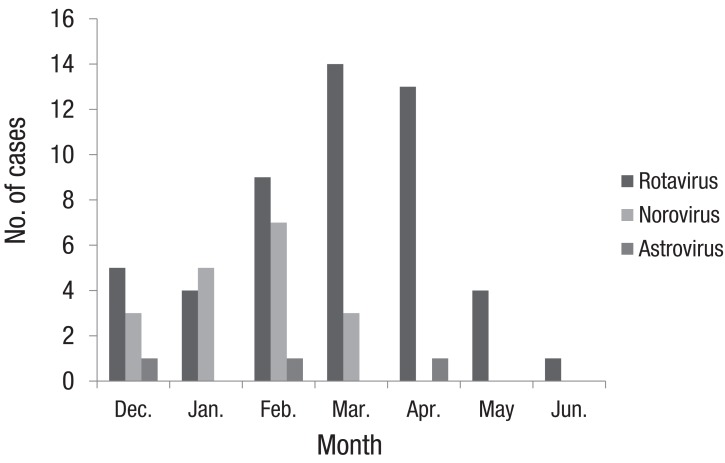
Rotaviral gastroenteritis was most commonly observed among children one year to less than two years of age; however, norovirus infection was most common in the less-than-1-year age group (Fig. 2). The male and female ratio was 1:0.7 for rotavirus and 1:0.4 for norovirus infections. Mean duration of hospitalization was 4.8±2.02 and 6.8±3.3 days for rotavirus and norovirus infections, respectively. The duration of hospitalization for acute diarrhea due to norovirus was longer than that of rotavirus (P=0.003) (Table 2). In our study, rotavirus was detected most commonly in March, such as 14 of the 51 cases (27%). In cases of norovirus, it was detected most commonly in February, such as 7 of the 19 cases (37%) (Fig. 3).
Fig. 2.
The age distribution of causative viruses in children with acute diarrhea. Exclude one case that rotavirus and norovirus were detected simultaneously.
Table 2.
Parameters of viral infection in children with acute diarrhea
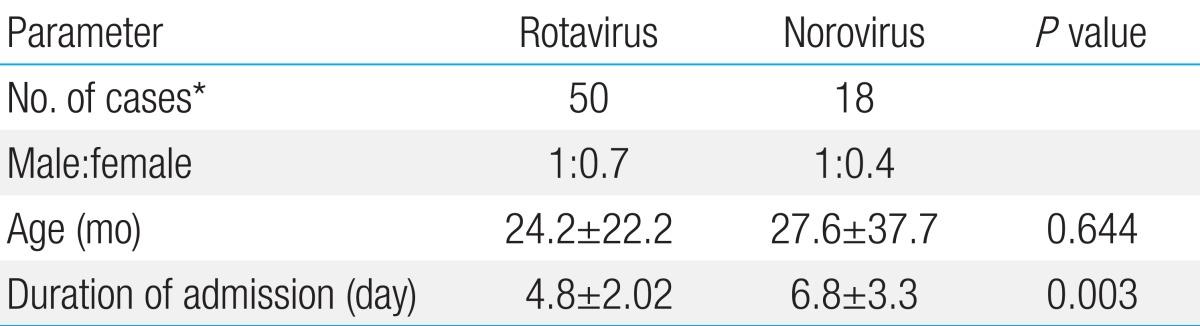
Values are presented as mean±standard deviation.
*Excluding one patient in whom rotavirus and norovirus were detected simultaneously.
Fig. 3.
The monthly distribution of causative viruses in children with acute diarrhea. Exclude one case that rotavirus and norovirus were detected simultaneously.
Clinical signs and symptoms such as the frequency and duration of diarrhea were not different between rotavirus and norovirus infections. However, vomiting was common in rotavirus infections and was observed in 30 of the 50 rotavirus infection cases (60%) compared to 6 of the 18 norovirus infection cases (32%), but it was not statistically significant (P=0.507) (Table 3). The mean hemoglobin and hematocrit levels in peripheral blood were within the normal reference range, and they were not statistically different between rotavirus and norovirus infection cases. WBC count (×1,000/mL) increased to 10.1±4.0 and 13.7±8.7 in rotavirus and norovirus infections, respectively; patients with norovirus infection showed considerably more leukocytosis than those with rotavirus infection (P=0.02). Moreover, AST levels in peripheral blood were higher in rotavirus infection (48.6±22.22) than in norovirus infection cases (37.8±10.69, P=0.047) (Table 4).
Table 3.
Clinical characteristics of acute diarrhea in children with viral infection
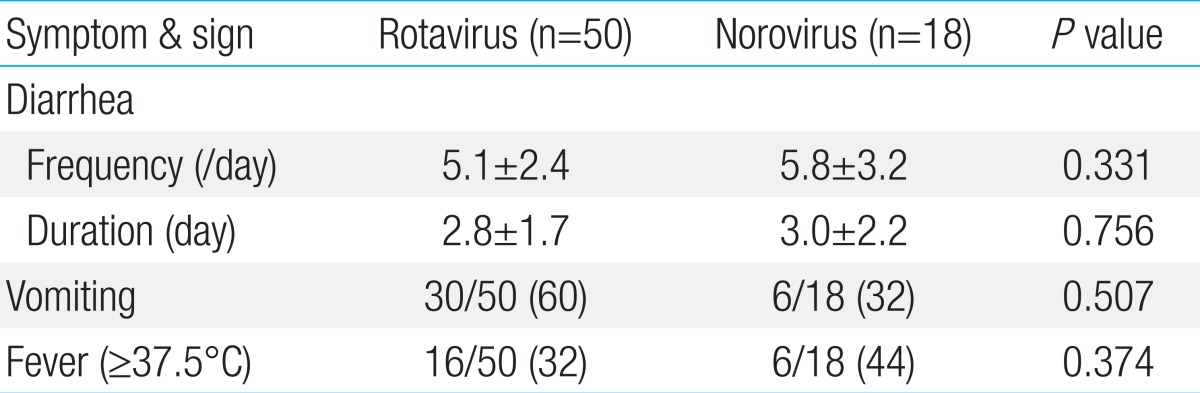
Values are presented as mean±standard deviation. Excluding one patient in whom rotavirus and norovirus were detected simultaneously.
Table 4.
Laboratory findings of acute diarrhea in children with viral infection
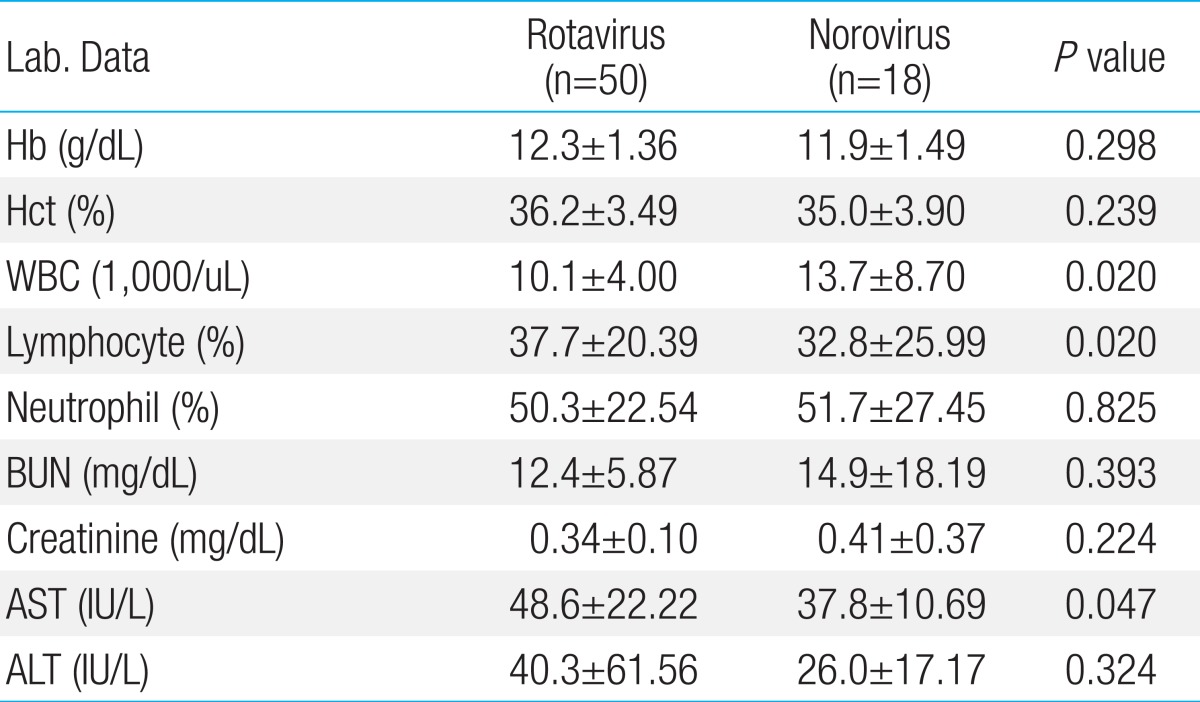
Values are mean±SD. Exclude one case that rotavirus and norovirus were detected simultaneously. Hb, Hemoglobin; Hct, hematocrit; WBC, white blood cell; BUN, blood urea nitrogen; AST, Aspartate aminotransferase; ALT, alanine aminotransferase.
Discussion
Relatively simple, easy, and rapid methods have been developed lately for the detection of viruses in the stool, including the more convenient multiplex PCR and multiplex RT-PCR method. Yan et al.9,10) developed multiplex RT-PCR methods to detect 8 viruses such as rotavirus (groups A-C), adenovirus, norovirus (genogroups GI and GII), sapovirus, and astrovirus. In the present study, we used multiplex RT-PCR to simultaneously detect viruses such as rotavirus, norovirus (genogroup I, II), astrovirus, and adenovirus in stool specimens of children with acute diarrhea, using a Seeplex Diarrhea-V ACE detection kit (Seegene Inc.). This kit is a novel commercial multiplex RT-PCR assay that can detect 5 viruses commonly associated with diarrhea, including rotavirus, norovirus (genogroup I and II), astrovirus, and adenovirus. The sensitivity and specificity of this method has been evaluated and confirmed by Higgins et al.3), who reported 100% diagnostic sensitivity for rotavirus, norovirus GI, and adenovirus, and 97% sensitivity for norovirus GII.
Viral etiology in acute diarrhea stool includes rotavirus, norovirus (genogroups I and II), astrovirus, and group F adenovirus (serotypes 40 and 41), and these viruses are the common cause of acute gastroenteritis in children. Therefore, rapid detection of these viral agents in acute gastroenteritis can help clinicians achieve efficient management of disease and avoid unnecessary antibiotic use11-13). Noroviruses and rotaviruses affect both adult and child populations, and are a primary concern for infection control14-16).
The rate of virus-positive stool samples was 47% according to a study conducted by Colomba et al.17), which is within the 31.5-59% range and is roughly similar to previously reported studies18,19). In 2011, the Korean Center for Disease Control (KCDC)4) reported detecting viruses in the stool of 30.6% of acute diarrhea patients less than 5 years of age; the most commonly detected virus was rotavirus (15.4%), followed by norovirus (11.6%), adenovirus (3.0%), and astrovirus (0.6%). These virus detection rates in stool from acute gastroenteritis patients were similar to those observed in other countries16,20,21). In Lanzhou, China, viral etiologies of acute gastroenteritis in hospitalized children with diarrhea were detected using enzyme-linked immunosorbent assay, RT-PCR, or PCR21). The detection rates of rotavirus, norovirus, saporovirus, astrovirus, and adenovirus were 54.0%, 9.2%, 1.1%, 3.3%, and 4.4%, respectively. In our study, the rate of virus-positive stool samples was 38.7%. Rotavirus was detected in 51/186 cases (27.4%), norovirus in 19/186 cases (10.2%), and astrovirus in 3/186 cases (1.6%); adenovirus was not detected in any of the cases. Norovirus genogroup I and II were detected in 4/19 (21.1%) and 15/19 cases (78.9%), respectively. We consider that the difference between the detection rates observed in our study and those reported by the KCDC4) is due to the difference in sample size and mean age of patients between both studies. Moreover, the detection rate of rotavirus in our study was lower than that reported by the study conducted in China (27.4% vs. 54%), which is, we believe, due to the detection method, socioeconomic, and geographic differences between both studies.
Bon et al.22) detected rotavirus, calicivirus, astrovirus, and adenovirus 40 and 41 using an enzyme immunoassay and RT-PCR. They reported the average ages of patients with rotavirus, calicivirus, astrovirus, and adenovirus 40 and 41 infections as 11, 14.8, 34, and 15 months, respectively. Lee et al.6) studied rotavirus and norovirus infections in children with gastroenteritis, and reported that the age most commonly detecting rotavirus (111 cases, 61.0%) and norovirus (37 cases, 50.1%) was 7 months to 2 years old age group. In our study, the age group most commonly detecting rotavirus was 12 months to under 2 years of age, and the age group for norovirus and astrovirus was under one year of age.
Lee et al.6) studied for 2 years from 2005 to 2006 to compare norovirus to rotavirus gastroenteritis, and they reported that rotavirus was most commonly detected in May and norovirus in December. However, Hwang et al.8)'s study on viral gastroenteritis showed that the peak incidence of rotaviral and noroviral gastroenteritis in 2009 were during March and April. In our study, the incidence of rotavirus infection gradually increased from December to March 2011, and the peak incidence was reported in March. After April, the incidence of rotavirus infection dropped significantly, and only 1 case was reported in June 2011. Norovirus infections showed a relatively similar pattern to rotavirus infections, but the peak incidence was noticed in February, and no cases were detected after March. There were some limitations in comparing our study to others, because our study was done in short term duration and for the abruptly increased cases.
Coinfection of viral agents in children with acute gastroenteritis was reported by several studies23-25). Simpson et al.24) reported the incidence of viral agents in diarrhea stool of American children as 28% for rotavirus, 13% for norovirus, and 9% for coinfection. The rate of rotavirus coinfection with other viruses was 6.3% among children living in Spain, according to a study conducted by Sanchez-Fauquier et al.23). Koh et al.25) reported a rotavirus coinfection rate of 39.7% and a norovirus coinfection rate of 41.1%. In our study, rotavirus and norovirus coinfection was only reported in one case. This is similar to the results reported by Yan et al.10) who did not observe coinfection in any of the cases they studied; they detected norovirus, saporovirus, and astrovirus in 42, 16, and 4 cases out of 62 cases of virus-positive stool samples, respectively.
The tetra-valent rotaviral vaccine was developed in 1998 and was authorized for clinical use in USA. However, vaccination was later prohibited because intussusceptions were reported within a year from vaccination26). Recently, new rotaviral vaccines, such as human-bovine reassortant rotavirus vaccine and live attenuated vaccine, were developed and reintroduced for clinical use27,28). These new rotavirus vaccines have been clinically administered in our country since 2007. In our study, among 50 patients with rotavirus gastroenteritis, 4 patients have been previously vaccinated against rotavirus. This result does not imply that rotavirus vaccination fails to protect against rotavirus infections, since patients included in this study were limited to those hospitalized for acute diarrhea.
The clinical symptoms and signs such as fever, vomiting, and diarrhea are known to be severe in rotavirus infections29), and they result in dehydration, which subsequently leads to prolonged hospitalization durations17,30,31). Ahn et al.32) reported fever, vomiting, and diarrhea in 67.8%, 57.1%, and 98% of rotavirus infections, as well as 68.9%, 55.1%, and 89.6% of norovirus infections, respectively. The signs and symptoms of norovirus infection were relatively mild compared to rotavirus infection. Hwang et al.8) reported that mean duration of hospitalization was 3.9 and 4.2 days for rotavirus and norovirus infections, respectively and no significant differences were noticed in the frequency and duration of vomiting in these gastroenteritis. In our study, the difference in the mean duration of diarrhea between rotaviral (4.8 days) and noroviral (6.8 days) infections was statistically significant. On the other hand, the frequency (times/day) and duration (days) of diarrhea in children with rotavirus and norovirus gastroenteritis were 5.1±2.4 vs. 5.8±3.2 and 2.8±1.7 vs. 3.0±2.2, respectively, and the difference was not statistically significant. However, vomiting occurred frequently in rotavirus infection than in norovirus infection cases, but it was not statistically significant.
Hwang et al.8) also reported leukocytosis in 4 cases (18.2%) of norovirus infection and 4 cases (8.9%) of rotavirus infection. Abnormal liver function test results were also observed in 14 cases (63.6%) of norovirus infection and 40 cases (88.9%) of rotavirus infection. In our study, mean WBC count was higher in norovirus infections compared to rotavirus infection cases, while mean AST levels were considerably higher in rotavirus infections compare to norovirus infection cases; the differences between both groups were statistically significant.
In conclusion, the leading infectious cause was rotavirus and the second most common viral agent was norovirus in the abruptly increased cases of viral gastroenteritis in our study. These results were similar with the other studies of long term duration in acute viral gastroenteritis. The symptoms, signs, and laboratory findings were not specific in our study compare to other studies of acute viral gastroenteritis. Further studies will provide well understandings of outbreaks of viral gastroenteritis and usefulness of multiplex RT-PCR for detection of viruses in these cases.
Acknowledgments
This work was supported by Wonkwang University in 2011.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Cohen MB. Etiology and mechanisms of acute infectious diarrhea in infants in the United States. J Pediatr. 1991;118(4 Pt 2):S34–S39. doi: 10.1016/s0022-3476(05)81423-4. [DOI] [PubMed] [Google Scholar]
- 2.Feeney SA, Armstrong VJ, Mitchell SJ, Crawford L, McCaughey C, Coyle PV. Development and clinical validation of multiplex TaqMan assays for rapid diagnosis of viral gastroenteritis. J Med Virol. 2011;83:1650–1656. doi: 10.1002/jmv.22162. [DOI] [PubMed] [Google Scholar]
- 3.Higgins RR, Beniprashad M, Cardona M, Masney S, Low DE, Gubbay JB. Evaluation and verification of the Seeplex Diarrhea-V ACE assay for simultaneous detection of adenovirus, rotavirus, and norovirus genogroups I and II in clinical stool specimens. J Clin Microbiol. 2011;49:3154–3162. doi: 10.1128/JCM.00599-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korea Centers for Disease Control and Prevention. Acute infectious agents laboratory surveillance reports [Internet] Cheongwon: Korea Centers for Disease Control and Prevention; 2012. [cited 2012 May 17]. Available from: http://www.cdc.go.kr. [Google Scholar]
- 5.Park SE, Kim KH, Kim JH, Shin SH, Oh SH, Lee HJ, et al. Rotavirus vaccine. Korean J Pediatr. 2007;50:803–810. [Google Scholar]
- 6.Lee YJ, Jeong SN, Yoo JH, Cho HM, Yoo EJ, Kim EY, et al. Clinical spectrum of norovirus gastroenteritis compared to rotavirus gastroenteritis at a single center in Gwangju, Korea during 2005-2006; Compared to rotaviral gastroenteritis. Korean J Pediatr Infect Dis. 2009;16:61–72. [Google Scholar]
- 7.Suzuki H, Sakai T, Tanabe N, Okabe N. Peak rotavirus activity shifted from winter to early spring in Japan. Pediatr Infect Dis J. 2005;24:257–260. doi: 10.1097/01.inf.0000154327.00232.4d. [DOI] [PubMed] [Google Scholar]
- 8.Hwang PJ, Kwak JH, Lee TJ, Jeong SJ. Clinical features of acute noroviral gastroenteritis in children : comparison with rotaviral gastroenteritis. Korean J Pediatr. 2009;52:453–457. [Google Scholar]
- 9.Yan H, Yagyu F, Okitsu S, Nishio O, Ushijima H. Detection of norovirus (GI, GII), Sapovirus and astrovirus in fecal samples using reverse transcription single-round multiplex PCR. J Virol Methods. 2003;114:37–44. doi: 10.1016/j.jviromet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Yan H, Nguyen TA, Phan TG, Okitsu S, Li Y, Ushijima H. Development of RT-multiplex PCR assay for detection of adenovirus and group A and C rotaviruses in diarrheal fecal specimens from children in China. Kansenshogaku Zasshi. 2004;78:699–709. doi: 10.11150/kansenshogakuzasshi1970.78.699. [DOI] [PubMed] [Google Scholar]
- 11.Logan C, O'Leary JJ, O'Sullivan N. Real-time reverse transcription PCR detection of norovirus, sapovirus and astrovirus as causativeagents of acute viral gastroenteritis. J Virol Methods. 2007;146:36–44. doi: 10.1016/j.jviromet.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Tran A, Talmud D, Lejeune B, Jovenin N, Renois F, Payan C, et al. Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in northern France. J Clin Microbiol. 2010;48:1943–1946. doi: 10.1128/JCM.02181-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Maarseveen NM, Wessels E, de Brouwer CS, Vossen AC, Claas EC. Diagnosis of viral gastroenteritis by simultaneous detection of Adenovirus group F, Astrovirus, Rotavirus group A, Norovirus genogroups I and II, and Sapovirus in two internally controlled multiplex real-time PCR assays. J Clin Virol. 2010;49:205–210. doi: 10.1016/j.jcv.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Feeney SA, Mitchell SJ, Mitchell F, Wyatt DE, Fairley D, McCaughey C, et al. Association of the G4 rotavirus genotype with gastroenteritis in adults. J Med Virol. 2006;78:1119–1123. doi: 10.1002/jmv.20671. [DOI] [PubMed] [Google Scholar]
- 15.Gallimore CI, Taylor C, Gennery AR, Cant AJ, Galloway A, Xerry J, et al. Contamination of the hospital environment with gastroenteric viruses: comparison of two pediatric wards over a winter season. J Clin Microbiol. 2008;46:3112–3115. doi: 10.1128/JCM.00400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimoldi SG, Stefani F, Pagani C, Chenal LL, Zanchetta N, Di Bartolo I, et al. Epidemiological and clinical characteristics of pediatric gastroenteritis associated with new viral agents. Arch Virol. 2011;156:1583–1589. doi: 10.1007/s00705-011-1037-5. [DOI] [PubMed] [Google Scholar]
- 17.Colomba C, De Grazia S, Giammanco GM, Saporito L, Scarlata F, Titone L, et al. Viral gastroenteritis in children hospitalised in Sicily, Italy. Eur J Clin Microbiol Infect Dis. 2006;25:570–575. doi: 10.1007/s10096-006-0188-x. [DOI] [PubMed] [Google Scholar]
- 18.Marie-Cardine A, Gourlain K, Mouterde O, Castignolles N, Hellot MF, Mallet E, et al. Epidemiology of acute viral gastroenteritis in children hospitalized in Rouen, France. Clin Infect Dis. 2002;34:1170–1178. doi: 10.1086/339807. [DOI] [PubMed] [Google Scholar]
- 19.Oh DY, Gaedicke G, Schreier E. Viral agents of acute gastroenteritis in German children: prevalence and molecular diversity. J Med Virol. 2003;71:82–93. doi: 10.1002/jmv.10449. [DOI] [PubMed] [Google Scholar]
- 20.Cunliffe NA, Booth JA, Elliot C, Lowe SJ, Sopwith W, Kitchin N, et al. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg Infect Dis. 2010;16:55–62. doi: 10.3201/eid1601.090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y, Cheng WX, Yang XM, Jin M, Zhang Q, Xu ZQ, et al. Viral agents associated with acute gastroenteritis in children hospitalized with diarrhea in Lanzhou, China. J Clin Virol. 2009;44:238–241. doi: 10.1016/j.jcv.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Bon F, Fascia P, Dauvergne M, Tenenbaum D, Planson H, Petion AM, et al. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J Clin Microbiol. 1999;37:3055–3058. doi: 10.1128/jcm.37.9.3055-3058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Fauquier A, Montero V, Colomina J, Gonzalez-Galan V, Aznar J, Aisa ML, et al. Global study of viral diarrhea in hospitalized children in Spain: results of structural surveillance of viral gastroenteritis network (VIGESS-net) 2006-2008. J Clin Virol. 2011;52:353–358. doi: 10.1016/j.jcv.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Simpson R, Aliyu S, Iturriza-Gomara M, Desselberger U, Gray J. Infantile viral gastroenteritis: on the way to closing the diagnostic gap. J Med Virol. 2003;70:258–262. doi: 10.1002/jmv.10386. [DOI] [PubMed] [Google Scholar]
- 25.Koh H, Baek SY, Shin JI, Chung KS, Jee YM. Coinfection of viral agents in Korean children with acute watery diarrhea. J Korean Med Sci. 2008;23:937–940. doi: 10.3346/jkms.2008.23.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim JO. Differential diagnosis of acute diarrheal disorders in children. J Korean Med Assoc. 2012;55:516–524. [Google Scholar]
- 27.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Palacios GM, Perez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 29.Ma SH. Acute infectious diarrhea in pediatirc patients. Korean J Pediatr. 2005;48:235–250. [Google Scholar]
- 30.Albano F, Bruzzese E, Bella A, Cascio A, Titone L, Arista S, et al. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr. 2007;166:241–247. doi: 10.1007/s00431-006-0237-6. [DOI] [PubMed] [Google Scholar]
- 31.Nakagomi T, Nakagomi O, Takahashi Y, Enoki M, Suzuki T, Kilgore PE. Incidence and burden of rotavirus gastroenteritis in Japan, as estimated from a prospective sentinel hospital study. J Infect Dis. 2005;192(Suppl 1):S106–S110. doi: 10.1086/431503. [DOI] [PubMed] [Google Scholar]
- 32.Ahn SH, Lim HC, Kim HM, Uh Y, Seok SW. Comparing the cause and symptom severity of children with acute gastroenteritis. Korean J Pediatr Infect Dis. 2008;15:138–145. [Google Scholar]