Abstract
Purpose
This single-center study was conducted to assess the changes in epidemiological and clinical characteristics and outcomes of patients with Kawasaki disease (KD) over the past 7 years.
Methods
This retrospective study included 135 children with KD, admitted to Chungnam National University Hospital, Daejeon, between 2004 and 2005 (group A, n=53) and between 2011 and 2012 (group B, n=82). Medical records were reviewed to obtain information regarding the presenting signs and symptoms, demographic characteristics, and laboratory and echocardiographic findings associated with KD.
Results
The hospital admission date after onset was significantly earlier in group B than in group A (P=0.008). The proportion of patients with incomplete KD was 45.3% and 65.9% in group A and B, respectively (P=0.018). The number of pretreatment coronary artery lesions (CALs) were significantly lesser in group B than in group A. (10/53 vs. 5/82, P=0.021). No significant differences was observed in the incidence of CALs at discharge, febrile phase duration, hospital stay duration, incidence of retreatment, and intravenous immunoglobulin dose between 2 groups. The total febrile phase was shorter in patients with incomplete KD than in those with complete KD in both groups.
Conclusion
The proportion of incomplete KD has become higher. Furthermore, early admission and management of patients with KD may be related to increased incomplete KD and decreased CALs. Therefore, we believe that a diagnostic strategy for incomplete KD should be established regardless of the presence of coronary lesions.
Keywords: Kawasaki disease, Incidence, Epidemiology
Introduction
Since Kawasaki disease (KD) was first reported as "mucocutaneous lymph node syndrome" in 19671), its etiology and pathophysiology have remained unknown and no specific diagnostic laboratory markers have been developed, despite intensive efforts to establish KD-specific markers. Currently, in order to make a diagnosis of KD, pediatricians must inevitably rely on the diagnostic criteria established by the Japanese Kawasaki Disease Research Committee or the American Heart Association. Clinicians sometimes encounter febrile children who do not fulfill the diagnostic criteria for incomplete KD but have several findings compatible with KD.
The average annual incidence of KD during 2009-2010 was 222.9 per 100,000 children under 5 years of age in Japan2). The average annual KD incidence during 2009-2011was 127.7 per 100,000 children under the age of 5 in Korea3), and it is the second highest incidence of KD in the world. The incidence of KD has increased in Korean and Japan since 2000.
A Japanese nationwide survey of KD from 2007 to 2008 indicated that the percentage of patients with an incomplete form of KD was as high as 15-20%4). In a recent Korean nationwide survey from 2009 to 2011, the percentage of patients with incomplete KD was about 42%3). This recent epidemiologic data suggests that the incidence of KD and the proportion of its incomplete form tend to increase annually. Incomplete KD has previously been shown to be associated with delayed diagnoses and treatments, which has also been reported to be a risk factor for the development of coronary artery lesions (CALs)5).
Sudo et al.6) suggested that the higher prevalence of CALs in patients with incomplete presentation was primarily a selection bias due to the use of echocardiography for its diagnostic process. Therefore, incomplete KD without CALs appeared to be missed. Another reason for increased prevalence of incomplete KD was delayed diagnosis and late administration of intravenous immunoglobulin (IVIG). Furthermore, there may have been some children with KD who had less than 4 principal symptoms in early stage. Therefore, restricting a diagnosis of incomplete KD to children with echocardiographic CALs may result in diagnostic errors. Manlhiot et al.7) reported the same incidence of CALs in patients with complete KD and in those with incomplete KD using their definition of incomplete presentation of KD in which the use of echocardiography has excluded.
The aim of this study was to determine the changes of epidemiological and clinical characteristics and treatment outcomes in patients presenting with KD over the past 7 years in our institution.
Materials and methods
1. Patients
A retrospective study was conducted on 135 KD children who were admitted to Chungnam National University Hospital in Daejeon, Korea. We reviewed patients' medical records between June 2004 and May 2005 (group A, n=53) and between June 2011 and May 2012 (group B, n=82).
The diagnosis of KD was based on clinical features established by the Japanese Kawasaki Disease Research Committee8).Complete KD is defined as having at least 5 of the 6 principal clinical signs that the Japanese Kawasaki disease Research Committee has validated: 1) fever persisting for 5 days or more; 2) bilateral conjunctival congestion; 3) changes in the lips and oral cavity, such as reddening of the lips, strawberry tongue, and diffuse injection of oral and pharyngeal mucosa; 4) polymorphous exanthema; 5) changes in the peripheral extremities, such as reddening of the palms and soles, indurative edema in initial stages, and membranous desquamation from the fingertips in convalescent stages; and 6) acute nonpurulent cervical lymphadenopathy. The Japanese diagnostic guidelines state that patients with 4 of the principal clinical signs can be diagnosed as having KD when CALs is recognized by 2-dimensional echocardiography. In this study, incomplete KD was defined as having 4 or fewer principal signs, with or without cardiac lesions once other diseases with similar findings had been excluded. To differentiate KD from other diseases that mimic KD, we checked blood culture, mycoplasma antibody, respiratory virus polymerase chain reaction, Epstein-barr virus and ASO (antistreptolysin O) if needed. We used the same guidelines during both 2004-2005 and 2011-2012 studies. Patients who had the remote history of KD were excluded.
2. Methods
Each patient's medical records were reviewed for presenting signs and symptoms, the duration of fever and demographic characteristics associated with KD. Patients with complete KD were treated with IVIG, 2 g/kg in a single infusion over 10-12 hours along with aspirin (30-50 mg/kg). Once fever was controlled for at least 48 hours, the aspirin dose was reduced to 3-5 mg/kg per day. If patients with incomplete KD had CALs, or showed increased white blood cell (WBC) counts (>10,000/mm3), neutrophil counts (>50%) and C-reactive protein (CRP) (>2 mg/dL), they were treated with IVIG. In early 2004, the American Heart Association (AHA) guideline was not established for treatment of incomplete KD. Park et al.9) reported that WBC counts, neutrophil counts and CRP levels increased more in patients with CALs than in those without CALs after IVIG treatment. So, these results were used as the treatment guideline in our study period. Aspirin was only administered to patients who had a duration of fever <5 days and less than 4 clinical signs when the fever subsided on the first and second days after admission. Patients with a fever of 38℃ or higher and without another possible source of fever 36 hours after completion of IVIG treatment were retreated with 1 g/kg of IVIG and continued to receive aspirin therapy.
Laboratory data was analyzed prior to IVIG treatment and the data included a complete blood count, erythrocyte sedimentation rate (ESR), and the level of albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum sodium, and CRP. We utilized the Harada10), Kobayashi et al.11), and Egami et al.12) risk scoring system to predict coronary arterial abnormalities and IVIG unresponsiveness.
Echocardiographic examination was performed prior to IVIG treatment, at discharge and 2 months after treatment using Aspen (ACUSON, Burnsville, MN, USA) and Vivid 7 (GE Vingmed, Horten, Norway). The dimensions of the left main coronary artery (CA), proximal left anterior CA, and right CA were adjusted for body surface area and expressed in standard deviation units (z scores)13). Patients were thought to have CALs if any CA segment had a z score of >2. "Dilatation" was defined as the enlargement of the internal lumen diameter of the CA segment by less than 1.5 times of the upper normal limit. CA aneurysm was considered "present" if the maximum internal lumen diameter was enlarged 1.5 times or more.
3. Statistical analysis
All statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation. Independent t test was used for comparison when the data was normally distributed, while the Mann-Whitney U test was used for nonparametric data. Fisher exact test and the chi-square test were used to compare individual proportions. A P value of less than 0.05 was considered statistically significant.
Results
1. Clinical characteristics before IVIG treatment
A total of 135 KD patients (group A, n=53; group B, n=82) were included in the study. The mean ages of the patients were 33.66±23.64 months in group A and 29.56±21.36 months in group B. The admission date after the onset of symptoms was significantly earlier in group B than in group A (group A, 6.00±2.18 days vs. group B, 5.00±2.04 days; P=0.008). The durations of fever prior to treatment were 6.43±2.3 days in group A and 5.66±2.02 days in group B. There were no significant differences between the 2 groups in age, gender, and the duration of fever. The incidences of incomplete KD were 45.3% (24/53) in group A and 65.9% (54/82) in group B, and the difference was statistically significant (P=0.018) (Table 1). The prevalence of CALs at admission in group A was higher than in group B (group A, 18.9% [10/53] vs. group B 6.1% [5/82]; P=0.021). The Harada10), Kobayashi et al.11), and Egami et al.12) scores were similar between two groups (Table 1).
Table 1.
Clinical characteristics before IVIG treatment
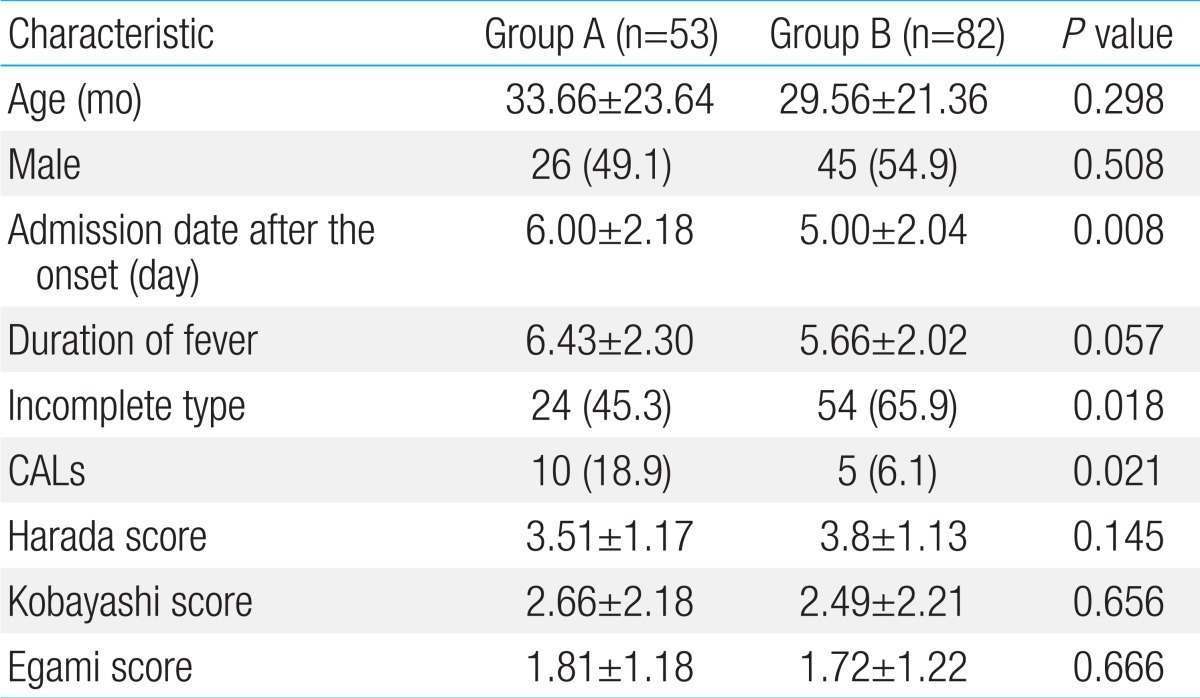
Values are presented as mean±standard deviation or number (%).
IVIG, intravenous immunoglobulin; CALs, coronary arterial lesions.
2. Laboratory findings before IVIG treatment
There were no significant differences in counts of WBC, hemoglobin, hematocrit, platelet count, ESR, and CRP between the 2 groups. Biochemical data showed no significant differences in the level of AST, ALT, serum sodium, and albumin (Table 2).
Table 2.
Laboratory findings before IVIG treatment
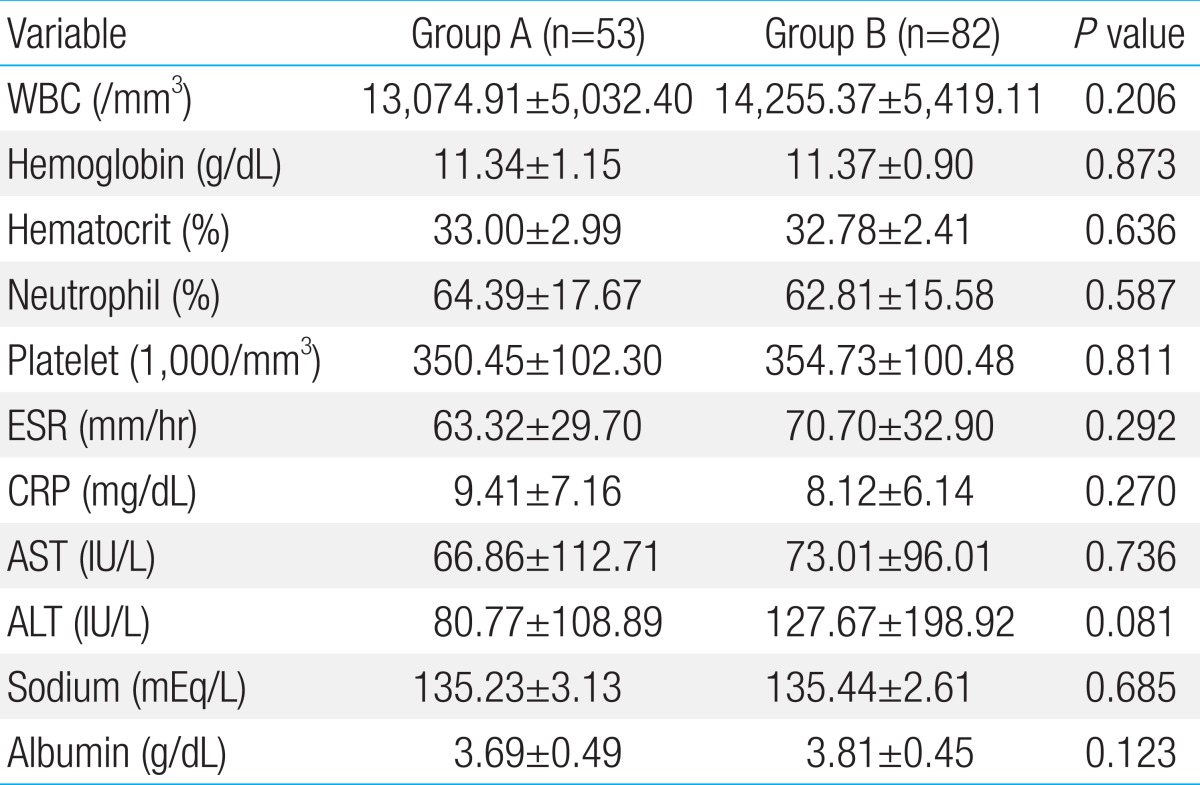
Values are presented as mean±standard deviation
IVIG, intravenous immunoglobulin; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
3. Clinical and cardiovascular outcomes
When the patients were classified according to the presence of CALs, the patients with CALs were admitted to the hospital significantly later than those without CALs (patients without CALs vs. those with CALs, 5.56±1.97 days vs. 7.90±2.13 days in group A, P=0.003; 4.84±1.91 days vs. 7.40±2.70 days in group B, P=0.026; table not shown).
In both groups, patients with incomplete KD had higher proportion of CALs at admission than those with complete KD. However, this was not statistically significant (incomplete vs. complete, 20.8% [5/24] vs. 17.2% [5/29] in group A; 7.4% [4/54] vs. 3.6% [1/28] in group B; table not shown).
At discharge and 2 months after treatment, there were no significant differences in the incidence of CALs between the 2 groups (Table 3). We compared the frequency of CALs and mitral regurgitation between patients with incomplete KD in group A to those in group B, but the difference was not statistically significant (data not shown). Furthermore, there were no significant differences in the total duration of fever, hospital stay, the incidence of retreatment, and the total dose of IVIG between the 2 groups (Table 4).
Table 3.
Cardiovascular sequelae on echocardiographic examination
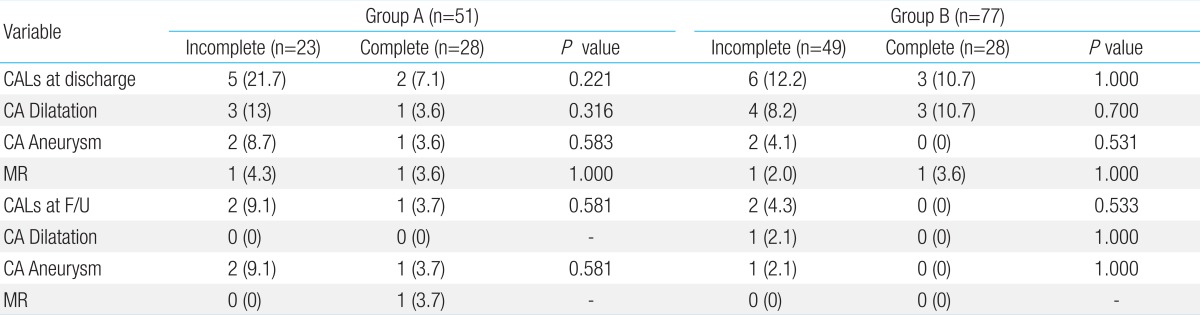
Values are presented as number (%).
CALs, coronary artery lesions; CA, coronary artery; MR, mitral regurgitation, mild to severe; F/U, follow-up at 2 months after treatment.
Table 4.
Clinical outcomes after treatment
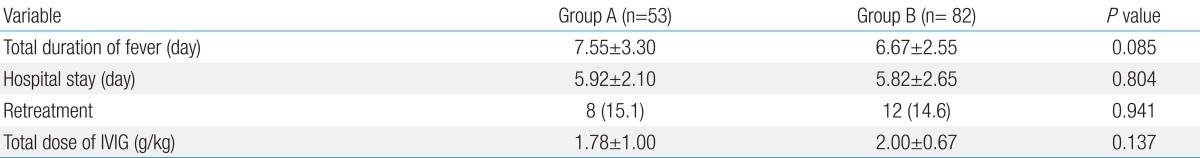
Values are presented as mean±standard deviation or number (%).
IVIG, intravenous immunoglobulin.
4. Demographic and clinical characteristics in patient with KD by clinical presentation
The characteristics and clinical outcomes of KD patients were compared according to the clinical forms of KD. In both groups, incomplete KD occurred at younger age than complete KD (incomplete vs. complete, 23.63±16.93 months vs. 42.03±25.37 months in group A; 26.63±21.74 months vs. 35.21±19.76 months in group B) (Table 5). In both groups, the total duration of fever was shorter in patients with incomplete KD than in those with complete KD (P=0.008 in group A, P=0.006 in group B).
Table 5.
Demographic and clinical characteristics of patients with Kawasaki disease according to the clinical presentation
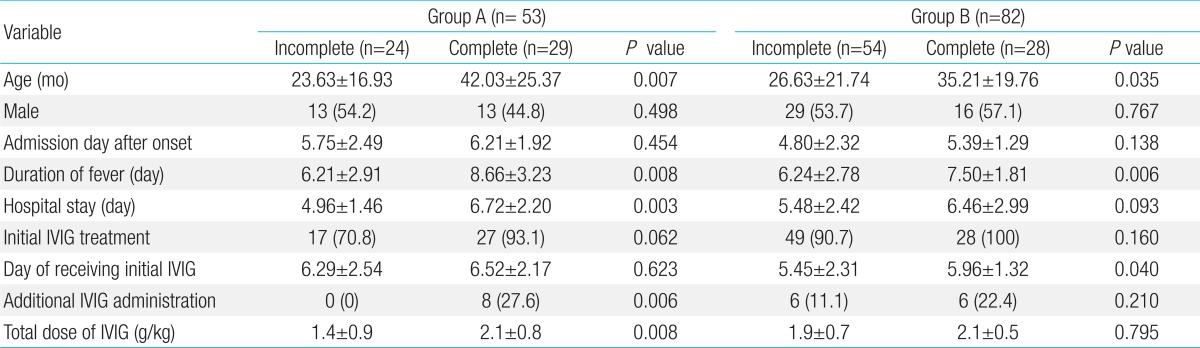
Values are presented as mean±standard deviation or number (%).
IVIG, intravenous immunoglobulin.
In both groups, the proportion of patients who received initial IVIG was higher in patients with complete KD than in those with incomplete KD, but the difference was not statistically significant (incomplete vs. complete, 70.8% [17/24] vs. 93.1% [27/29] in group A; 90.7% [49/54] vs. 100% [28/28] in group B) (Table 5). In group B, the date of initial IVIG was significantly earlier in patients with incomplete KD than in those with complete KD (5.45±2.31 days in incomplete cases vs. 5.96±1.32 days incomplete cases, P=0.040) (Table 5). In group A, the proportion of patients treated with additional IVIG was 27.6% (8/29) in patients with complete KD, which was higher than in those with incomplete KD (0% [0/24], P=0.006) (Table 5). Furthermore, in group A, the total dose of IVIG was significantly higher in patients with complete KD than in those with incomplete KD (P=0.008).
We also compared incomplete KD of two groups on the same set of items in Table 5, but the differences were not statistically significant except the proportion of initial IVIG treatment and total dose of IVIG (data not shown). The proportion of initial IVIG treatment in incomplete KD of group B was significantly larger than that of group A (group A vs. group B, 70.8% [17/24] vs. 90.7% [49/54]; P=0.025). The total dose of IVIG was significantly higher in incomplete KD of group B than in that of group A (group A vs. group B, 1.4±0.9 g/kg vs. 1.9±0.7g/kg; P=0.019). When comparing complete KD patients of group A with those of group B, there was no statistically different on all items in Table 5.
Discussion
This study was conducted to determine changes in epidemiological and clinical characteristics and treatment outcomes in patients presenting with KD over the past 7 years in our institution.
KD is an acute, self-limited vasculitis of unknown etiology that occurs predominantly in infants and young children. It is a major cause of pediatric acquired heart disease in Asia, including Korea. According to epidemiologic studies of KD in Korea from 2004 to 2011, the incidence of KD among children aged <5 years was 106 in 100,000 in 2004 and 134 in 100,000 in 20113). Approximately 15% to 25% of children with untreated KD develop coronary aneurysms or ectasia, which may lead to myocardial infarction, sudden death or ischemic heart disease1). Patients with delayed diagnoses of KD were at significantly high risk of developing CALs. Therefore, it is necessary to develop a strategy for KD diagnosis and educate health care providers and parents on early recognition of KD.
In 2004, the AHA suggested a new algorithmic approach for the evaluation of children with suspected KD who did not meet preexisting clinical diagnostic guidelines1). Increased detection of incomplete KD has been reported in a prospective study using the AHA guidelines14,15), but the validity of such diagnostic criteria for incomplete KD should be tested. In 1994, Fukushige et al.16) reported that 10% of the 242 KD Japanese patients were diagnosed with incomplete KD. The incidence of incomplete KD appears to be greater in infants younger than six months of age17,18). According to the 2007-2008 nationwide survey of KD patients in Japan, 20% of the KD patients had incomplete KD6). In a recent Korean nationwide survey of KD from 2009 to 2011, the incidence of incomplete KD was 42%3). Our study found that the proportions of incomplete KD were 45.3% in 2004-2005 and 65.9% in 2011-2012. When patients were also classified as having incomplete or complete KD according to 2004 AHA diagnostic criteria (data were not shown), the proportion of incomplete KD was 41.5% (22/53) in group A and 62.2% (51/82) in group B, and the difference was statistically significant (P=0.019). According to AHA guideline, a diagnosis of KD can be made on day 4 of illness in the presence of more than 4 principal criteria1). In this situation, the proportion of incomplete KD was 34% (18/53) in group A and 45.1% (37/82) in group B (P=0.198). These classifications were not considered CALs, and therefore the proportion of incomplete KD might be overestimated. These proportions of incomplete KD are significantly higher than those of previous studies, hence the indicative of increased proportions of incomplete KD over the last 7 years. Possible reason for this is the use of laboratory values as supplemental criteria as well as environmental factors. Another reason may be related to increased levels of awareness of KD among physicians and parents. When comparing the characteristics of patients in 2004-2005 and 2011-2012, the admission date after onset was significantly earlier in 2011-2012 than in 2004-2005. Moreover, the incidence of CALs was lower in the recent period than in the past. In both periods, the patients with CALs were hospitalized significantly later than in those without CALs. The previous reports have suggested that delayed diagnosis and treatment are the risk factors of CALs.
There are some risk scoring systems for predicting IVIG resistance, which are derived from the data observed in Japanese populations10-12,19). We used 3 Japanese risk scoring systems to predict risks of IVIG resistance and compare the risks between two groups; however, there were no significant differences. This result allowed us to conclude that demographic and laboratory findings may be similar between the 2 groups.
Numerous studies have shown that 13% to 21% of all KD patients have IVIG resistance11,12,19,20). In our study, the proportions of additional IVIG administration were 15.1% in 2004-2005 and 14.6% in 2011-2012. This is similar to the results of previous studies. In the Korean national surveys of KD, the incidences of CALs were 25.2% in 1991-199321),18.8% in 2003-200522) and 16.4% in 2009-20113). Our study showed that the incidences of CALs at an acute phase (at discharge) were 13.7% and 11.7% in 2004-2005 and 2011-2012, respectively, and these findings are similar to the results of previous Korean national surveys.
Sudo et al.6) have demonstrated that the higher incidence of CALs in patients with incomplete KD is attributed to the diagnostic process and delays in treatment due to difficulty in making a diagnosis. In contrast to the results of previous studies, our study demonstrated that the incidence of CALs was not higher in incomplete KD than in those with complete KD over the last 7 years. There are at least 2 possible reasons for this discrepancy. First, we did not include CALs as a criterion for the diagnosis of incomplete KD, hence possible prompt diagnosis. Second, incomplete KD patients received IVIG treatment at the same time or earlier than complete KD patients over the past 7 years. Based on the results of our study, incomplete KD patients had lower incidence of CALs in 2011-2012 than in 2004-2005. Moreover, the proportion of patients who received initial IVIG was higher in 2011-2012 (91%) than in 2004-2005 (71%).
Some previous studies have reported that the incidence of incomplete KD is higher in younger patients6,23,24), while others have reported that there are no age-related incidence differences7,25). In our study, we found that patients with incomplete KD were younger than those with complete KD.
This study has some limitations. First, this is a retrospective study, not a continuous longitudinal study. We analyzed clinical symptoms with the patients' medical records only, and not all case reports had the necessary details about clinical symptoms. Second, the number of subjects is relatively small. Therefore, this may not be an accurate representative of recent Korean national surveys. Lastly, there is the possibility of overdiagnosis due to the application of the KD diagnostic criteria regardless of the presence of coronary lesions.
The number of patients with incomplete KD has increased without any changes in the incidence of CALs over the last 7 years at our hospital. Although the present study was conducted in a single institution, it is assumed that this trend also exists in Korea. The current definition of incomplete KD in which coronary arterial abnormalities are included as a necessary condition, may be restrictive. The results of this study suggest that a diagnostic strategy for incomplete KD should be established regardless of the presence of coronary lesions.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 2.Abstracts of the 10th International Kawasaki Disease Symposium. February 7-10, 2012. Kyoto, Japan. Pediatr Int. 2012;54(Suppl 1):38–142. doi: 10.1111/j.1442-200X.2012.03534.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim GB, Han JW, Hong YM. Epidemiological features of Kawasaki disease in Korea, 2009-2011; The 56th Annual Scientific Meeting of the Korean Society of Cardiology; 2012 Nov 16-17; Daejeon, Korea. [Google Scholar]
- 4.Nakamura Y, Yashiro M, Uehara R, Sadakane A, Chihara I, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2007-2008 nationwide survey. J Epidemiol. 2010;20:302–307. doi: 10.2188/jea.JE20090180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juan CC, Hwang B, Lee PC, Lin YJ, Chien JC, Lee HY, et al. The clinical manifestations and risk factors of a delayed diagnosis of Kawasaki disease. J Chin Med Assoc. 2007;70:374–379. doi: 10.1016/S1726-4901(08)70023-6. [DOI] [PubMed] [Google Scholar]
- 6.Sudo D, Monobe Y, Yashiro M, Mieno MN, Uehara R, Tsuchiya K, et al. Coronary artery lesions of incomplete Kawasaki disease: a nationwide survey in Japan. Eur J Pediatr. 2012;171:651–656. doi: 10.1007/s00431-011-1630-3. [DOI] [PubMed] [Google Scholar]
- 7.Manlhiot C, Christie E, McCrindle BW, Rosenberg H, Chahal N, Yeung RS. Complete and incomplete Kawasaki disease: two sides of the same coin. Eur J Pediatr. 2012;171:657–662. doi: 10.1007/s00431-011-1631-2. [DOI] [PubMed] [Google Scholar]
- 8.Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition) Pediatr Int. 2005;47:232–234. doi: 10.1111/j.1442-200x.2005.02033.x. [DOI] [PubMed] [Google Scholar]
- 9.Park MY, Lee KY, Han JW, Lee HS, Hong JH, Whang KT. Laboratory values in patients with Kawasaki disease after intravenous immunoglobulin: comparison of patients with coronary artery lesions to those without coronary artery lesions. J Korean Pediatr Soc. 2003;46:162–166. [Google Scholar]
- 10.Harada K. Intravenous gamma-globulin treatment in Kawasaki disease. Acta Paediatr Jpn. 1991;33:805–810. doi: 10.1111/j.1442-200x.1991.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 12.Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149:237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 13.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–258. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 14.Heuclin T, Dubos F, Hue V, Godart F, Francart C, Vincent P, et al. Increased detection rate of Kawasaki disease using new diagnostic algorithm, including early use of echocardiography. J Pediatr. 2009;155:695–699.e1. doi: 10.1016/j.jpeds.2009.04.058. [DOI] [PubMed] [Google Scholar]
- 15.Ghelani SJ, Sable C, Wiedermann BL, Spurney CF. Increased incidence of incomplete Kawasaki disease at a pediatric hospital after publication of the 2004 American Heart Association guidelines. Pediatr Cardiol. 2012;33:1097–1103. doi: 10.1007/s00246-012-0232-9. [DOI] [PubMed] [Google Scholar]
- 16.Fukushige J, Takahashi N, Ueda Y, Ueda K. Incidence and clinical features of incomplete Kawasaki disease. Acta Paediatr. 1994;83:1057–1060. doi: 10.1111/j.1651-2227.1994.tb12985.x. [DOI] [PubMed] [Google Scholar]
- 17.Joffe A, Kabani A, Jadavji T. Atypical and complicated Kawasaki disease in infants. Do we need criteria? West J Med. 1995;162:322–327. [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld EA, Corydon KE, Shulman ST. Kawasaki disease in infants less than one year of age. J Pediatr. 1995;126:524–529. doi: 10.1016/s0022-3476(95)70344-6. [DOI] [PubMed] [Google Scholar]
- 19.Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166:131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 20.Son MB, Gauvreau K, Ma L, Baker AL, Sundel RP, Fulton DR, et al. Treatment of Kawasaki disease: analysis of 27 US pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8. doi: 10.1542/peds.2008-0730. [DOI] [PubMed] [Google Scholar]
- 21.Park YW, Park IS, Kim CH, Ma JS, Lee SB, Kim CH, et al. Epidemiologic study of Kawasaki disease in Korea, 1997-1999: comparison with previous studies during 1991-1996. J Korean Med Sci. 2002;17:453–456. doi: 10.3346/jkms.2002.17.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park YW, Han JW, Park IS, Kim CH, Cha SH, Ma JS, et al. Kawasaki disease in Korea, 2003-2005. Pediatr Infect Dis J. 2007;26:821–823. doi: 10.1097/INF.0b013e318124aa1a. [DOI] [PubMed] [Google Scholar]
- 23.Chang FY, Hwang B, Chen SJ, Lee PC, Meng CC, Lu JH. Characteristics of Kawasaki disease in infants younger than six months of age. Pediatr Infect Dis J. 2006;25:241–244. doi: 10.1097/01.inf.0000202067.50975.90. [DOI] [PubMed] [Google Scholar]
- 24.Tseng CF, Fu YC, Fu LS, Betau H, Chi CS. Clinical spectrum of Kawasaki disease in infants. Zhonghua Yi Xue Za Zhi (Taipei) 2001;64:168–173. [PubMed] [Google Scholar]
- 25.Perrin L, Letierce A, Guitton C, Tran TA, Lambert V, Kone-Paut I. Comparative study of complete versus incomplete Kawasaki disease in 59 pediatric patients. Joint Bone Spine. 2009;76:481–485. doi: 10.1016/j.jbspin.2008.11.015. [DOI] [PubMed] [Google Scholar]


