Abstract
Aging is associated with a variety of pathophysiological changes, including development of insulin resistance, progressive decline in β-cell function, and chronic inflammation, all of which affect metabolic homeostasis in response to nutritional and environmental stimuli. SIRT1, the mammalian nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylase, and nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting NAD biosynthetic enzyme, together comprise a novel systemic regulatory network, named the “NAD World,” that orchestrates physiological responses to internal and external perturbations and maintains the robustness of the physiological system in mammals. In the past decade, an accumulating body of evidence has demonstrated that SIRT1 and NAMPT, two essential components in the NAD World, play a critical role in regulating insulin sensitivity and insulin secretion throughout the body. In this review article, we will summarize the physiological significance of SIRT1 and NAMPT-mediated NAD biosynthesis in metabolic regulation and discuss the ideas of functional hierarchy and frailty in determining the robustness of the system. We will also discuss the potential of key NAD intermediates as effective nutriceuticals for the prevention and the treatment of age-associated metabolic complications, such as type 2 diabetes.
Introduction
Aging is one of the most serious risk factors for many metabolic complications, including obesity, atherosclerosis, and type 2 diabetes. For example, among US residents aged 65 years and older, 10.9 million or 26.9% of all people in this age group suffered diabetes in 2010, based on the 2011 National Diabetes Fact Sheet from the Center for Disease Control and Prevention. Indeed, it has been well known that insulin resistance develops over time [1]. It has also been shown that β-cell function declines progressively during aging [2], contributing to the pathogenesis of type 2 diabetes. Another important factor of aging that affects metabolic homeostasis is chronic inflammation. It has been known that levels of inflammatory cytokines and markers, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP), elevate with age in healthy old individuals [3]. The elevation of these inflammatory cytokines and markers is tightly associated with the development of insulin resistance and β-cell dysfunction [4,5]. Therefore, one would speculate that factors that contribute to the regulation of systemic metabolic robustness and anti-inflammatory responses could play a crucial role in the pathogenesis of these age-associated metabolic complications, such as atherosclerosis and type 2 diabetes.
One such factor is the mammalian nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylase SIRT1, one of the seven family members of mammalian sirtuins [6,7]. In the past decade, an accumulating body of evidence suggests that SIRT1 plays an important role in the regulation of glucose and lipid metabolism, providing a hope that SIRT1 will be a promising therapeutic target for age-associated metabolic complications, particularly type 2 diabetes [8]. Because SIRT1 requires NAD for its enzymatic activity, understanding the regulation of mammalian NAD biosynthesis has also become a critical issue in the field of metabolism and aging research. Particularly, nicotinamide phosphoribosyltransferase (NAMPT), a key NAD biosynthetic enzyme in mammals, has recently become a focus of intensive investigation [9]. In this review, we will focus on the importance of a systemic regulatory network, named the “NAD World,” mediated by these two major players, SIRT1 and NAMPT. We will also discuss the translational aspect of the studies on SIRT1 and NAMPT for the treatment and prevention of type 2 diabetes.
SIRT1, a key mediator that regulates metabolic responses to nutritional input
The biology of SIR2 (silent information regulator 2) family proteins, called “sirtuins,” has been evolving dramatically in the past decade since the discovery of their unique NAD-dependent deacetylase activity [8]. Through these tremendous amounts of studies on sirtuins, it has become clear that sirtuins function to maintain and/or enhance the robustness of the physiological system and secure the survival of individuals when organisms are exposed to internal and external perturbations [6,7]. Among these perturbations, energy limitation, such as fasting and diet restriction, is one of the most important ones that are known to increase sirtuin dosages and/or enhance their activities. Sirtuins also respond to other types of stresses and damages, such as oxidative stresses and DNA damages. In this regard, it is likely that sirtuins have evolved primarily to prevent the physiological system from any destruction due to a wide variety of environmental perturbations, such as a famine. This particular evolutional trait of sirtuins might have likely put themselves to a universal position of mediating anti-aging effects in many different organisms.
Indeed, in yeast, worms, and flies, sirtuins have been shown to play an important role in the regulation of aging and longevity [10-12]. However, some of those results, particularly the effects of SIR2 orthologs, sir-2.1 and dSir2, on longevity in worms and flies, respectively, have been called into a question [13], generating a serious controversy regarding the importance of sirtuins for aging and longevity. Although this controversy still remains in the field, recent studies in yeast, flies, and mice have provided further supportive evidence for the importance of sirtuins as key regulators of aging and longevity [14-16]. In mammals, it has been firmly established that sirtuins are critical metabolic mediators in multiple tissues [6,7]. In particular, SIRT1, the mammalian SIR2 ortholog, regulates a variety of metabolic responses to changes in nutritional input in multiple tissues, including the liver, skeletal muscle, adipose tissue, pancreatic islets, and the brain (Figure 1). SIRT1 also plays a critical role in the regulation of phenotypes induced by diet restriction (DR), a well-known regimen that delays aging and extends life span in a wide variety of organisms. Whereas the whole-body and brain-specific Sirt1 knockout mice fail to respond to DR [17-19], Sirt1-overexpresing transgenic mice display phenotypes similar to DR mice [20] or prevention against metabolic complications caused by high-fat diet (HFD) and aging [21,22]. However, whole-body Sirt1-overexpressing mice have been reported to fail to show life span extension, although they show a lower incidence of spontaneous carcinomas and sarcomas and a reduced susceptibility to HFD/carcinogen-induced liver tumors [23]. Therefore, whether and how sirtuins, SIRT1 in particular, regulate aging and longevity in mammals still remains a critical question, and intensive investigation is currently in progress in the field of sirtuin biology to address this long-standing question.
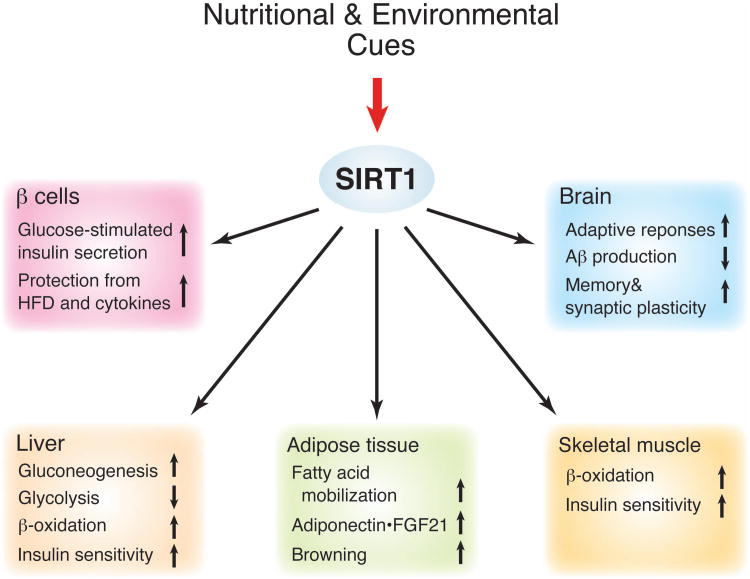
Figure 1. The function of SIRT1 as a key mediator that coordinates metabolic responses to nutritional and environmental cues and maintains physiological robustness in mammals.
Major roles of SIRT1 in the liver, adipose tissue, skeletal muscle, pancreatic β cells, and the brain are summarized in this scheme. Through these functions illustrated here, SIRT1 regulates the balance between insulin sensitivity and insulin secretion throughout the body and provides protection against nutritional and environmental perturbations, such as high-fat diet (HFD) and chronic inflammation. More details are given in the text.
Regulation of insulin sensitivity by SIRT1
Nonetheless, a number of genetic studies have so far strongly suggested that SIRT1 is important to maintain both insulin sensitivity and insulin secretion throughout the body. For example, in the liver, hepatic deletion of SIRT1 impairs peroxisome proliferator-activated receptor (PPAR) α function and decreases fatty acid β-oxidation, causing hepatic steatosis, inflammation and endoplasmic reticulum stress when exposed to a HFD [24]. Another study has demonstrated that hepatic Sirt1 deficiency impairs the mammalian target of rapamycin complex 2 (mTORC2)/AKT signaling pathway, causing chronic hyperglycemia, oxidative stress, and systemic insulin resistance on a regular diet [25]. In adipose tissue, adipose tissue-specific Sirt1 deficiency causes increased adiposity and leads to insulin resistance under a HFD and during aging [26]. Interestingly, Sirt1 deficiency in adipose tissue causes changes in gene expression that largely overlap with those caused by a HFD [26]. Most recently, it has been shown that SIRT1 promotes “browning” of white adipose tissue by deacetylating PPARγ and recruiting Prdm16, a key coactivator for the development and function of brown adipose tissue, to PPARγ, potentially contributing to the improvement of insulin sensitivity [27]. In skeletal muscle, DR increases SIRT1 activity and enhances insulin-stimulated phosphoinositide 3-kinase (PI3K) signaling and glucose uptake through SIRT1-mediated STAT3 deacetylation [28]. These adaptive responses in skeletal muscle are completely abrogated by skeletal muscle-specific Sirt1 deletion. These findings clearly demonstrate that SIRT1 plays a critical role in maintaining and improving insulin sensitivity in response to nutritional perturbations in major insulin sensitive tissues.
Regulation of insulin secretion by SIRT1
On the other hand, SIRT1 has also been demonstrated to positively regulate glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells. Our group has previously demonstrated that an increased dosage of SIRT1 in β-cells significantly enhances GSIS and improves glucose tolerance in pancreatic β-cell specific SIRT1-overexpressing (BESTO) transgenic mice [29]. Given that DR enhances postprandial insulin secretion [30], the BESTO phenotype is an interesting phenocopy of this DR-induced response of insulin secretion. Contrarily, Sirt1-deficient mice and islets show blunted GSIS [31], further supporting the importance of SIRT1 in the regulation of GSIS in pancreatic β-cells. SIRT1 is also important for β-cell adaptation in response to increasing insulin resistance. Our group has shown that BESTO mice are able to maintain improved glucose tolerance with enhanced GSIS even under a long-term (up to 30 weeks) HFD treatment [32]. Consistent with our finding, other groups have also shown that SIRT1 protects pancreatic β-cells from metabolic stress- and cytokine-induced β-cell death by deacetylating FOXO1 and the p65 subunit of NF-κB, respectively [33,34]. Therefore, these findings indicate that SIRT1 is critical to protect pancreatic β-cells from dysfunction caused by metabolic and age-induced perturbations.
Regulation of central adaptive response by SIRT1
In addition to regulating the balance between insulin sensitivity and insulin secretion in peripheral tissues and organs, SIRT1 is also required to regulate central adaptive responses to acute and chronic energy limitations. For example, DR significantly increases SIRT1 protein levels and induces neural activation in the dorsomedial and lateral hypothalamic nuclei (DMH and LH, respectively) [19]. Brain-specific SIRT1-overexpressing (BRASTO) mice mimics DR-induced neural activation in the DMH and LH, promotes physical activity, and counteract the decrease in body temperature in response to different diet-restricting paradigms [19]. These adaptive responses are all abrogated in Sirt1−/− mice. In the DMH and LH, SIRT1 upregulates expression of the orexin type 2 receptor to mediate these adaptive responses. Furthermore, it has been reported that SIRT1 decreases the production of Aβ amyloid [35], prevents tau-mediated neurodegeneration [36], and maintain memory and synaptic plasticity [37], contributing to the prevention of age-associated cognitive disorders. These findings also provide strong support for the notion that SIRT1 functions to maintain physiological robustness against different kinds of perturbations, mediating beneficial effects against aging.
Expanding roles of other sirtuins in metabolic regulation
Recent studies have clearly proven the importance of other sirtuins in the regulation of insulin sensitivity and insulin secretion. For example, SIRT3, one of the mitochondrial sirtuin family members (SIRT3-5), regulates insulin sensitivity in skeletal muscle [38], and Sirt3 deficiency is associated with the pathogenesis of metabolic syndrome including insulin resistance [39]. SIRT4, another mitochondrial sirtuin, controls amino acid-stimulated insulin secretion in pancreatic β-cells [40]. It has also been reported that SIRT6 promotes glucose-stimulated insulin secretion in β-cells [41], whereas it suppresses gluconeogenesis in the liver [42]. Currently, intensive investigation is being undertaken to understand the roles of mitochondrial sirtuins and SIRT6 in cancer metabolism [43,44]. It has now become apparent that sirtuins have extensively divergent, complex functions in the regulation of metabolism under many different pathophysiological conditions. Given that they all require NAD for their functions, sirtuins are likely the critical keys to connect between NAD availability and metabolic regulation.
NAMPT, a key NAD biosynthetic enzyme that functions as a pacemaker and fine-tunes SIRT1 activity
The pathophysiological significance of SIRT1 and other sirtuins has fueled more enthusiasm to investigate NAD biosynthetic pathways. NAD is a universal and essential coenzyme involved in many cellular redox reactions. To generate NAD, mammals utilize four different precursors, including tryptophan, nicotinamide and nicotinic acid (also known as two forms of vitamin B3), and nicotinamide riboside (NR) [45]. It is known that the salvage pathway starting from nicotinamide is a predominant NAD biosynthetic pathway in mammals [46]. In this pathway, NAMPT produces nicotinamide mononucleotide (NMN) from nicotinamide and 5′-phosphoribosyl-1-pyrophosphate. NMN, together with ATP, is then converted into NAD by the second enzyme, nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) (Figure 2). Studies have demonstrated that NAMPT is a dimeric type II phosphoribosyltransferase which functions as the rate-limiting enzyme in mammalian NAD biosynthetic pathway and directly regulates SIRT1 activity [47,48]. Interestingly, mammals possess two different forms of NAMPT: intracellular and extracellular NAMPT (iNampt and eNampt, respectively) (Figure 2) [9,49]. In the past decade, the role of iNAMPT has been extensively studied in many biological processes. Particularly, iNAMPT plays an important role in regulating metabolic function by modulating SIRT1 activity. For example, FoxOs regulate Nampt transcription in the liver, and overexpression of iNAMPT reduces hepatic triglyceride levels [50]. It has also been reported that the levels of hepatic iNAMPT protein decrease in the patients with nonalcoholic fatty liver disease (NAFLD) [51]. In skeletal muscle, both glucose restriction and exercise increase iNAMPT protein levels [52,53], and iNAMPT levels correlate with mitochondrial contents in humans [52]. iNAMPT also plays a protective role in the stress associated with aging and high-glucose in endothelial cells [54]. Interestingly, we and other groups have reported that the Nampt gene is regulated by the core clock machinery and thereby iNAMPT and NAD levels display circadian oscillation patterns in peripheral metabolic tissues, such as the liver and adipose tissue [55-57]. In turn, SIRT1 negatively regulates the transcriptional activation of clock genes. In other words, iNAMPT and SIRT1 together comprises a novel circadian-regulatory feedback loop, connecting circadian rhythm regulation to metabolic regulation. These findings suggest that NAD functions as a “metabolic oscillator” that dynamically influences rhythmic regulation of metabolic responses [55]. We have recently found that both iNAMPT and NAD levels are reduced in multiple metabolic tissues and organs by HFD feeding and aging, contributing to the pathogenesis of type 2 diabetes [58]. It appears that inflammatory cytokines and oxidative stress cause the reduction in iNAMPT-mediated NAD biosynthesis, implicating an interesting connection between chronic inflammation and NAMPT-mediated NAD biosynthesis.
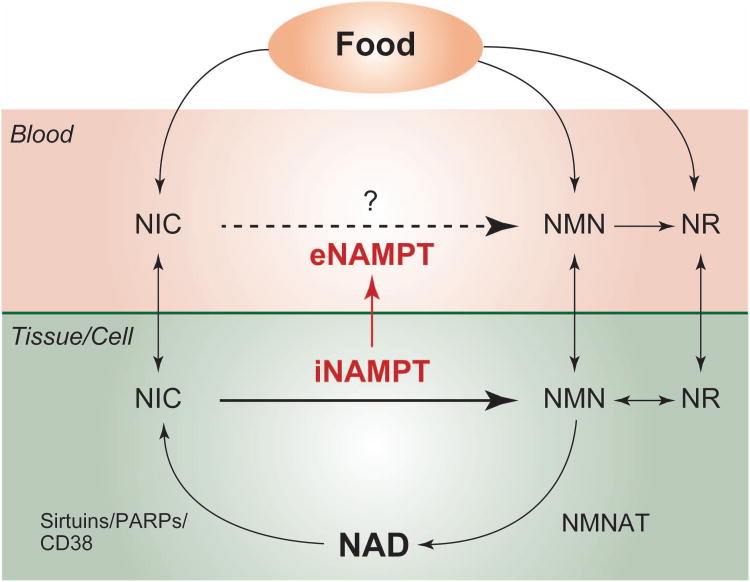
Figure 2. NAMPT-mediated NAD biosynthetic pathways in mammals.
In mammals, nicotinamide (NIC) is converted to nicotinamide mononucleotide (NMN) by the rate-limiting enzyme, nicotinamide phosphoribosyltranferase (NAMPT). There are two forms of NAMPT: intracellular and extracellular NAMPT (iNampt and eNampt, respectively). eNAMPT has significantly higher enzymatic activity than iNAMPT and likely synthesizes NMN in blood circulation. Extracellular NMN might be directly transferred into tissues/cells or is first converted to nicotinamide riboside (NR) and then transferred into tissues/cells. NR is re-converted to NMN by nioctinamide riboside kinase and utilized for NAD biosynthesis. NAD is generated from NMN by nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) and consumed by sirtuins, poly(ADP-ribose) polymerases (PARPs), and CD38.
The physiological function of eNAMPT is still under debate. It has been reported that eNAMPT is positively secreted from fully differentiated adipocytes [49], mononuclear cells [59], hepatocytes [60], and cardiomyocytes [61]. Our previous study has demonstrated that eNAMPT, secreted from adipocytes, has higher enzymatic activity compared to iNAMPT, and it might be involved in the extracellular synthesis of NMN, possibly in the blood circulation (Figure 2) [49]. However, one recent study shows the contradictory data that recombinant NAMPT is not capable of producing NMN in plasma in vitro [62]. To further elucidate the function of eNAMPT in vivo, employing genetic approach, as well as biochemical approach, is important, and the detailed analysis of adipose tissue-specific Nampt knockout mice is currently in progress.
The NAD World: A systemic regulatory network connecting metabolism and aging
As summarized above, the functional interplay between SIRT1 and NAMPT-mediated NAD biosynthesis plays a crucial role in the regulation of a variety of biological processes, particularly metabolic regulation. NAMPT-mediated NAD biosynthesis functions as a “pacemaker” that controls a novel transcriptional-enzymatic arm of circadian regulation and fine-tunes SIRT1 activity. In response to the alterations in NAMPT-mediated NAD biosynthesis, SIRT1 functions as a key mediator that coordinates a number of metabolic responses throughout the body. Through this tight interplay, NAMPT-mediated NAD biosynthesis and SIRT1 together comprise a novel systemic regulatory network, named the “NAD World,” that orchestrates physiological responses to internal and external perturbations and maintains the robustness of the physiological system in mammals [55,63-65].
The significance of this concept is that it conveys the ideas of functional hierarchy and frailty in determining the robustness of the systemic regulation of metabolism and aging. In this concept of the NAD World, critical frailty points are the tissues and organs that have very low levels of iNAMPT. Such tissues and organs likely rely on extracellular sources of NAD intermediates, such as NMN and NR, to maintain sufficient NAD levels for their functions (Figure 3). In this regard, pancreatic β-cells and neurons are likely the most critical frailty points in the mammalian physiological system because both cell types have very low levels of iNAMPT compared to other cell types. Our previous studies have clearly shown that pancreatic β-cells are indeed an important frailty point in the NAD World that is susceptible to changes in NAMPT-mediated NAD biosynthesis. Genetic, pharmacological, and pathophysiological reductions in NAMPT-mediated NAD biosynthesis all impairs β-cell function, causing defects in GSIS and impaired glucose tolerance in vivo [32]. Similarly, neurons are also likely another critical frailty point in the NAD World. It has been demonstrated that SIRT1 regulates memory and synaptic plasticity in the hippocampus [37] and neurobehavioral adaptation in the hypothalamus [19]. Therefore, NAD supply for SIRT1 in these brain regions must be critical, and its reduction could cause neurological problems, including dementia and neurobehavioral complications.
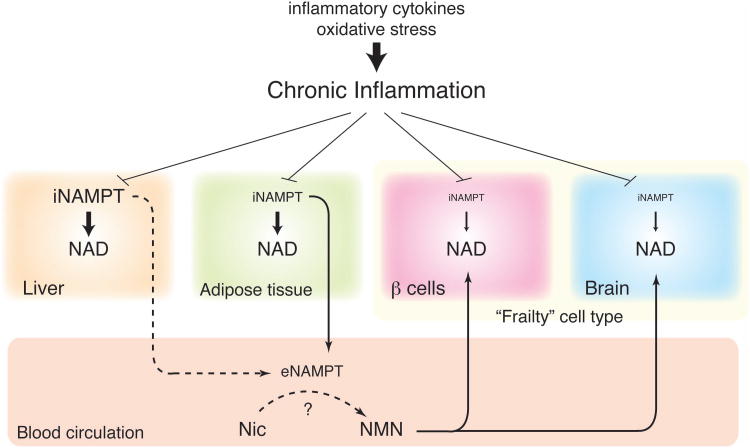
Figure 3. The concept of the NAD World and the possible effect of chronic inflammation.
Pancreatic β-cells and neurons (the brain) are two major frailty points in the NAD World because these two cell types have very low levels of iNAMPT. These particular cell types likely depend on extracellular NMN, which is speculated to be synthesized by eNAMPT secreted by adipose tissue, and maintain optimal NAD levels for their functions. Chronic inflammation, which is caused by inflammatory cytokines and oxidative stress, decreases NAMPT and NAD levels in multiple tissues, contributing to the pathogenesis of age-associated metabolic complications, such as type 2 diabetes. It still remains unclear whether chronic inflammation in adipose tissue also decreases plasma eNAMPT levels and remotely affects the functions of “frailty” cell types. More details for the concept of the NAD World are given in the text.
As briefly described in the previous section, we have shown that TNF-α and oxidative stress significantly reduce NAMPT and NAD levels in primary hepatocytes [58]. Given that both inflammatory cytokines and oxidative stress contribute to age-associated chronic inflammation [3], the development of chronic inflammation could be the reason why NAMPT-mediated NAD biosynthesis is compromised during aging, leading to reduction in SIRT1 activity and thereby a variety of metabolic complications in multiple tissues. Therefore, it will be of great interest to examine whether inflammatory cytokines, such as TNF-α, and/or oxidative stress indeed affects NAD levels in pancreatic β-cells and central neurons. If this is the case, serious dysfunction of these two cell types would be caused by chronic inflammation through defects in NAMPT-mediated NAD biosynthesis. Such dysfunction of pancreatic β-cells and central neurons would affect many other tissues and organs through insulin action and central metabolic regulation, resulting in the gradual deterioration of physiological robustness over time. We speculate that this cascade of robustness breakdown triggered by defects in NAMPT-mediated NAD biosynthesis underlies in the induction of age-associated pathophysiology. If so, is it possible to prevent this systemic robustness breakdown by enhancing NAD biosynthesis at a systemic level? We will discuss this interesting possibility in the next section.
Key NAD intermediates: A translational perspective
Given the importance of SIRT1 in the regulation of metabolic responses in multiple tissues and organs, it has been speculated that modulating NAD levels may influence metabolic functions and provide an effective intervention to treat metabolic disorders such as type 2 diabetes, obesity, and insulin resistance [66]. Indeed, recent studies, including our own, show that enhancing NAD biosynthesis has beneficial effects on glucose and lipid metabolism by increasing SIRT1 activity. For example, genetically engineered mouse models demonstrate that inactivation of poly(ADP-ribose) polymerase-1 (NAD consuming enzyme) [67] or CD38 (NAD degrading enzyme) [68] significantly improves mitochondrial function in skeletal muscle and prevents diet-induced obesity by enhancing energy expenditure. Slow Wallerian degeneration (WldS) mutant mice that contain a spontaneous mutation containing full-length NMNAT1 enhance insulin secretion, and they are also protective against HFD-induced glucose intolerance and streptozotocin-induced hyperglycemia in a SIRT1 dependent manner [69]. Furthermore, administration of apigenin (a potent CD38 inhibitor) [70] and leucine [71] also increases tissue NAD levels and improves metabolic complications, such as glucose intolerance and insulin resistance, in HFD-fed mice.
Our group has demonstrated that administration of a key NAD intermediate, NMN, treats the pathophysiology of metabolic disorders associated with haplodeficiency of Nampt, HFD-feeding, and aging. NMN is a product of NAMPT enzymatic reaction and found in our daily food sources (our unpublished finding). NMN administration restores GSIS in Nampt heterozygous knockout mice [49] and old BESTO and wild-type mice [32]. Furthermore, NMN increases GSIS and insulin sensitivity in HFD-fed type 2 diabetic model mice by restoring the defects in NAMPT-mediated NAD biosynthesis [58]. Interestingly, NMN appears to ameliorate inflammatory response, leading to the improvement in hepatic insulin sensitivity in HFD-fed mice. Indeed, NMN administration enhances the deacetylation of the p65 subunit of NF-κB through the activation of SIRT1 in the liver. Our bioinformatics analyses confirm that biological pathways associated with inflammatory response and NF-κB target genes, such as interleukin-1β (IL-1β) and SA100 calcium binding proteins A8 and A9 (S100a8 and S100a9), are also reduced by NMN treatment. Consistent with our findings, NMN administration reduces the expression of IL-1β and restores β-cell function in fructose-rich diet-fed mice [72]. Additionally, NMN restores insulin secretion in pro-inflammatory cytokine-treated islets [72,73]. These findings indicate that NMN treatment has anti-inflammatory effects in pancreatic islets and the liver, improving insulin secretion and action in diabetic model mice. NR is another promising NAD intermediate to treat metabolic disorders. It has been reported that NR administration also improves mitochondrial function in skeletal muscle and brown adipose tissue, glucose tolerance, insulin secretion and action, plasma lipid panel, and energy expenditure through activating SIRT1 and SIRT3 in HFD-fed mice [74]. Because SIRT1 functions to prevent a variety of age-associated diseases [6,8], it is likely that enhancing NAD levels through supplementation with NAD intermediates, such as NMN and NR, could prevent not only metabolic disorders but also other age-associated diseases (Figure 4). One recent study has shown that NR treatment significantly attenuates cognitive deterioration in the AD mouse model [75]. Furthermore, it is also possible that NMN/NR supplementation directly affects NAD-dependent redox metabolism such as β-oxidation and glycolysis. Therefore, it will be of great importance to investigate the effect of NMN/NR supplementation on those biological processes. Given that both NMN and NR are natural compounds (unpublished data) and derivatives of vitamin B3, these compounds are expected to be translatable as effective anti-aging nutriceuticals into humans in the near future. To this end, it will be of great importance to carefully evaluate the effects of long-term NMN/NR supplementation, as well as their potential side effects, on metabolism and other pathophysiological parameters in rodents and then possibly humans.
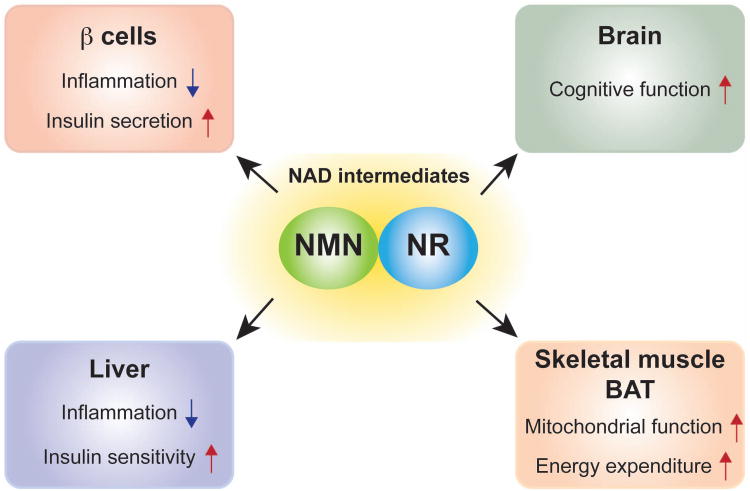
Figure 4. Therapeutic potential of key NAD intermediates against age-associated diseases.
Supplementation of key NAD intermediates, nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR), improves insulin secretion, insulin action, energy expenditure, and cognitive function and prevents inflammatory reactions in mice. These NAD intermediates are expected to be translatable as effective anti-aging nutriceuticals into humans in the near future.
Concluding remarks
On the verge of historically unprecedented increases in elderly demographics through the globe, it is now of great importance to understand the spatial and temporal dynamics of the systemic regulatory network that integrates metabolic regulation to the aging/longevity control in mammals. In this regard, the concept of the NAD World provides critical insight into how to dissect such system dynamics, focusing on two critical components, namely SIRT1 and NAMPT-mediated NAD biosynthesis. Several important questions still remain unanswered. If chronic inflammation is a major cause for defects in NAMPT-mediated biosynthesis at a systemic level, how and where does it happen during the process of aging? Are “frailty” cell types, such as pancreatic β-cells and neurons, indeed sensitive to inflammation-induced dysfunction of NAD biosynthesis? Can we reverse this destruction process by enhancing NAD biosynthesis with key NAD intermediates as nutriceuticals? Can we really improve the quality of life and eventually achieve longevity by administering these NAD intermediates in humans? Further investigation will be definitely required to address these questions. We hope that understanding the NAD World will guide us towards reasonable solutions for social and economic problems caused by heavily aging societies.
Acknowledgments
We thank members of the Imai laboratory for their daily, stimulating discussions. This work was supported in part by grants from the National Institute on Aging (AG024150, AG037457), the National Heart, Lung, and Blood Institute (HL097817), the Ellison Medical Foundation, and the Longer Life Foundation to S.I and by institutional support from the Washington University Nutrition Obesity Research Center (P30DK056341) and the Washington University Diabetes Research Center (P60DK020579). J.Y. was supported by the Japan Research Foundation for Clinical Pharmacology, the Manpei Suzuki Diabetes Foundation, and the Kanae Foundation for the Promotion of Medical Science.
Footnotes
Conflict of Interest statement: S.I. serves as a scientific advisory board member for Sirtris, a GSK company, and has a Sponsored Research Agreement with Oriental Yeast Co., Tokyo, Japan. J.Y. has no conflict of interest.
References
- 1.Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest. 1983;71:1523–1535. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu R, Breda E, Oberg AL, Powell CC, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 3.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donath MY, Ehses JA, Maedler K, et al. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005;54(Suppl 2):S108–S113. doi: 10.2337/diabetes.54.suppl_2.s108. [DOI] [PubMed] [Google Scholar]
- 5.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 6.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh A, Stein L, Imai S. The role of mammalian sirtuins in the regulation of metabolism, aging, and longevity. Handb Exp Pharmacol. 2011;206:125–162. doi: 10.1007/978-3-642-21631-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai S. Nicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseases. Curr Pharm Des. 2009;15:20–28. doi: 10.2174/138161209787185814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 13.Burnett C, Valentini S, Cabreiro F, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam U. dSir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep. 2012;2:1485–1491. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–21. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 16.Stumpferl SW, Brand SE, Jiang JC, et al. Natural genetic variation in yeast longevity. Genome Res. 2012;22:1963–1973. doi: 10.1101/gr.136549.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 18.Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satoh A, Brace CS, Ben-Josef G, et al. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordone L, Cohen D, Robinson A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 21.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herranz D, Munoz-Martin M, Canamero M, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477–4490. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16:180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiang L, Wang L, Kon N, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–32. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenk S, McCurdy CE, Philp A, et al. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest. 2011;121:4281–4288. doi: 10.1172/JCI58554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Richardson A, Liu F, Adamo ML, Van Remmen H, Nelson JF. The role of insulin and insulin-like growth factor-I in mammalian ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in β cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Song MY, Song EK, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–351. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Min SW, Cho SH, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao J, Wang WY, Mao YW, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing E, Emanuelli B, Hirschey MD, et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirschey MD, Shimazu T, Jing E, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haigis MC, Mostoslavsky R, Haigis KM, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 41.Kanfi Y, Peshti V, Gil R, et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9:162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 42.Dominy JE, Jr, Lee Y, Jedrychowski MP, et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol Cell. 2012;48:900–913. doi: 10.1016/j.molcel.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finley LW, Carracedo A, Lee J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sebastian C, Zwaans BM, Silberman DM, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–1199. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23:420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- 47.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Zhang X, Bheda P, Revollo JR, Imai S, Wolberger C. Structure of Nampt/PBEF/visfatin, a mammalian NAD(+) biosynthetic enzyme. Nat Struct Mol Biol. 2006;13:661–662. doi: 10.1038/nsmb1114. [DOI] [PubMed] [Google Scholar]
- 49.Revollo JR, Körner A, Mills KF, et al. Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao R, Wei D, Gao H, Liu Y, DePinho RA, Dong XC. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J Biol Chem. 2011;286:14681–14690. doi: 10.1074/jbc.M110.201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahl TB, Haukeland JW, Yndestad A, et al. Intracellular nicotinamide phosphoribosyltransferase protects against hepatocyte apoptosis and is down-regulated in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:3039–3047. doi: 10.1210/jc.2009-2148. [DOI] [PubMed] [Google Scholar]
- 52.Costford SR, Bajpeyi S, Pasarica M, et al. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298:E117–E126. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fulco M, Cen Y, Zhao P, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borradaile NM, Pickering JG. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell. 2009;8:100–112. doi: 10.1111/j.1474-9726.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- 55.Imai S. “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim Biophys Acta. 2010;1804:1584–1590. doi: 10.1016/j.bbapap.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friebe D, Neef M, Kratzsch J, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011;54:1200–1211. doi: 10.1007/s00125-010-2042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garten A, Petzold S, Barnikol-Oettler A, et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem Biophys Res Commun. 2009;391:376–381. doi: 10.1016/j.bbrc.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 61.Pillai VB, Sundaresan NR, Kim G, et al. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circ Physiol. 2013;304:H415–H426. doi: 10.1152/ajpheart.00468.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hara N, Yamada K, Shibata T, Osago H, Tsuchiya M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS One. 2011;6:e22781. doi: 10.1371/journal.pone.0022781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imai S. From heterochromatin islands to the NAD World: A hierarchical view of aging through the functions of mammalian Sirt1 and systemic NAD biosynthesis. Biochim Biophys Acta. 2009;1790:997–1004. doi: 10.1016/j.bbagen.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imai S. The NAD World: a new systemic regulatory network for metabolism and aging - Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imai S. Dissecting systemic control of metabolism and aging in the NAD World: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett. 2011;585:1657–1662. doi: 10.1016/j.febslet.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imai S. A possibility of nutriceuticals as an anti-aging intervention: Activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol Res. 2010;62:42–47. doi: 10.1016/j.phrs.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai P, Canto C, Oudart H, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbosa MT, Soares SM, Novak CM, et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.25) is necessary for the development of diet-induced obesity. Faseb J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- 69.Wu J, Zhang F, Yan M, et al. WldS enhances insulin transcription and secretion via a SIRT1-dependent pathway and improves glucose homeostasis. Diabetes. 2011;60:3197–3207. doi: 10.2337/db11-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Escande C, Nin V, Price NL, et al. Flavonoid apigenin is an inhibitor of the NAD+ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–1093. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H, Xu M, Lee J, He C, Xie Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am J Physiol Endocrinol Metab. 2012;303:E1234–E1244. doi: 10.1152/ajpendo.00198.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia. 2011;54:3083–3092. doi: 10.1007/s00125-011-2288-0. [DOI] [PubMed] [Google Scholar]
- 73.Caton PW, Richardson SJ, Kieswich J, et al. Sirtuin 3 regulates mouse pancreatic beta cell function and is suppressed in pancreatic islets isolated from human type 2 diabetic patients. Diabetologia. 2013;56:1068–1077. doi: 10.1007/s00125-013-2851-y. [DOI] [PubMed] [Google Scholar]
- 74.Canto C, Houtkooper RH, Pirinen E, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong B, Pan Y, Vempati P, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models. Neurobiol Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]