Abstract
Cancer progression is characterized by rapidly proliferating cancer cells that are in need of increased protein synthesis. Therefore, enhanced endoplasmic reticulum (ER) activity is required to facilitate the folding, assembly and transportation of membrane and secretory proteins. These functions are carried out by ER chaperones. It is now becoming clear that the ER chaperones have critical functions outside of simply facilitating protein folding. For example, cancer progression requires GRP78 for cancer cell survival and proliferation, as well as angiogenesis in the microenvironment. GRP78 can translocate to the cell surface acting as a receptor regulating oncogenic signaling and cell viability. Calreticulin, another ER chaperone, can translocate to the cell surface of apoptotic cancer cells and induce immunogenic cancer cell death and antitumor responses in vivo. Tumor-secreted GRP94 has been shown to elicit antitumor immune responses when used as antitumor vaccines. Protein disulfide isomerase is another ER chaperone that demonstrates pro-oncogenic and pro-survival functions. Due to intrinsic alterations of cellular metabolism and extrinsic factors in the tumor microenvironment, cancer cells are under ER stress, and they respond to this stress by activating the unfolded protein response (UPR). Depending on the severity and duration of ER stress, the signaling branches of the UPR can activate adaptive and pro-survival signals, or induce apoptotic cell death. The PERK signaling branch of the UPR has a dual role in cancer proliferation and survival, and is also required for ER stress-induced autophagy. The activation of the IRE1α branch promotes tumorigenesis, cancer cell survival, and regulates tumor invasion. In summary, perturbance of ER homeostasis plays critical roles in tumorigenesis, and therapeutic modulation of ER chaperones and/or UPR components presents potential antitumor treatments.
Introduction
The endoplasmic reticulum (ER) is a perinuclear, cytosolic compartment essential for the synthesis, folding and modification of secretory and membrane proteins. It is also the site for lipid synthesis and a major intracellular site for Ca2+ storage. Physiological and pathologic conditions that perturb the ER, such as nutrient deprivation, hypoxia, ER Ca2+ depletion, impaired glycosylation or disulfide bond formation, oxidative stress, and viral or bacterial infection, may lead to ER stress. ER stress occurs when the protein load exceeds the ER capacity to fold or degrade them, and is manifested by the accumulation of malfolded proteins in the ER. ER stress triggers an evolutionarily conserved quality control mechanism, the unfolded protein response (UPR), which aims at restoring ER homeostasis by activating a cascade of signaling molecules to transiently arrest protein translation, to induce ER molecular chaperones and enzymes that enhance the protein folding capacity, and to initiate a process to export and degrade the misfolded ER proteins1,2. The most abundant and well-characterized ER chaperone proteins include GRP78, GRP94, CRT, and PDI. Due to increased protein synthesis in proliferating cancer cells, tumor cells require increased ER capacity and function. ER chaperones serve a host of important roles in maintaining ER homeostasis contributing to cancer cell survival and progression. For example, GRP78 is critical for tumorigenesis and therapeutic resistance. Recently, much progress has been made in identifying diverse roles for ER chaperones in cancer. Due to the interconnectivity of the ER with other cellular compartments, it is now becoming clear that chaperones traditionally thought to remain in the ER, can function beyond this compartment and are involved in processes beyond protein folding and posttranslational modification. In particular, the cell surface form of GRP78, GRP94, CRT and PDI assume novel signaling functions that regulate proliferation, apoptosis and immunity. In view of the many excellent general reviews on the UPR, this review focuses on the diverse functions of major ER chaperones inside and outside of the ER and updates the contribution of the UPR signaling pathways within the context of cancer progression and anti-cancer therapy.
Endoplasmic reticulum homeostasis and the unfolded protein response
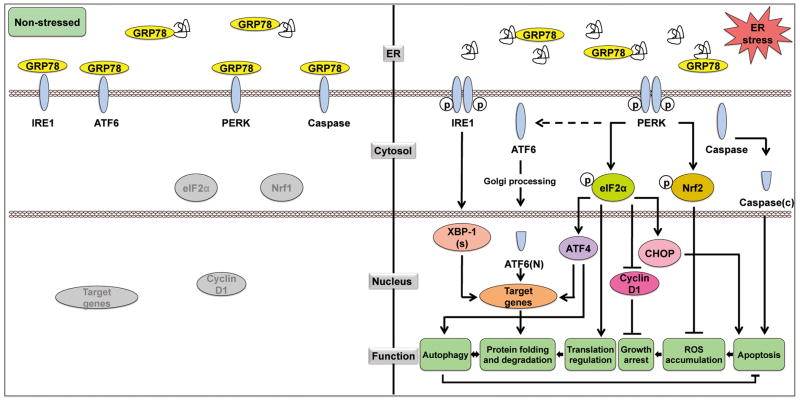
There are three canonical branches in the UPR signaling pathway, which are mediated by three ER stress sensors: protein kinase RNA (PKR)-like ER kinase (PERK), inositol-requiring kinase 1 (IRE1α), and the activating transcription factor 6 (ATF6). A major ER chaperone, the 78 kDa glucose regulated protein (GRP78), also referred to as BiP/HSPA5, acts as a master regulator of the UPR through direct interaction with all three sensors and maintains them in an inactive form in non-stressed situations (Figure 1)3–5. Upon ER stress, GRP78 is titrated away by the accumulated malfolded proteins, releasing the UPR sensors, which allows the activation and transduction of UPR signals across the ER membrane to the cytosol and the nucleus. PERK is an ER transmembrane protein with an ER luminal stress-sensing domain and a cytosolic kinase domain. An important function of PERK in the UPR is to facilitate the attenuation of global protein synthesis via the phosphorylation of eIF2α, which suppresses 80S ribosome assembly. This results in the inhibition of cyclin D1 translation and cell cycle arrest6. While global translation is suppressed under conditions of eIF2α phosphorylation, select mRNAs containing regulatory sequences in the open reading frame in 5′-untranslated regions require the phosphorylation of eIF2α for translation. The transcription factor ATF4 is one example, and the translational upregulation of ATF4 can induce the expression of UPR target genes that promote ER folding capacity and adaptation to stress.
Figure 1.
Unfolded protein response and its regulation on cell activities. Left panel: Under non-stress condition, ER lumenal GRP78, in addition to folding proteins, binds to IRE1α, ATF6, PERK and caspase-12 and -7, quenching their activation. Right panel: When cells are under ER stress, GRP78 is titrated away through binding to the malfolded proteins, resulting in activation of the IRE1α, ATF6 and PERK signaling pathway. IRE1α activates its RNase activity to cleave the mRNA of XBP1, resulting in a spliced form of XBP1 (XBP1-s). ATF6 translocates from the ER to the Golgi apparatus, where it is cleaved into the active nuclear form ATF6(N). PERK dimerizes and autophosphorylates, and thereby phosphorylates its two major substrates eukaryotic translation initiation factor 2α (eIF2α) and nuclear factor-like 2 (Nrf2). eIF2α phosphorylation attenuates global protein synthesis, and inhibits cyclin D1 translation through which contributes to cell cycle arrest. The phosphorylation of eIF2α also activates the transcription of ATF4. XBP1(s), ATF6(N) and ATF4 act in concert to induce transcription of target genes mediating protein folding and degradation. Autophagy is also triggered. Another function of phosphorylated eIF2α is to activate CHOP which promotes apoptosis. The phosphorylation of Nrf2 activates the expression of enzymes required for ROS quenching, and thereby inhibits ROS accumulation. Procaspase-12 and -7, upon released from GRP78, are cleaved into their activated forms triggering apoptosis.
IRE1α is a transmembrane Ser/Thr protein kinase that also has site-specific endoribonuclease (RNase) activity. Upon ER stress, IRE1α dimerizes and autophosphorylates, and thereby activates its RNase activity to cleave a 26 base intron from the mRNA encoding X-box-binding protein 1 (XBP1), resulting in a translational frameshift and a translation of a spliced form of XBP1 (XBP1-s) which is a more stable and potent transcription factor of target genes including DnaJ, p58, ERdj4, EDEM and PDI, all involved in protein folding and ER-associated degradation (ERAD)7,8. ATF6, a basic leucine zipper (bZIP) transcription factor, when released from GRP78 upon ER stress, translocates from the ER to the Golgi apparatus, where it is cleaved by S1P and S2P proteases to generate the active nuclear form of ATF6 (p50). Cleaved ATF6 and spliced XBP1 act in parallel to mainly induce the transcription of genes encoding ER chaperones and enzymes that facilitate protein folding and maturation. Interestingly, while the PERK/peIF2α/ATF4 pathway is canonically regarded as the translational controlling arm of the UPR, a recent report suggests that it is also required for the activation of ATF6 and its target genes, thereby fully integrating the regulatory networks of the UPR9.
In addition to these ER stress pathways, autophagy is activated upon ER stress and has been implicated as a defensive mechanism for maintaining ER homeostasis that promotes survival10,11. Autophagy is an intracellular protein degradation system required for the normal turnover of cellular components and for the starvation response. When autophagy is induced, a double-membrane structure called autophagosome is formed de novo or from existing membranes, possibly from the ER, to enclose the subcellular components. Loss of the integrity of the ER, for example through depletion of GRP78, suppresses autophagosome formation induced by ER stress as well as nutrient starvation12. How might autophagy relieve ER stress? As shown in the yeast system, upon ER stress when the ERAD system is saturated, autophagy removes both soluble and aggregated forms of unfolded proteins13. It has also been suggested that autophagy counterbalances ER expansion in the face of continuously accumulating unfolded proteins14. On the other hand, the onset of autophagy upon ER stress requires the UPR. Studies using MEFs with deficiency in various UPR stress sensors showed that ER stress- induced autophagy was inhibited15–17. While the precise requirement of specific UPR pathways to induce autophagy under different stress conditions awaits further resolution, the UPR and autophagy are integrated processes playing important roles in restoring homeostasis upon stress, counteracting apoptotic mechanisms (Figure 1).
While UPR activation leads to adaptations that may sustain cell survival, under severe and prolonged ER stress conditions where the cells fail to restore ER homeostasis, the UPR activates pathways that lead to apoptotic cell death2,3. Such measures protect the organism by eliminating damaged cells beyond repair. ER stress-induced apoptosis can be carried out in a number of ways. Examples include the translocation of the death effectors BAX and BAK from the ER to the mitochondria triggered by ER Ca2+ flux, caspase activation (C-12 and C-4 in mice and human, respectively) by tumor necrosis factor receptor (TNFR)-associated factor-2, and the activation of c-Jun-N-terminal kinase (JNK) by IRE1 via phosphorylation and the inactivation of the anti-apoptotic protein BCL-2. Additionally, the synthesis of BCL-2 can be suppressed by CHOP, downstream of the PERK-eIF2α-ATF4 pathway. However, it has been proposed that in stressed cells the pro-apoptotic effect of CHOP may be attributed more to its activation of GADD34 which promotes eIF2α dephosphorylation, thereby acting in a negative feedback loop which leads to excessive recovery of protein synthesis that exceeds the ER folding capacity2.
ER stress in tumorigenesis
Neoplastic progression is a multistep process resulting from genetic alterations that drive the progressive transformation of normal cells into malignant states by overriding growth arrest or senescence controls, and at the same time, suppressing the pro-apoptotic signals. Rapidly proliferating cancer cells require increased ER activity to facilitate the folding, assembly and transport of membrane and secretory proteins, and are thereby subjected to ER stress. Due to inadequate vascularization and rapid growth, tumor cells encounter growth-limiting conditions such as hypoxia and nutrient deprivation. An inadequate supply of glucose affects protein glycosylation and the production of ATP, both of which could lead to the accumulation of unfolded proteins in the ER, resulting in ER stress. Thus, in response to ER stress, activation of the UPR has been observed in various tumors18. In the case of leukemic cells, retroviral infection, the pathological expression of fusion protein PML-RARα and the accumulation of reactive oxygen species (ROS) can all induce ER stress19,20. The hypoxic bone marrow (BM) environment and the high glucose demands resulting from rapid proliferation also contribute to ER stress in leukemia cells. Indeed, ER stress signaling has been implicated in the spontaneous apoptosis of leukemia cells21,22 and inducible heterozygous knockout of GRP78 in the BM suppresses PTEN-mediated leukemogenesis23.
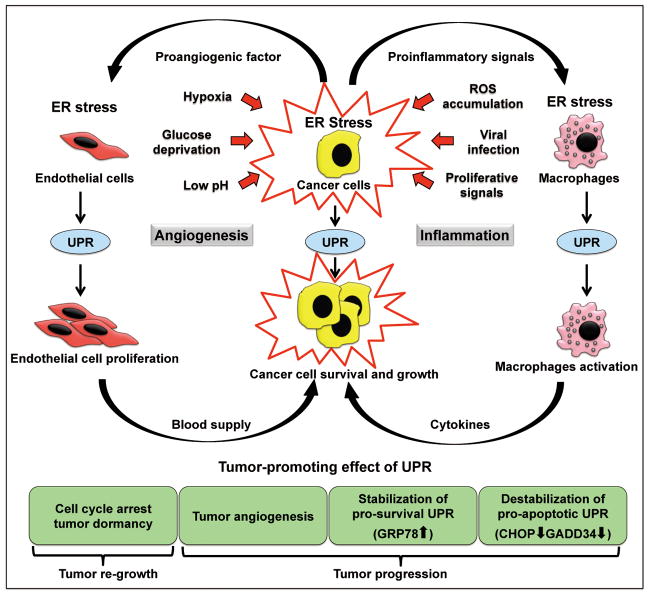
During the early stages of tumor development, ER stress and the activation of the UPR could affect tumorigenesis in multiple ways (Figure 2). For example, ER stress promotes angiogenesis through stimulating VEGF expression and secretion24,25. It also induces cancer cell dormancy through G1 arrest in response to decreased cyclin D1 downstream of PERK activation26,27. During tumorigenesis, the robust upregulation of GRP78 and other ER chaperones by the UPR can enhance the ER protein folding capacity and maintain ER homeostasis. Additionally, the anti-apoptotic property of GRP78 can also counterbalance the cell death pathways that are still functional in the cancer cells. This, coupled with the induced dormancy, dually protects the cancer cells from apoptosis and allows for recurrence once favorable growth conditions return. A recent report further suggests a novel role of the tumor ER stress response in promoting macrophage activation and inflammation in the tumor microenvironment28. Macrophages cultured in conditioned medium from ER stressed tumor cells became activated, and they undergo ER stress with upregulation of GRP78, GADD34, CHOP and XBP-1 splicing, and were able to recapitulate, amplify and expand the proinflammatory response of tumor cells, favoring tumor progression (Figure 2).
Figure 2.
The vicious circle of ER stress and unfolded protein response in promoting tumor progression. Cancer cells are under ER stress due to the growth signaling and factors from the microenvironments. Adaptive UPR is activated to support tumor cell survival and growth. Cancer cells under ER stress secrete proangiogenic factors to stimulate the proliferation of endothelial cells, which in return promotes cancer cell survival and tumor growth. Cancer cells under ER stress also secrete proinflammatory signals to the stromal cells in the vicinity, mostly tumor-associated macrophages, which in turn are activated and secrete inflammatory cytokines that promote tumor growth, angiogenesis, invasion and metastasis.
Despite the protective effects of the UPR, severe, persistent ER stress leads to cell death. Thus, overactivation of the ER stress pathways in hypoxic tumor cells has been shown to render them more sensitive to proteasome inhibitors such as bortezomib, resulting in increased cytotoxicity29. Interestingly, HSP90-inibitor IPI504, another agent known to enhance proteotoxic stress, induces tumor regression only when combined with rapamycin, by promoting irresolvable ER and oxidative stress that result in catastrophic ER and mitochondria damage30. Nonetheless, in cancer cells where mutations often inactivate their apoptotic potential, mild, chronic ER stress could be beneficial for cancer cell survival. In a reconstituted cell culture system, it was demonstrated that chronic ER stress can be a predominantly adaptive pathway, primarily by maintenance of expression of UPR targets that facilitate survival, in particular ER chaperones such as GRP78 by ATF6 activation31. At the same time, mild, chronic ER stress leads to intrinsic instabilities of mRNAs and proteins that promote apoptosis such as CHOP and GADD34 (Figure 2). Thus, the UPR can be structured to allow cells to avert death as they adapt and both posttranscriptional and posttranslational mechanisms influence this outcome. Nonetheless, considering the complexity and heterogeneity within the tumor as well as the microenvironment around the tumor, the interplay between UPR and the various signaling pathways will likely corporate to dictate whether apoptosis, growth arrest or proliferation will occur.
The pleiotrophic role of the ER master chaperone GRP78 in cancer
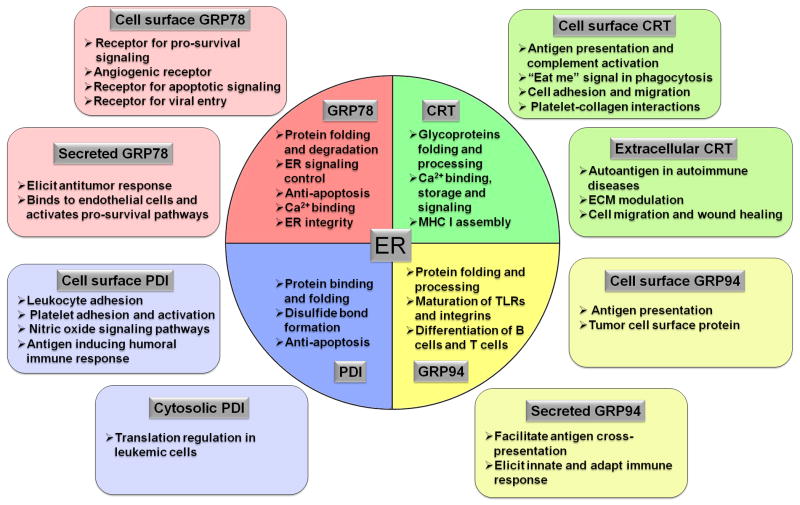
GRP78 is a multifunctional protein that can impact a wide range of human diseases via diverse mechanisms4,5,32–34. While traditionally GRP78 is regarded as a lumen ER chaperone whose major function is to fold and process ER proteins, bind ER Ca2+ and maintain ER homeostasis, recent studies have established that in specific cell types or when subjected to stress, GRP78 can be located in compartments outside the ER, including the cell surface, the cytosol, the mitochondria and the nucleus, and it can even be secreted, where it binds interacting partners and exerts new effects on cell growth and signaling35–37. These discoveries change the paradigm on GRP78 function and offers novel therapeutic approaches to target GRP78 (Figure 3).
Figure 3.
The different localizations and functions of major ER chaperones. The circle in the center indicates ER, where the majority of ER chaperones are located. The squares outside of ER indicate non-traditional localization of these chaperones and their functions.
GRP78 regulates cell survival and proliferation
GRP78 is widely observed to be upregulated in cancer cells4,5,33,38. One major function of GRP78 is protection against stress-induced apoptosis. The ability of GRP78 to block apoptosis includes binding and inactivating pro-apoptotic components such as BIK and caspase-7 that localize to the ER, as well as suppressing the induction of CHOP, which mediates the apoptotic arm of the UPR39,40. Recently, a novel mechanism for the anti-apoptotic function of GRP78 has been uncovered involving functional interactions between GRP78, BIK, NOXA and BCL-2 (ref. 41). This new study showed that GRP78 is able to negate apoptosis resulting from BIK induction, even if it is assisted by another BH-3 protein such as NOXA. GRP78 binds to BIK in a BH-3 domain independent manner through its amino portion, consistent with previous findings that a subfraction of GRP78 may exist in a transmembrane configuration with the amino portion exposed to the cytosol39,42. Interestingly, GRP78 and BCL-2 form separate complexes with different domains of BIK, and increasing amounts of GRP78 expressed in cells leads to a reduction of BCL-2 binding to BIK and vice versa41. These results suggest that when the expression of BIK is elevated under circumstances such as estrogen starvation or anti-estrogen treatment of breast cancer cells, it promotes the formation of BIK/BCL-2 heterodimers at the outer surface of the ER. The high ratio of BIK:BCL-2 changes the set of proteins BCL-2 interacts with the ER, leading to Ca2+ release and initiation of the apoptosis including the translocation of BAX to the mitochondria and the release of cytochrome c to the cytosol. However, when GRP78 is expressed at high levels in cancer cells due to ER/metabolic stress associated with long-term estrogen deprivation, GRP78 binds to and sequesters BIK through complex formation. With reduced binding to BIK, BCL-2 is able to suppress ER Ca2+ release, thereby suppressing apoptosis. This mechanistic explanation is consistent with another report implicating BCL-2 in the survival pathway activated by GRP78 overexpression43. Considering that the apoptotic function of BIK has also been implicated in response to chemotherapy and proteasome inhibitors44,45, this mechanism may also apply in these systems. In addition to being a regulator of apoptosis, GRP78 is also identified as a regulator of autophagy due to its role in maintaining ER integrity and homeostasis12.
Evidence is accumulating that GRP78 is critical for cell proliferation. Genetic knockout of Grp78 leads to a much reduced embryonic cell proliferation as well as massive apoptotic death of the inner cell mass, leading to embryonic lethality at day E3.5 (ref. 46). This provides the first hint that GRP78 may be important for pluripotent cell homeostasis and survival. In adult cells, GRP78 is expressed at low levels in the normal adult brain but is highly elevated in malignant glioma specimens and human malignant cell lines, correlating with their rate of proliferation, and knockdown of GRP78 by small interfering RNA leads to a slowdown in glioma cell growth38. This is further supported by the observation that in an endogenous breast cancer model, GRP78 haploinsufficiency inhibits tumor cell proliferation47. How might GRP78 facilitate cell proliferation? This could be due to the ER chaperone function of GRP78 in growth factor secretion and/or the maturation of growth factor receptors. Another major discovery is that ER stress actively promotes the localization of GRP78 to the cell surface48, and selected cell types, notably cancer cells, express cell surface GRP78 which acts as a multifunctional receptor36,49. One function of cell surface GRP78 is to enhance P13K/AKT signaling, leading to both increased proliferation and survival. It is recently reported that ligation of prostate cancer cell surface GRP78 by activated α2-macroglobulin (*α2-M) upregulates the prostate-specific antigen (PSA), which is then secreted into the medium and binds *α2-M. The resultant PSA/*α2-M complex then binds to cell surface GRP78, causing activation of multiple kinase signaling pathways, coupling with an increase in proliferation and protein synthesis50. Additionally, GRP78 and Cripto can form a complex at the cell surface and collaborate to inhibit transforming growth factor β-signaling and enhance cell growth51. Cell surface GRP78 is also reported to complex with Cripto in adult hematopoietic stem cells (HSCs) and maintains them in the hypoxic endosteal niche in the BM52.
GRP78 is required for solid tumor progression
Fast proliferating solid tumors are characterized with increasing hypoxia, nutrient starvation and acidosis, factors that activate the UPR. Correspondingly, GRP78 has been reported to be upregulated in solid tumors in various organs including breast, liver, gastric, esophagus, brain, prostate, head and neck and melanoma, correlating with aggressive tumor behavior and recurrence5,18,33,38,53–57. While xenograft studies with knockdown of GRP78 supported its requirement for tumor formation and progression58, the pathophysiologic role of GRP78 in tumorigenesis has been directly validated using the heterozygous Grp78 mice and mouse models of conditional knockout of the Grp78 in specific tissues. These studies revealed that without affecting mouse growth rate, organ development, and antibody production, GRP78 haploinsufficiency prolongs the latency period and retards the progression of the oncogene-induced mammary tumors in the MMTV-PyVT breast adenocarcinoma mouse model47. The underlying mechanisms for this suppression include lowered tumor proliferation, increased apoptosis and dramatically reduced tumor angiogenesis. Likewise, heterozygous or homozygous deletion of Grp78 specifically in the mouse prostate epithelium suppresses prostate tumorigenesis mediated by a loss of the tumor suppressor gene PTEN without affecting postnatal prostate development and growth59. Strikingly, AKT activation in the PTEN-null prostate epithelium was potently suppressed by the loss of GRP78, and a similar suppression of stress-mediated AKT activation was observed in human prostate cancer cells where GRP78 was knocked down by siRNA. Since PTEN mutation and AKT activation are key drivers of human cancer, inactivation of GRP78 may represent a novel approach to block tumorigenesis resulting from loss of PTEN tumor suppression or activation of oncogenic AKT, or both.
Cancer initiating cells (CICs) with self-renewal capacity have recently been implicated as the root cause of cancer metastasis, therapeutic resistance, and recurrence. GRP78 has been reported to be highly elevated in breast disseminated tumor cells, which shared similar biological properties of CICs60. In agreement, differential systemic analysis revealed elevated GRP78 expression in head and neck cancer initiating cells (HN-CICs)56. This same study also reported that such cells with cell surface expression of GRP78 contain cancer stemness properties of self-renewal, differentiation and radioresistance; whereas knockdown of GRP78 by shRNA promoted HN-CIC cell differentiation and apoptosis and impaired their tumorigenic properties both in vitro and in vivo. These observations, though still at an early stage, suggest that GRP78 could play a critical role in regulating CIC proliferation and survival, as well as in stem cell biology in general, as evidenced by the previous observation that homozygous knockout of Grp78 led to massive apoptosis of cells within the inner cell mass which are precursors of embryonic stem cells46.
GRP78 is an effector for leukemogenesis and AKT oncogenic signaling
While GRP78 is established to protect cancer cells against the adverse hypoxic and nutrient-deprived microenvironment of solid tumors, its role in the initiation and progression of hematologic cancers is just emerging. Proteomic analysis reveals that GRP78 is differentially expressed in the HSC-like fractions from the BM of leukemic patients61. In addition to being a tumor suppressor, PTEN also contributes to the maintenance of the HSCs. Induced deletion of PTEN in the hematopoietic system of postnatal mice exhausted normal HSCs and promoted exhaustive proliferation of leukomogenic stem cells, resulting in the development of myeloproliferative disorders and eventually leukemia62,63. These studies further showed that the mTOR inhibitor rapamycin effectively suppressed growth of the leukemia initiating cells and prevented the exhaustion of normal HSCs. Through creation of a biallelic conditional knockout mouse model of GRP78 and PTEN in the hematopoietic system, it was demonstrated that GRP78 haploinsufficiency potently suppresses leukemogenesis and AKT/mTOR signaling in PTEN null BM cells23. Importantly, Grp78 heterozygosity by itself has no apparent effect on development or survival, nor does it alter total BM cell number or HSC population23,46,47. Thus, partial GRP78 expression is sufficient to maintain normal organ homeostasis whereas tumor progression requires an optimal level of GRP78, both in solid and blood tumors.
How might GRP78 contribute to the PTEN-null mediated leukemogenesis? While GRP78 is able to confer multiple anti-apoptotic effects on cancer cells, a key mechanistic explanation may be that the activation of AKT, a prominent effector that is activated by the loss of PTEN, is compromised by the reduction of GRP78 in the hematopoietic system. Thus, PTEN-null mediated AKT/mTOR signaling is potently suppressed in the BM of the Pten null Grp78 heterozygous mice. It has been reported that ligation of cell surface GRP78 in human cancer cells with antibodies directed against its carboxyl domain suppresses PI3K/AKT/mTOR signaling64. The observation that knockdown of GRP78 in leukemia cells suppresses serum-stimulated phosphorylation of the p85 regulatory subunit of PI3K and inhibits PI(3,4,5)P3 production further suggests that GRP78 regulates AKT activation through functional regulation of PI3K23. Upregulation of Wnt signaling is also suggested to be associated with leukemogenesis65. Hypoxia-induced ER stress was reported to inhibit normal Wnt protein processing and secretion as ER stress causes dissociation between GRP78 and Wnt which is essential for its correct posttranslational processing65. Hence, the delayed onset of leukemogenesis observed in the Pten null, Grp78 heterozygous mice may be partially attributed to the attenuation of Wnt protein processing due to GRP78 knockdown, which awaits further investigation. The expression of anti-apoptotic protein BCL-2 has been implicated in hematologic malignancies66. Considering that GRP78 level may influence the binding of pro-apoptotic BH-3 protein BIK to BCL-2 (ref. 41), one speculation is that the suppressed leukemic phenotype in the Pten null, Grp78 heterozygous mice may in part be due to modulation of the BCL-2 activity. While the role of GRP78 in the development of human leukemia remains to be validated, emerging evidence shows elevated Grp78 mRNA and protein expression in patient leukemic blasts compared to normal controls, notably in AML and in high grade B-lineage lymphoid malignancies21–23,67. With the Grp78 knockout leukemic mouse model providing proof-of-principle that a partial reduction of GRP78 can arrest leukemogenesis while having no harmful effect on the hematopoietic system, targeting GRP78 may represent a novel therapeutic target against leukemia and other stem cell related diseases.
GRP78 is required for angiogenesis in the tumor microenvironment
The tumor microenvironment contains a plethora of cells that support tumor growth and progression. Tumor vasculature is essential for tumor growth and metastasis, as it supplies the nutrients and oxygen that are critical for the growth and maintenance of the tumor. Endothelial cells are therefore the requisite members of the tumor microenvironment. The first hint that GRP78 may play a critical role in tumor angiogenesis is the constitutive high-level expression of GRP78 within the tumor vasculature of glioblastoma, suggestive of the constant state of stress of these activated tumor associated endothelial cells68. Multiple lines of evidence support the critical requirement of GRP78 for tumor neoangiogenesis. In the MMTV-PyVT transgene induced mammary tumor model, Grp78 heterozygosity showed a dramatic reduction in the microvessel density (MVD) of endogenous mammary tumors, while having no effect on the MVD of normal organs47. Wild-type syngeneic mammary tumor cells injected into Grp78 heterozygous host mice showed suppressed tumor growth and the early phase of angiogenesis. As neoangiogenesis is critical for supporting metastatic growth, injection of WT, syngeneic melanoma cells in the Grp78 heterozygous host mice also resulted in potent suppression of pulmonary metastatic lesions69. Furthermore, creation of a conditional heterozygous deletion of GRP78 in the host endothelial cells showed a severe reduction of tumor angiogenesis and metastatic growth, again with minimal effect on normal tissue MVD69. Knockdown of GRP78 expression by siRNA in immortalized human endothelial cells revealed that GRP78 regulates endothelial cell proliferation, survival and migration69. While intracellular GRP78 can contribute to tumor angiogenesis, other forms of GRP78 may also be important. Cell surface GRP78 is induced by VEGF and is required for its angiogenic signaling70. Interestingly, some tumor cell lines are capable of secreting a high amount of GRP78 into the tumor microenvironment. By binding to the cell surface receptors of endothelial cells, extracellular GRP78 activates the ERK and AKT pathways, and protects endothelial cells from anti-angiogenic effects of bortezomib35. Angiogenesis inhibitors targeting VEGF and other pro-angiogenic growth factor pathways, while showing antitumor effects, concomitantly elicit tumor adaptations leading to accelerated metastasis71,72. Considering that GRP78 regulates a much larger repertoire of cellular function than a single pro-angiogenic pathway and is a key modulator of cellular adaptation to stress, targeting GRP78 may offer a new approach to dually suppress tumor growth and angiogenesis. The role of GRP78 in the microenvironment may well extend beyond the endothelial cells. The observation that GRP78 expression is upregulated in tumor-associated macrophages but not in normal organs73 suggests that stromal cells supporting tumor growth are likely to experience stress associated with the tumor microenvironment and require GRP78 for proliferation, survival and migration. Thus, suppression of the stress induction of GRP78 may then have the added benefit of also impeding the ability of tumor-associated macrophages and other stromal cells to support tumor growth and this warrants future investigation.
Targeting GRP78 sensitizes cancer cells to therapy
Therapeutic resistance remains a major challenge in the treatment of cancer. While this issue is complex and involves multiple mechanisms, the induction of the protective elements of the UPR, such as GRP78, in both the tumor and the tumor microenvironment, particularly at the necrotic borders that are highly chemoresistant, can be a major contributing factor18,32,73. In agreement, an elevated expression of GRP78 correlates with resistance to a wide range of therapies including chemotoxic, anti-hormonal, DNA damaging and anti-angiogenesis agents in a variety of cancers, in both proliferating and dormant cancer cells as well as tumor associated endothelial cells and inversely, knockdown of GRP78 sensitizes the cells to these treatments27,38,39,68,74. As the role of intracellular and cell surface GRP78 in cancer therapy and drug resistance has been summarized in previous reviews5,33, here we focus on some new, unanticipated findings.
Histone deacetylase (HDAC) inhibitors represent a new class of anti-cancer compounds with great therapeutic potential through their ability to modulate the expression of both histone and non-histone genes resulting in growth arrest, increased differentiation and apoptosis75. However, this treatment could also lead to resistance that is not well understood. It was recently discovered that HDAC inhibitors specifically induce GRP78 without concomitantly inducing an ER or heat shock stress response76. This is due to HDAC1 binding to and acting as a repressor for the basal transcription of Grp78 and HDAC inhibitors such as trichostatin A and MS-275 relieve this suppression. Overexpression of GRP78 confers resistance to HDAC inhibitor induced apoptosis, whereas knockdown of GRP78 sensitizes cancer cells to the treatment. This provides an example that drugs that do not induce ER stress can still upregulate GRP78 through mechanisms distinct from the UPR. Another interesting observation is that in the case of estrogen-receptor positive breast cancer cells, while estrogen starvation initially lowers GRP78 expression, prolonged exposure to this adverse condition, which mimics the action of aromatase inhibitors, leads to the upregulation of GRP78, associating with hormonal resistance, which can be suppressed by the knockdown of GRP78 (ref. 41). In prostate cancer, GRP78 upregulation associates with androgen receptor status and the development of castration resistance and recurrence53,57.
With regard to drug resistance in leukemia, PI3K/AKT is constitutively active in primary AML cells from patients and blocking PI3K with inhibitor (LY294002) potentiates the response to AraC77. GRP78 is required for PI3K/AKT signaling in leukemia cells and modulation of GRP78 expression alters sensitivity to AraC23. Since knockdown of GRP78 by siRNA downregulates both intracellular and cell surface GRP78 (ref. 69), both forms of GRP78 could contribute to AraC resistance, with mechanisms involving cell surface GRP78 promoting AKT survival signaling and intracellular GRP78 suppressing caspase-7 activation by AraC23. Resistance of B-ALL cells to the anti-leukemic drug vincristine was suppressed by (−)-epigallocatechin gallate (EGCG), which inhibits the anti-apoptotic function of GRP78 by targeting its ATP-binding domain67,78. Chemoresistant B-ALL cells underwent apoptosis when exposed to a doxorubicin-conjugated penetrating cyclic anti-GRP78 peptide that targets cell surface GRP78 (ref. 67). There is also new evidence linking GRP78 over-expression to early relapse in childhood ALL23, as well as GRP78 overexpression in relapsed ALL compared to initial diagnosis67. Furthermore, ER stress induces alternative splicing of the Grp78 transcript, leading to the production of a cytosolic isoform of GRP78 (GRP78va) that also protects leukemic cells from ER stress-induced cell death79.
Collectively, these discoveries identify GRP78 as a novel therapeutic target against chemoresistance in cancer cells. In agreement, recent studies showed that therapies capable of reducing the level of GRP78 in cancer cells are effective in enhancing cancer cell death, sensitizing them to chemotherapy treatment and reducing xenograft roles5,33,67,80–85.
Cell surface GRP78 is a novel anti-cancer target
Evidence is emerging that a subfraction of GRP78 can be localized on the cell surface of cancer cells as well as cells undergoing ER stress36,48. The preferential expression of GRP78 on the surface of tumor cells in vivo enables specific tumor targeting by peptidic GRP78 ligands linked to cytotoxic agents for anti-cancer therapy without harmful effects on normal organs86–89. This is consistent with a report that a phage display-derived human monoclonal antibody that recognizes GRP78 binds strongly to multiple types of cancer cells, but shows weak or no binding to the corresponding normal tissues90. However, it should be cautioned that studies on cell surface GRP78 expression performed in tissue culture show much variability among different cell lines36. This is probably in part due to the inherent differences among cell lines that have been maintained long term in tissue culture. In addition, the stressful conditions of tissue culture may artificially induce cell surface localization of GRP78 in non-transformed cells. Another possibility is rapid endocytosis of cell surface GRP78 making its detection by acute methods not being reliable. Thus far, in vivo studies consistently showed preferential expression of surface GRP78 in tumors compared to normal tissues. A human monoclonal IgM antibody (SAM6) that was isolated from a gastric cancer patient bound to an O-linked glycosylated form of cell surface GRP78 in malignant cells and induced intracellular lipids accumulation prior to apoptosis induction in cancer cells91. Currently, a Phase I melanoma study is being conducted to test the efficacy of this antibody. Furthermore, hypoxic/stressed endothelial cells express cell surface GRP78, which is induced by VEGF70. Taken together, these findings raise the exciting idea that agents against cell surface GRP78 can dually target tumor cells and tumor vasculature while sparing normal organs.
Cell surface calreticulin and immunogenic chemotherapy
Calreticulin (CRT) is an evolutionarily conserved 46 kDa ER luminal protein traditionally regarded as a Ca2+ homeostasis regulator and an ER chaperone92–95. Additionally, CRT regulates other cellular processes, including cell adhesion, MHC class I molecules assembly and hormone-sensitive gene expression. While the majority of CRT resides in the ER, evidence from various studies indicates that CRT is also found on the cell surface and can be secreted from cells under specific conditions92,96.
Cell surface CRT induces immunogenic tumor cell death
CRT was demonstrated to be expressed on the surface of tumor cells treated with apoptotic inducing agents such as anthracyclines, oxaliplatin, and ionizing irradiation97,98. Additionally, cell surface CRT (ecto-CRT) functions as an “eat me” signal that is recognized by dendritic cells (DC), and elicits DC-mediated phagocytosis of tumor cells99. Phagocytosis leads to increased tumor antigen cross-presentation, which activates cytotoxic T cells and triggers an antitumor immune response and immunogenic tumor cell death. In human cancer samples, ecto-CRT expression is enhanced in both solid tumors and hematologic malignancies when compared to the corresponding normal tissues, which could possibly explain the increased susceptibility of tumors in immune-based cancer therapy compared to normal cells100. In acute myeloid leukemia patients, the presence of ecto-CRT on leukemia blast cells undergoing anthracyclin treatment positively correlates with the cellular anti-cancer immune response101.
The recent discovery that CRT is presented on the cell surface offers a new anti-cancer therapeutic strategy based on ecto-CRT mediated immunogenic chemotherapy102. For example, human colon cancer cells that were treated with anthracycline in culture, and then injected into mice, prevented the tumor formation induced by the administration of non-anthracycline treated colon cancer cells. Furthermore, intratumoral injection of recombinant CRT combined with the chemotherapeutic agent Mitomycin C cured already established tumors in the same mouse model102. These observations were also confirmed in fibrosarcoma and mammary carcinoma cells. Importantly, this tumor immunity was specific against autologous tumors, as opposed to unrelated tumors102. Interestingly, the immunogenic tumor cell death induced by ecto-CRT appears to require both cell surface CRT and apoptotic signals. CRT exposure driven by ER stress restores the immunogenicity caused by cisplatin, an apoptosis inducer that fails to induce CRT exposure by itself103. To induce immunogenicity, ecto-CRT must present on the surface of apoptotic cells, as opposed to the surface of adjacent live cells. Recombinant CRT that was absorbed onto the surface of tumor cells could trigger immunogenic tumor cell death when substituted for endogenous CRT that has translocated to the cell surface99,102, demonstrating the direct requirement of cell surface CRT in triggering immunogenic tumor cell death. Collectively, such tumor specificity raises the possibility of personalized tumor vaccination where primary tumor cells from a patient could be treated with anthracyclines ex vivo, and subsequently injected back into the patient in order to both eradicate existing tumor cells and to induce long-term antitumor immunity.
Cell surface CRT exposure requires PERK activation and apoptotic effectors
The cell surface exposure of CRT in immunogenic cell death results from the translocation of endogenous CRT and is unrelated to the enhanced expression of CRT within the cell104. Upon anthracycline treatment, only CRT and ERp5 are detected on the surface of tumor cells while other ER chaperones, including GRP78, GRP94, PDI or CNX are not. ERp57 and CRT form a complex in the lumen of the ER, and translocate together to the cell surface105,106. The translocation process requires the activation of PERK, but not IRE1α or ATF6. Blocking the function of PERK-eIF2α activation using PERK-specific shRNA or non-phosphorylatable eIF2α-S51A mutant abolished CRT translocation, whereas inhibiting the function of GADD34 and PP1 complex, which is involved in eIF2α dephosphorylation, enhanced the surface exposure of CRT98,103,104,107. However, ER stress alone does not induce CRT/ERp57 translocation; additional apoptotic effectors are required. Caspase-8 cleavage downstream of PERK-activated p-eIF2α phosphorylation is required for the cleavage of an ER-sessile protein called Bap31. Bap31 is a Bcl-2 binding protein and cleaved Bap31 binds to Bcl-2 (ref. 108). This binding may compete with the binding of Bcl-2 to Bax, and could potentially lead to the activation of Bax and Bak, which serve as the apoptotic effectors required for CRT/ERp57 translocation downstream of PERK104. The physical translocation of CRT/ERp57 to the cell surface can be blocked by the anterograde protein transport inhibitor brefeldin A, the actin skeleton inhibitor latrunculin B, or the knockdown of SNAREs, suggesting a role for the ER-Golgi secretory pathway104. Nonetheless, the precise roles of Bap31, Bax and Bak, or how CRT/ERp57 translocates from the ER to the cell surface have not been well elucidated.
The role of GRP94 and other chaperones in antitumor vaccination
GRP94, also known as gp96, is a major glycoprotein in the ER with functions beyond protein folding and processing4. GRP94 plays an important role in immune responses by facilitating MHC class I molecule mediated antigen presentation, and by inducing the maturation and activation of various cells involved in innate and adapted immune responses, including macrophages, DCs, T cells and B cells109–111. Therefore, it might be expected that GRP94 could elicit an antitumor response. Indeed, several studies demonstrated that a vaccination of lethally irradiated cancer cells expressing various non-ER-retainable autologous GRP94 fusion proteins (devoid of the ER retention/retrieval signal KDEL) protected mice from primary tumor growth as well as metastasis112–114. One explanation for this effect could be that autologous tumor-derived secretory GRP94 stimulates the maturation of macrophages and DCs, enhances the antigen cross-presentation and amplifies the inflammatory signals109,110. As an alternative to autologous GRP94, a pooled GRP94 vaccine derived from different multiple myeloma cells was shown to be as effective as the autologous GRP94 vaccine when tested in mouse models115. Taken together, these studies demonstrate that the use of GRP94 as an antitumor vaccination can prevent cancer development. However, GRP94 vaccination in mice appears less promising in the treatment of established tumors114,116,117, and clinical trials across various types of tumors suggest that autologous tumor-derived GRP94 vaccination may have limited efficacy118–121.
Tumor-derived GRP78 is also capable of eliciting an antitumor response and inhibiting tumor growth in mouse models with established tumors as well as inducing an antitumor memory response in mice rechallenged with the tumor cells122. One explanation for this effect could be that KDEL-deleted secretable GRP78 is recognized by DCs and cross-presented with associated antigens by MHC class I molecules, leading to the activation of cytotoxic T cells. Additionally, heat shock proteins HSP70, HSP110 and GRP170 derived from tumors have been shown to function as antitumor vaccines123–126. Evidence suggests that vaccination with mixed heat shock proteins or heat shock proteins with other antigens may increase the antitumor effect. In a mouse model injected with sarcoma cells, tumor regression and long-term survival is shown in the mice treated with a mixed heat shock protein antitumor vaccine (GRP94, HSP60, HSP70 and HSP110)127.
The role of PDI in tumorigenesis
Protein disulfide isomerase (PDI) is a thiol-disulfide oxidoreductase that is recognized for catalyzing the formation of disulfide bonds in newly synthesized proteins in the lumen of the ER128–130. PDI is also found in the cytosol, nucleus, on the cell surface, and can be secreted131 (Figure 3). Studies on the role of PDI in cancer have demonstrated a pro-oncogenic, pro-survival function for PDI in cancer and therapeutic resistance. Similar to other ER chaperones, PDI upregulation in response to ER stress helps ameliorate misfolded proteins and stress-induced apoptosis132,133. Thus, inhibiting PDI activity sensitizes cells to stress-induced apoptosis. Treatment of melanoma cells with the PDI inhibitor bacitracin enhanced the stress response and apoptosis in the presence of chemotherapeutic drugs134. PDI is highly expressed in invasive glioma cells and the invasive edge of human glioblastomas. Furthermore, suppression of PDI activity with an anti-PDI antibody, or the specific PDI inhibitor bacitracin, reduced tumor cell migration and invasion in vitro135. Beyond these roles in tumor progression, PDI may also be important for tumor formation. A recent study demonstrated a novel role of PDI in AML tumorigenesis, in which PDI suppressed the expression of CEBPA by binding to the stem loop region of its mRNA, and therefore blocking normal neutrophil differentiation136. PDI has also been reported to induce antibody production and evoke a potent humoral immune response in GM-CSF secreting cancer cells137. Therefore, because PDI is expressed on the surface of various murine and human cancer cell lines, and plays important roles in tumorigenesis, it presents a potential immune target which may enhance the efficacy of anti-cancer therapy135,137.
The role of PERK in tumorigenesis
PERK is an ER transmembrane serine/threonine protein kinase that is activated in response to ER stress1,2. Upon UPR activation, PERK phosphorylates its two major substrates: eukaryotic translation initiation factor 2α (eIF2α) and nuclear factor-like 2 (Nrf2)138 (Figure 1). The phosphorylation of eIF2α acts to attenuate global protein translation, while at the same time enhancing the translation of select mRNAs, such as ATF4 (ref. 139), The phosphorylation of Nrf2 activates the expression of enzymes that are required for ROS quenching140,141.
PERK plays a critical role during fetal development since the PERK−/− mice that survive are characterized by severe postnatal growth retardation, skeletal dysplasia and neonatal diabetes, all of which are also symptoms presented in the human Wolcott-Rallison syndrome142. PERK is required for osteoblast proliferation, differentiation and bone matrix collagen I secretion143. PERK is also required for pancreatic β cell development. Despite displaying a morphologically and functionally normal pancreas at birth, mice with global PERK knockout rapidly developed hyperglycemia and died142,144,145. It was demonstrated that β cell development occurs at E13.5–16.5, and impaired β cell proliferation and differentiation was observed in PERK−/− mice from day E13.5 to 18.5 (ref. 145). Interestingly, mice with β cell specific PERK knockout expressed postnatally are normo-glycemic and maintain glycemia throughout the lifespan145. Taken together, these studies suggest that the defect of PERK−/− β cell development at the embryonic stage is permanent such that when β cells begin to play an important role in regulating blood glucose, the PERK deficiency starts to exert its effect and eventually leads to the hyperglycemic and neonatal diabetic phenotypes.
Dual role of PERK in tumor proliferation and survival
In the case of tumorigenesis and cancer progression, PERK exhibits both pro- and antitumor properties. PERK promoted tumor survival and angiogenesis under conditions of ER stress caused by hypoxia, as was demonstrated in a xenograft tumor model derived from K-Ras or Ki-RasV12 transformed Perk−/− mouse embryonic fibroblasts (MEFs)146,147. Similar observations were made in a xenograft model using human colorectal carcinoma cells expressing a dominant negative PERK146. In these models, PERK-deficient tumors showed increased apoptosis in hypoxic regions, were poorly vascularized, and these effects were attributed to the loss of eIF2α and ATF4. The requirement of PERK in tumor angiogenesis was further confirmed using a mouse PERK−/− insulinoma model, in which PERK−/− tumors had lower vascularity compared to size-matched PERK+/+ tumors148. PERK is not the only kinase that can phosphorylate eIF2α and regulate cell survival. GCN2 is another eIF2α kinase demonstrated to work in parallel to PERK to regulate eIF2α-mediated cell survival under conditions of hypoxia and nutrient deprivation in immortalized MEFs, human fibrosarcoma, and colorectal adenocarcinoma cells149–151. Mechanisms by which eIF2α phosphorylation promoted cell survival were attributed to cell cycle G1 arrest, mediated by the downregulation of cyclin D1 and the induction of HIF-1α and p21WAF1 (refs. 149,150), and autophagy, mediated by ATF4 and CHOP152–154. Moreover, it has been shown that the expression of asparagine synthetase (ASNS), which is a downstream target of ATF4, partially restored growth in tumors with compromised ATF4 function151. Although phosphorylation of eIF2α attenuates protein translation and protects cells from apoptosis under ER stress, under severe stress such as bortezomib treatment, the downstream pro-apoptotic factor CHOP was activated and the cancer cells underwent apoptosis155. Bortezomib-resistant multiple myeloma cells displayed attenuated eIF2α phosphorylation, and became susceptible when eIF2α dephosphorylation was inhibited.
The role of PERK in cell proliferation has been controversial. In early studies, PERK-eIF2α was indicated to function as a barrier in the transformation of normal cells to cancer cells. Expression of a nonphosphorylatable eIF2α mutant led to the transformation of mouse fibroblasts, and facilitated the transformation of human fibroblasts in conjunction with hTERT and the SV40 large T-antigen156,157. Non-tumorigenic mammary epithelial cells with a dominant negative PERK or a kinase dead PERK mutant showed increased proliferation, displayed hyperplastic growth, and developed into small tumor nodules after orthotopic implantation158. In human squamous carcinoma cells, pharmacologically induced PERK activation caused growth arrest in vitro and suppressed tumor growth in vivo159. This study implies that PERK activation in established tumors is sufficient to inhibit tumor proliferation. This suppression of tumor cell proliferation by PERK may be attributed to the eIF2α-mediated inhibition of cyclin D1 synthesis26,149,159. However, other studies indicate that PERK is required for cancer cell proliferation. PERK−/− insulinoma induced by β-cell specific expression of SV40 large T-antigen displayed reduced proliferation compared to PERK+/+ controls148. Cell cycle arrest was observed upon PERK knockdown by shRNA in human breast carcinoma and esophageal carcinoma cells via reduced Nrf2 activity. The same study demonstrated that mammary gland specific PERK deletion delayed tumor onset in MMTV-Neu breast cancer mice160. Phosphorylated Nrf2 activates the expression of enzymes required for ROS elimination. Therefore, reduced Nrf2 activity leads to ROS accumulation which causes cell cycle arrest mediated by increased DNA damage. Collectively, these observations demonstrate the complexity and specificity of PERK in tumorigenesis and cancer progression, and illustrate that the role of PERK is dependent on its expression and activation, as well as cancer cell type, proliferation status, tumor microenvironment, and the model system studied. Therefore, one can imagine the challenges involved in targeting PERK as an effective and generalized anti-cancer therapy. For instance, the suppression of tumor growth by either over-activation or depletion of PERK might also drive proliferative cancer cells into dormancy and thereby protect them from chemotherapy.
The role of PERK in autophagy
Autophagy can be activated upon ER stress as a defensive mechanism for maintaining ER homeostasis10,16,161. Depending on the context, autophagy either enhances cell survival by counterbalancing ER expansion or commits the cell to non-apoptotic death 162. Studies have shown that autophagy alleviated ER stress-induced cell death in cancer cells while promoting cell death in non-transformed cells10. As two important mechanisms regulating ER homeostasis, the UPR and autophagy are coupled via the PERK-eIF2α-ATF4 pathway. The requirement of the PERK-eIF2α-ATF4 pathway in mediating autophagy is shown in different models, in which autophagy is induced by polyglutamine, radiation, ECM detachment and hypoxia17,152–154,163. In response to ER stress, PERK phosphorylates eIF2α and activates its downstream transcription factor ATF4, which induces the expression of another transcription factor CHOP. Studies have shown that ATF4 and CHOP bind to the promoter of two genes essential for autophagy. ATF4 activates the expression of MAP1LC3B, while CHOP activates the transcription of ATG5 (ref. 152). MAP1LC3B and ATG5 are two essential proteins for autophagy. ATG5-dependent conversion of free LC3-I to membrane-bound LC3-II is an essential step for autophagosome formation17, whereas MAP1LC3B induction is necessary for replenishing the rapid degradation of MAP1LC3B during the prolonged autophagy152. Interestingly, ATF4 also induces autophagy in a PERK-independent manner. The induction of autophagy by Bortezomib treatment was dependent on the proteasome stabilization of ATF4 and the upregulation of LC3B by ATF4 (ref. 164). However, it was also suggested that the UPR and autophagy are linked by IRE1, rather than PERK. The accumulation of LC3-positive vesicles triggered by tunicamycin or thapsigargin was shown to depend on IRE1 but not PERK or ATF6, using MEFs or embryonic stem cells deficient for IRE1α, ATF6 or PERK16.
The role of IRE1-XBP1 axis in tumorigenesis
XBP1 is a transcription factor downstream of the IRE1α endonuclease165–167. It has been shown to be essential for the development and function of various organs, especially secretory organs. XBP1 knockout mouse embryos died of liver failure and severe anemia. Mice with liver-specific overexpression of XBP1 were rescued from the embryonic lethality, but died shortly postnatal with impaired secretory apparati including the pancreas and salivary glands165. XBP1 and its splicing are also essential for plasma cell differentiation and antibody secretion7,168.
XBP1 splicing is widely observed in both solid and hematological tumors, and enhanced XBP-1 splicing has been associated with more malignant phenotypes and poor survival169–171. XBP1 activation was required for tumor survival in solid tumors that undergo hypoxic stress172. In breast cancer cells, XBP1 was shown to be a key factor affecting estrogen dependency. Overexpression of XBP1-s in estrogen-dependent breast cancer cell lines conferred an increased expression of estrogen receptors and estrogen-independent growth173. In the case of virus-associated cancers such as the Epstein-Barr virus associated nasopharyngeal carcinoma, XBP1 was shown to bind to the promoter of EBV oncoprotein LMP1 and activate its expression174. Other than promoting cancer cell survival and facilitating tumorigenesis along with other oncogenes, XBP1 splicing itself may lead to tumorigenesis, as the expression of XBP1-s alone in B cells and plasma cells drove the pathogenesis of multiple myeloma with features mimicking the classic hallmarks of human multiple myeloma175.
Since XBP1 is the only known substrate of IRE1α167, studies on IRE1α also shed light on the role of the IRE1α-XBP1 pathway in tumorigenesis. Using prostate cancer cell lines with IRE1α mutants that are either kinase-defective or with drug-inducible RNase activity, IRE1α was reported to promote cell proliferation in vitro by regulating the expression of cyclin A1 through XBP1 splicing176. In a xenograft model with glioma cells expressing a dominant-negative IRE1α (dnIRE1α), dnIRE1α expressing cancer cells had a lower proliferation rate in vitro and in vivo177. This xenograft model also revealed that IRE1α was required for angiogenesis and functioned as a key switch between the angiogenesis and the invasion of glioma. Wild-type gliomas showed the angiogenic/massive phenotype whereas tumors expressing dnIRE1α displayed the avascular/diffuse phenotype. Inhibition of IRE1α function diminished the expression of potent pro-angiogenic factors, leading to a compensatory response in glioma cells to migrate and hijack host blood vessels in response to their poor angiogenesis ability. The requirement of IRE1 in tumor angiogenesis under hypoxia or glucose deprivation could be attributed to its role in regulating VEGF-A expression178. However, the link between the IRE1-XBP1 axis, angiogenesis, and tumor growth can also occur in a VEGF-independent manner. It was shown that secretion of VEGF was not compromised when XBP1 was inhibited using an shRNA approach172,179. Additionally, IRE1α was recently reported to regulate the survival/apoptosis switch in the UPR180. Under mild ER stress conditions, IRE1α preferentially splices XBP1 to promote cell survival; while under conditions of overwhelming stress or chronic ER stress, it causes endonucleolytic decay of ER-localized mRNA, including those encoding ER chaperones such as GRP78, and renders apoptosis180–182.
The important role of the IRE1α-XBP1 pathway in cancer progression presents a potential therapeutic target in anti-cancer therapy. Recently a small-molecule inhibitor targeting the endonuclease activity of IRE1α was identified and tested in a multiple myeloma model183. This inhibitor blocked XBP1 splicing induced by ER stress both in vitro and in vivo. Treatment with this small molecule inhibited the growth of human multiple myeloma cells xenografted in host mice, and displayed cytotoxicity specifically on the multiple myeloma cells rather than normal blood cells freshly isolated from patients. Along with this small molecule inhibitor, another IRE1α inhibitor that is salicylaldehyde-based was used in rhabdovirus mediated oncolytic viral therapy184, in which natural or genetically engineered replicating viruses selectively targeted tumor cells based on their altered genetic properties185. Blockage of the UPR by this IRE1α inhibitor sensitized cancer cells to apoptosis in response to rhabdovirus infection and dramatically improved the oncolytic efficacy184.
Conclusion
While extensive cell culture studies have clearly implicated the contribution of ER chaperones and the UPR in cancer cell proliferation, survival and therapeutic resistance, the fruition of mouse models of cancer which allowed for the dissection of the physiological roles of various components of the UPR in relation to their effects on normal organ development and homeostasis validated their unique roles in tumorigenesis as well as in tumor angiogenesis. Evidence is also emerging that ER stress not only arms cancer cells with prosurvival adaptive pathways to combat the host surveillance mechanisms, it also promotes a vicious feedback cycle where cancer cells and stroma cells in the tumor microenvironment cross stimulate each other leading to tumor progression and metastasis. Knowledge of the involvement of ER stress and the UPR in the tumor microenvironment is at an early stage and offers exciting new opportunities for investigation. The discovery that major ER chaperones such as GRP78, GRP94, CRT and PDI all exhibit a cell surface form with activities distinct from those of the ER forms changes the paradigm of their function and expands their role in tumor progression and therapeutic resistance. While ER stress can be exploited to enhance tumor cell death, tumor cells are likely to acquire resistance to such therapy, in part by upregulating the pro-survival arms of the UPR such as the ER chaperones. With therapeutics targeting ER chaperones and UPR pathways currently in development, the next major breakthrough will come from directly testing whether these agents will be effective in reversing the course of cancer, either alone or in combination with existing therapy, and their impact on normal cell physiology.
Acknowledgments
B. L. is a recipient of the University of Southern California Norris Comprehensive Cancer Center Wang Scholarship in Cancer Research. We thank Costas Koumenis, Albert Koong, Kyle Pfaffenbach and Kate Ott for helpful discussions. This work was supported in part by National Institutes of Health grants CA027607 and 1 P01 AG034906 to A.S.L.
Footnotes
Conflict of Interest
None
References
- 1.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 4.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diehl JA, Fuchs SY, Koumenis C. The cell biology of the unfolded protein response. Gastroenterology. 2011;141:38–41. 41 e31–32. doi: 10.1053/j.gastro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 8.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22:4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 11.Yorimitsu T, Klionsky DJ. Endoplasmic reticulum stress: a new pathway to induce autophagy. Autophagy. 2007;3:160–162. doi: 10.4161/auto.3653. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruse KB, Brodsky JL, McCracken AA. Characterization of an ERAD gene as VPS30/ATG6 reveals two alternative and functionally distinct protein quality control pathways: one for soluble Z variant of human alpha-1 proteinase inhibitor (A1PiZ) and another for aggregates of A1PiZ. Mol Biol Cell. 2006;17:203–212. doi: 10.1091/mbc.E04-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Momoi T. Conformational diseases and ER stress-mediated cell death: apoptotic cell death and autophagic cell death. Curr Mol Med. 2006;6:111–118. doi: 10.2174/156652406775574596. [DOI] [PubMed] [Google Scholar]
- 16.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 19.Khan MM, Nomura T, Chiba T, Tanaka K, Yoshida H, Mori K, et al. The fusion oncoprotein PML-RARalpha induces endoplasmic reticulum (ER)-associated degradation of N-CoR and ER stress. J Biol Chem. 2004;279:11814–11824. doi: 10.1074/jbc.M312121200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Soboloff J, Zhu Z, Berger SA. Inhibition of Ca2+ influx is required for mitochondrial reactive oxygen species-induced endoplasmic reticulum Ca2+ depletion and cell death in leukemia cells. Mol Pharmacol. 2006;70:1424–1434. doi: 10.1124/mol.106.024323. [DOI] [PubMed] [Google Scholar]
- 21.Tanimura A, Yujiri T, Tanaka Y, Hatanaka M, Mitani N, Nakamura Y, et al. The anti-apoptotic role of the unfolded protein response in Bcr-Abl-positive leukemia cells. Leuk Res. 2009;33:924–928. doi: 10.1016/j.leukres.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Rosati E, Sabatini R, Rampino G, De Falco F, Di Ianni M, Falzetti F, et al. Novel targets for endoplasmic reticulum stress-induced apoptosis in B-CLL. Blood. 2010;116:2713–2723. doi: 10.1182/blood-2010-03-275628. [DOI] [PubMed] [Google Scholar]
- 23.Wey S, Luo B, Tseng CC, Ni M, Zhou H, Fu Y, et al. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Blood. 2012;119:817–825. doi: 10.1182/blood-2011-06-357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira ER, Liao N, Neale GA, Hendershot LM. Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012521. pii: e12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci USA. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranganathan AC, Adam AP, Zhang L, Aguirre-Ghiso JA. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5:729–735. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci USA. 2011;108:6561–6566. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, et al. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer Res. 2008;68:9323–9330. doi: 10.1158/0008-5472.CAN-08-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20:400–413. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 33.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–156. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kern J, Untergasser G, Zenzmaier C, Sarg B, Gastl G, Gunsilius E, et al. GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood. 2009;114:3960–3967. doi: 10.1182/blood-2009-03-209668. [DOI] [PubMed] [Google Scholar]
- 36.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouyang YB, Xu LJ, Emery JF, Lee AS, Giffard RG. Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion. 2011;11:279–286. doi: 10.1016/j.mito.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 39.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 40.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen-starvation induced apoptosis. Cancer Res. 2007;67:3734–3740. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 41.Zhou H, Zhang Y, Fu Y, Chan L, Lee AS. Novel mechanism of anti-apoptotic function of 78 kDa glucose-regulated protein (GRP78) J Biol Chem. 2011;286:25687–25696. doi: 10.1074/jbc.M110.212944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, et al. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penas C, Font-Nieves M, Fores J, Petegnief V, Planas A, Navarro X, et al. Autophagy, and BiP level decrease are early key events in retrograde degeneration of motoneurons. Cell Death Differ. 2011;18:1617–1627. doi: 10.1038/cdd.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4:443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- 45.Oppermann M, Geilen CC, Fecker LF, Gillissen B, Daniel PT, Eberle J. Caspase-independent induction of apoptosis in human melanoma cells by the proapoptotic Bcl-2-related protein Nbk/Bik. Oncogene. 2005;24:7369–7380. doi: 10.1038/sj.onc.1208890. [DOI] [PubMed] [Google Scholar]
- 46.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Liu R, Ni M, Gill P, Lee AS. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 2010;285:15065–15075. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299–2306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 50.Misra UK, Payne S, Pizzo SV. Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop: a role for secreted prostate-specific antigen. J Biol Chem. 2011;286:1248–1259. doi: 10.1074/jbc.M110.129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol. 2008;28:666–677. doi: 10.1128/MCB.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miharada K, Karlsson G, Rehn M, Rorby E, Siva K, Cammenga J, et al. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell. 2011;9:330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Pootrakul L, Datar RH, Shi SR, Cai J, Hawes D, Groshen SG, et al. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–5993. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 54.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang L, Scolyer RA, Lee CS, McCarthy SW, Cooper WA, Zhang XD, et al. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–470. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 56.Wu MJ, Jan CI, Tsay YG, Yu YH, Huang CY, Lin SC, et al. Elimination of head and neck cancer initiating cells through targeting glucose regulated protein78 signaling. Mol Cancer. 2010;9:283. doi: 10.1186/1476-4598-9-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan SS, Ahmad I, Bennett HL, Singh L, Nixon C, Seywright M, et al. GRP78 up-regulation is associated with androgen receptor status, Hsp70-Hsp90 client proteins and castrate-resistant prostate cancer. J Pathol. 2011;223:81–87. doi: 10.1002/path.2795. [DOI] [PubMed] [Google Scholar]
- 58.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci USA. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, et al. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci U S A. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartkowiak K, Effenberger KE, Harder S, Andreas A, Buck F, Peter-Katalinic J, et al. Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J Proteome Res. 2010;9:3158–3168. doi: 10.1021/pr100039d. [DOI] [PubMed] [Google Scholar]
- 61.Ota J, Yamashita Y, Okawa K, Kisanuki H, Fujiwara S, Ishikawa M, et al. Proteomic analysis of hematopoietic stem cell-like fractions in leukemic disorders. Oncogene. 2003;22:5720–5728. doi: 10.1038/sj.onc.1206855. [DOI] [PubMed] [Google Scholar]
- 62.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 64.Misra UK, Pizzo SV. Ligation of cell surface GRP78 with antibody directed against the COOH-terminal domain of GRP78 suppresses Ras/MAPK and PI 3-kinase/AKT signaling while promoting caspase activation in human prostate cancer cells. Cancer Biol Ther. 2010;9:142–152. doi: 10.4161/cbt.9.2.10422. [DOI] [PubMed] [Google Scholar]
- 65.Verras M, Papandreou I, Lim AL, Denko NC. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:7212–7224. doi: 10.1128/MCB.00947-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002;118:521–534. doi: 10.1046/j.1365-2141.2002.03637.x. [DOI] [PubMed] [Google Scholar]
- 67.Uckun FM, Qazi S, Ozer Z, Garner AL, Pitt J, Ma H, et al. Inducing apoptosis in chemotherapy-resistant B-lineage acute lymphoblastic leukaemia cells by targeting HSPA5, a master regulator of the anti-apoptotic unfolded protein response signalling network. Br J Haematol. 2011;153:741–752. doi: 10.1111/j.1365-2141.2011.08671.x. [DOI] [PubMed] [Google Scholar]
- 68.Virrey JJ, Dong D, Stiles C, Patterson JB, Pen L, Ni M, et al. Stress chaperone GRP78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res. 2008;6:1268–1275. doi: 10.1158/1541-7786.MCR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong D, Stapleton C, Luo B, Xiong S, Ye W, Zhang Y, et al. A critical role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Cancer Res. 2011;71:2848–2857. doi: 10.1158/0008-5472.CAN-10-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katanasaka Y, Ishii T, Asai T, Naitou H, Maeda N, Koizumi F, et al. Cancer antineovascular therapy with liposome drug delivery systems targeted to BiP/GRP78. Int J Cancer. 2010;127:2685–2698. doi: 10.1002/ijc.25276. [DOI] [PubMed] [Google Scholar]
- 71.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong D, Dubeau L, Bading J, Nguyen K, Luna M, Yu H, et al. Spontaneous and controllable activation of suicide gene expression driven by the stress-inducible grp78 promoter resulting in eradication of sizable human tumors. Hum Gene Ther. 2004;15:553–561. doi: 10.1089/104303404323142006. [DOI] [PubMed] [Google Scholar]
- 74.Dong D, Ko B, Baumeister P, Swenson S, Costa F, Markland F, et al. Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res. 2005;65:5785–5791. doi: 10.1158/0008-5472.CAN-05-0754. [DOI] [PubMed] [Google Scholar]
- 75.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 76.Baumeister P, Dong D, Fu Y, Lee AS. Transcriptional induction of GRP78/BiP by histone deacetylase inhibitors and resistance to histone deacetylase inhibitor-induced apoptosis. Mol Cancer Ther. 2009;8:1086–1094. doi: 10.1158/1535-7163.MCT-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grandage VL, Gale RE, Linch DC, Khwaja A. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kappaB, Mapkinase and p53 pathways. Leukemia. 2005;19:586–594. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- 78.Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma WY, et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9269. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 79.Ni M, Zhou H, Wey S, Baumeister P, Lee AS. Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP. PLoS One. 2009;4:e6868. doi: 10.1371/journal.pone.0006868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu DH, Macdonald J, Liu G, Lee AS, Ly M, Davis T, et al. Pyrvinium targets the unfolded protein response to hypoglycemia and its anti-tumor activity is enhanced by combination therapy. PLoS One. 2008;3:e3951. doi: 10.1371/journal.pone.0003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Backer JM, Krivoshein AV, Hamby CV, Pizzonia J, Gilbert KS, Ray YS, et al. Chaperone-targeting cytotoxin and endoplasmic reticulum stress-inducing drug synergize to kill cancer cells. Neoplasia. 2009;11:1165–1173. doi: 10.1593/neo.09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li M, Wang J, Jing J, Hua H, Luo T, Xu L, et al. Synergistic promotion of breast cancer cells death by targeting molecular chaperone GRP78 and heat shock protein 70. J Cell Mol Med. 2009;13:4540–4550. doi: 10.1111/j.1582-4934.2008.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim JY, Hwang JH, Cha MR, Yoon MY, Son ES, Tomida A, et al. Arctigenin blocks the unfolded protein response and shows therapeutic antitumor activity. J Cell Physiol. 2010;224:33–40. doi: 10.1002/jcp.22085. [DOI] [PubMed] [Google Scholar]
- 84.Martin S, Hill DS, Paton JC, Paton AW, Birch-Machin MA, Lovat PE, et al. Targeting GRP78 to enhance melanoma cell death. Pigment Cell Melanoma Res. 2010;23:675–682. doi: 10.1111/j.1755-148X.2010.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roue G, Perez-Galan P, Mozos A, Lopez-Guerra M, Xargay-Torrent S, Rosich L, et al. The Hsp90 inhibitor IPI-504 overcomes bortezomib resistance in mantle cell lymphoma in vitro and in vivo by down-regulation of the prosurvival ER chaperone BiP/Grp78. Blood. 2011;117:1270–1279. doi: 10.1182/blood-2010-04-278853. [DOI] [PubMed] [Google Scholar]
- 86.Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 87.Kim Y, Lillo AM, Steiniger SC, Liu Y, Ballatore C, Anichini A, et al. Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry. 2006;45:9434–9444. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Steiniger SC, Kim Y, Kaufmann GF, Felding-Habermann B, Janda KD. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 2007;4:435–447. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sato M, Yao VJ, Arap W, Pasqualini R. GRP78 signaling hub a receptor for targeted tumor therapy. Adv Genet. 2010;69:97–114. doi: 10.1016/S0065-2660(10)69006-2. [DOI] [PubMed] [Google Scholar]
- 90.Jakobsen CG, Rasmussen N, Laenkholm AV, Ditzel HJ. Phage display derived human monoclonal antibodies isolated by binding to the surface of live primary breast cancer cells recognize GRP78. Cancer Res. 2007;67:9507–9517. doi: 10.1158/0008-5472.CAN-06-4686. [DOI] [PubMed] [Google Scholar]
- 91.Rauschert N, Brandlein S, Holzinger E, Hensel F, Muller-Hermelink HK, Vollmers HP. A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab Invest. 2008;88:375–386. doi: 10.1038/labinvest.2008.2. [DOI] [PubMed] [Google Scholar]
- 92.Johnson S, Michalak M, Opas M, Eggleton P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 2001;11:122–129. doi: 10.1016/s0962-8924(01)01926-2. [DOI] [PubMed] [Google Scholar]
- 93.Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37:260–266. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 94.Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. J Biol Chem. 2008;283:10221–10225. doi: 10.1074/jbc.R700048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- 96.Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 98.Martins I, Kepp O, Galluzzi L, Senovilla L, Schlemmer F, Adjemian S, et al. Surface-exposed calreticulin in the interaction between dying cells and phagocytes. Ann N Y Acad Sci. 2010;1209:77–82. doi: 10.1111/j.1749-6632.2010.05740.x. [DOI] [PubMed] [Google Scholar]
- 99.Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L, et al. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res. 2007;67:7941–7944. doi: 10.1158/0008-5472.CAN-07-1622. [DOI] [PubMed] [Google Scholar]
- 100.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wemeau M, Kepp O, Tesniere A, Panaretakis T, Flament C, De Botton S, et al. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis. 2010;1:e104. doi: 10.1038/cddis.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 103.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–1158. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 104.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Obeid M. ERP57 membrane translocation dictates the immunogenicity of tumor cell death by controlling the membrane translocation of calreticulin. J Immunol. 2008;181:2533–2543. doi: 10.4049/jimmunol.181.4.2533. [DOI] [PubMed] [Google Scholar]
- 106.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 107.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 108.Ng FW, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA, et al. p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139:327–338. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Podack ER, Raez LE. Allogeneic tumor-cell-based vaccines secreting endoplasmic reticulum chaperone gp96. Expert Opin Biol Ther. 2007;7:1679–1688. doi: 10.1517/14712598.7.11.1679. [DOI] [PubMed] [Google Scholar]
- 110.Strbo N, Podack ER. Secreted heat shock protein gp96-Ig: an innovative vaccine approach. Am J Reprod Immunol. 2008;59:407–416. doi: 10.1111/j.1600-0897.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 111.di Pietro A, Tosti G, Ferrucci PF, Testori A. The immunological era in melanoma treatment: new challenges for heat shock protein-based vaccine in the advanced disease. Expert Opin Biol Ther. 2011;11:1395–1407. doi: 10.1517/14712598.2011.605353. [DOI] [PubMed] [Google Scholar]
- 112.Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med. 2002;196:1447–1459. doi: 10.1084/jem.20020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu S, Wang H, Yang Z, Kon T, Zhu J, Cao Y, et al. Enhancement of cancer radiation therapy by use of adenovirus-mediated secretable glucose-regulated protein 94/gp96 expression. Cancer Res. 2005;65:9126–9131. doi: 10.1158/0008-5472.CAN-05-0945. [DOI] [PubMed] [Google Scholar]
- 114.Schreiber TH, Deyev VV, Rosenblatt JD, Podack ER. Tumor-induced suppression of CTL expansion and subjugation by gp96-Ig vaccination. Cancer Res. 2009;69:2026–2033. doi: 10.1158/0008-5472.CAN-08-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qian J, Hong S, Wang S, Zhang L, Sun L, Wang M, et al. Myeloma cell line-derived, pooled heat shock proteins as a universal vaccine for immunotherapy of multiple myeloma. Blood. 2009;114:3880–3889. doi: 10.1182/blood-2009-06-227355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pakravan N, Hassan ZM. Comparison of adjuvant activity of N- and C-terminal domain of gp96 in a Her2-positive breast cancer model. Cell Stress Chaperones. 2011;16:449–457. doi: 10.1007/s12192-011-0258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pakravan N, Hashemi SM, Hassan ZM. Adjuvant activity of GP96 C-terminal domain towards Her2/neu DNA vaccine is fusion direction-dependent. Cell Stress Chaperones. 2011;16:41–48. doi: 10.1007/s12192-010-0219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oki Y, McLaughlin P, Fayad LE, Pro B, Mansfield PF, Clayman GL, et al. Experience with heat shock protein-peptide complex 96 vaccine therapy in patients with indolent non-Hodgkin lymphoma. Cancer. 2007;109:77–83. doi: 10.1002/cncr.22389. [DOI] [PubMed] [Google Scholar]
- 119.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100–21 Study Group. J Clin Oncol. 2008;26:955–962. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 120.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 121.Eton O, Ross MI, East MJ, Mansfield PF, Papadopoulos N, Ellerhorst JA, et al. Autologous tumor-derived heat-shock protein peptide complex-96 (HSPPC-96) in patients with metastatic melanoma. J Transl Med. 2010;8:9. doi: 10.1186/1479-5876-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tamura Y, Hirohashi Y, Kutomi G, Nakanishi K, Kamiguchi K, Torigoe T, et al. Tumor-produced secreted form of binding of immunoglobulin protein elicits antigen-specific tumor immunity. J Immunol. 2011;186:4325–4330. doi: 10.4049/jimmunol.1004048. [DOI] [PubMed] [Google Scholar]
- 123.Gong J, Zhang Y, Durfee J, Weng D, Liu C, Koido S, et al. A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol. 2010;184:488–496. doi: 10.4049/jimmunol.0902255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ren F, Xu Y, Mao L, Ou R, Ding Z, Zhang X, et al. Heat shock protein 110 improves the antitumor effects of the cytotoxic T lymphocyte epitope E7(49–57) in mice. Cancer Biol Ther. 2010;9:134–141. doi: 10.4161/cbt.9.2.10391. [DOI] [PubMed] [Google Scholar]
- 125.Wang XY, Sun X, Chen X, Facciponte J, Repasky EA, Kane J, et al. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J Immunol. 2010;184:6309–6319. doi: 10.4049/jimmunol.0903891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qian J, Yi H, Guo C, Yu X, Zuo D, Chen X, et al. CD204 suppresses large heat shock protein-facilitated priming of tumor antigen gp100-specific T cells and chaperone vaccine activity against mouse melanoma. J Immunol. 2011;187:2905–2914. doi: 10.4049/jimmunol.1100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo QY, Yuan M, Peng J, Cui XM, Song G, Sui X, et al. Antitumor activity of mixed heat shock protein/peptide vaccine and cyclophosphamide plus interleukin-12 in mice sarcoma. J Exp Clin Cancer Res. 2011;30:24. doi: 10.1186/1756-9966-30-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noiva R. Protein disulfide isomerase: the multifunctional redox chaperone of the endoplasmic reticulum. Semin Cell Dev Biol. 1999;10:481–493. doi: 10.1006/scdb.1999.0319. [DOI] [PubMed] [Google Scholar]
- 129.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gruber CW, Cemazar M, Heras B, Martin JL, Craik DJ. Protein disulfide isomerase: the structure of oxidative folding. Trends Biochem Sci. 2006;31:455–464. doi: 10.1016/j.tibs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 131.Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 132.Ko HS, Uehara T, Nomura Y. Role of ubiquilin associated with protein-disulfide isomerase in the endoplasmic reticulum in stress-induced apoptotic cell death. J Biol Chem. 2002;277:35386–35392. doi: 10.1074/jbc.M203412200. [DOI] [PubMed] [Google Scholar]
- 133.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 134.Lovat PE, Corazzari M, Armstrong JL, Martin S, Pagliarini V, Hill D, et al. Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res. 2008;68:5363–5369. doi: 10.1158/0008-5472.CAN-08-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goplen D, Wang J, Enger PO, Tysnes BB, Terzis AJ, Laerum OD, et al. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res. 2006;66:9895–9902. doi: 10.1158/0008-5472.CAN-05-4589. [DOI] [PubMed] [Google Scholar]
- 136.Haefliger S, Klebig C, Schaubitzer K, Schardt J, Timchenko N, Mueller BU, et al. Protein disulfide isomerase blocks CEBPA translation and is up-regulated during the unfolded protein response in AML. Blood. 2011;117:5931–5940. doi: 10.1182/blood-2010-08-304485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fonseca C, Soiffer R, Ho V, Vanneman M, Jinushi M, Ritz J, et al. Protein disulfide isomerases are antibody targets during immune-mediated tumor destruction. Blood. 2009;113:1681–1688. doi: 10.1182/blood-2007-09-114157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schroder M, Kaufman RJ. Divergent roles of IRE1alpha and PERK in the unfolded protein response. Curr Mol Med. 2006;6:5–36. doi: 10.2174/156652406775574569. [DOI] [PubMed] [Google Scholar]
- 139.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 140.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 142.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei J, Sheng X, Feng D, McGrath B, Cavener DR. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J Cell Physiol. 2008;217:693–707. doi: 10.1002/jcp.21543. [DOI] [PubMed] [Google Scholar]
- 144.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 145.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 146.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gupta S, McGrath B, Cavener DR. PERK regulates the proliferation and development of insulin-secreting beta-cell tumors in the endocrine pancreas of mice. PLoS One. 2009;4:e8008. doi: 10.1371/journal.pone.0008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hamanaka RB, Bennett BS, Cullinan SB, Diehl JA. PERK and GCN2 contribute to eIF2alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol Biol Cell. 2005;16:5493–5501. doi: 10.1091/mbc.E05-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y, Laszlo C, Liu W, Chen X, Evans SC, Wu S. Regulation of G(1) arrest and apoptosis in hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation. Neoplasia. 2010;12:61–68. doi: 10.1593/neo.91354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, et al. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 2010;29:4424–4435. doi: 10.1038/onc.2010.191. [DOI] [PubMed] [Google Scholar]
- 154.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schewe DM, Aguirre-Ghiso JA. Inhibition of eIF2alpha dephosphorylation maximizes bortezomib efficiency and eliminates quiescent multiple myeloma cells surviving proteasome inhibitor therapy. Cancer Res. 2009;69:1545–1552. doi: 10.1158/0008-5472.CAN-08-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Donze O, Jagus R, Koromilas AE, Hershey JW, Sonenberg N. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828–3834. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perkins DJ, Barber GN. Defects in translational regulation mediated by the alpha subunit of eukaryotic initiation factor 2 inhibit antiviral activity and facilitate the malignant transformation of human fibroblasts. Mol Cell Biol. 2004;24:2025–2040. doi: 10.1128/MCB.24.5.2025-2040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sequeira SJ, Ranganathan AC, Adam AP, Iglesias BV, Farias EF, Aguirre-Ghiso JA. Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation. PLoS ONE. 2007;2:e615. doi: 10.1371/journal.pone.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ranganathan AC, Ojha S, Kourtidis A, Conklin DS, Aguirre-Ghiso JA. Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer Res. 2008;68:3260–3268. doi: 10.1158/0008-5472.CAN-07-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 163.Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Endoplasmic reticulum stress mediates radiation-induced autophagy by perk-eIF2alpha in caspase-3/7-deficient cells. Oncogene. 2010;29:3241–3251. doi: 10.1038/onc.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Milani M, Rzymski T, Mellor HR, Pike L, Bottini A, Generali D, et al. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009;69:4415–4423. doi: 10.1158/0008-5472.CAN-08-2839. [DOI] [PubMed] [Google Scholar]
- 165.Koong AC, Chauhan V, Romero-Ramirez L. Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol Ther. 2006;5:756–759. doi: 10.4161/cbt.5.7.2973. [DOI] [PubMed] [Google Scholar]
- 166.Yoshida H. Unconventional splicing of XBP-1 mRNA in the unfolded protein response. Antioxid Redox Signal. 2007;9:2323–2333. doi: 10.1089/ars.2007.1800. [DOI] [PubMed] [Google Scholar]
- 167.Glimcher LH. XBP1: the last two decades. Ann Rheum Dis. 2010;69 (Suppl 1):i67–71. doi: 10.1136/ard.2009.119388. [DOI] [PubMed] [Google Scholar]
- 168.Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fujimoto T, Yoshimatsu K, Watanabe K, Yokomizo H, Otani T, Matsumoto A, et al. Overexpression of human X-box binding protein 1 (XBP-1) in colorectal adenomas and adenocarcinomas. Anticancer Res. 2007;27:127–131. [PubMed] [Google Scholar]
- 170.Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R, et al. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. Int J Cancer. 2008;123:85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 171.Maestre L, Tooze R, Canamero M, Montes-Moreno S, Ramos R, Doody G, et al. Expression pattern of XBP1(S) in human B-cell lymphomas. Haematologica. 2009;94:419–422. doi: 10.3324/haematol.2008.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 173.Gomez BP, Riggins RB, Shajahan AN, Klimach U, Wang A, Crawford AC, et al. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. 2007;21:4013–4027. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- 174.Hsiao JR, Chang KC, Chen CW, Wu SY, Su IJ, Hsu MC, et al. Endoplasmic reticulum stress triggers XBP-1-mediated up-regulation of an EBV oncoprotein in nasopharyngeal carcinoma. Cancer Res. 2009;69:4461–4467. doi: 10.1158/0008-5472.CAN-09-0277. [DOI] [PubMed] [Google Scholar]
- 175.Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11:349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thorpe JA, Schwarze SR. IRE1alpha controls cyclin A1 expression and promotes cell proliferation through XBP-1. Cell Stress Chaperones. 2010;15:497–508. doi: 10.1007/s12192-009-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Auf G, Jabouille A, Guerit S, Pineau R, Delugin M, Bouchecareilh M, et al. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc Natl Acad Sci U S A. 2010;107:15553–15558. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Drogat B, Auguste P, Nguyen DT, Bouchecareilh M, Pineau R, Nalbantoglu J, et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 2007;67:6700–6707. doi: 10.1158/0008-5472.CAN-06-3235. [DOI] [PubMed] [Google Scholar]
- 179.Romero-Ramirez L, Cao H, Regalado MP, Kambham N, Siemann D, Kim JJ, et al. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl Oncol. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 182.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mahoney DJ, Lefebvre C, Allan K, Brun J, Sanaei CA, Baird S, et al. Virus-tumor interactome screen reveals ER stress response can reprogram resistant cancers for oncolytic virus-triggered caspase-2 cell death. Cancer Cell. 2011;20:443–456. doi: 10.1016/j.ccr.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 185.Sinkovics JG, Horvath JC. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancers. Arch Immunol Ther Exp (Warsz) 2008;56 (Suppl 1):3s–59s. doi: 10.1007/s00005-008-0047-9. [DOI] [PubMed] [Google Scholar]