Abstract
Background
Acute kidney injury (AKI) following cardiac surgery is associated with worse outcomes. However, it is not known how adverse long-term consequences vary according to the duration of AKI. We sought to determine the association between duration of AKI and survival.
Methods
4,987 cardiac surgery patients from 2002 through 2007 with serum creatinine (SCr) collection at a medical center in northern New England. AKI was defined as a ≥0.3 (mg/dL) or ≥50% increase in SCr from baseline and further classified into AKI network stages. Duration of AKI was defined by the number of days AKI was present and categorized by: no AKI, AKI for 1–2, 3–6 and ≥7 days.
Results
39% of patients developed AKI. Long-term survival was significantly different by AKI duration (p < 0.001). The proportion of patients with AKI duration, adjusted hazard ratios (HR) and 95% confidence intervals for mortality (no AKI as referent), were: 1–2 days (18%, HR 1.66; 1.32–2.09), 3–6 days (11%, HR 1.94; 1.51–2.49), ≥7 days (9%, HR 3.40; 2.73–4.25). This graded relationship of duration of AKI with long-term mortality persisted when patients who died during hospitalization were excluded from analysis (p < 0.001). Propensity matched analysis confirmed results.
Conclusion
The duration of AKI after cardiac surgery is directly proportional to long-term mortality. This AKI dose-dependent effect on long-term mortality helps to close the gap between association and causation, whereby AKI stages and AKI duration have important implications for patient care and can aid clinicians in evaluating the risk of in-hospital and post discharge death.
Keywords: acute kidney injury, acute renal failure, clinical epidemiology, epidemiology and outcomes, survival, coronary artery disease
Introduction
Acute kidney injury (AKI) is common in hospitalized patients and is independently associated with an increased risk of morbidity, mortality, and length of stay.[1] AKI occurs in 2 to 30% of patients undergoing cardiac surgery, depending on the definition.[2] Long-term survival in patients that undergo cardiac surgery is proportional to severity of AKI, at least as assessed by peak changes in serum creatinine (SCr).[3] The dose-response relationship between severity of AKI and long-term survival has been demonstrated in several other settings as well.[4] Most recent epidemiologic studies of AKI have utilized the consensus definitions of Risk Injury Failure Loss End-stage (RIFLE) or Acute Kidney Injury Network (AKIN) to classify severity of AKI.[3] These definitions assess the magnitude of serum creatinine elevation as the primary dimension to grade severity (change from baseline to peak creatinine). The AKIN definition also incorporates tempo as a prerequisite (rise must occur over a period of 48 hours), but both definitions ignore a potentially important third dimension of severity of AKI: duration. Not only does duration of serum creatinine elevation intuitively denote severity of AKI in the clinical arena, but the duration of serum creatinine elevation has also been suggested as a better endpoint for trials of AKI.[5] However, to our knowledge, the value of duration of AKI for predicting long-term survival has not been thoroughly studied.
Thus, we sought to determine the association between duration of SCr elevation and long-term survival in cardiac surgery patients. Cardiac surgery is an ideal setting in which to assess this relationship, as AKI is common in this setting, the timing of the insult is known, and daily SCr measurement is generally conducted on a daily basis for the first two postoperative days. Subsequent SCr measurements are done according to clinical indications until discharge.
Methods
We prospectively enrolled 4,987 consecutive cardiac surgery patients from 2002 through 2007. Patients with a history of dialysis were excluded from the analysis (N=70). Eighty-five patients were excluded that did not have valid procedure dates and thus AKI duration was not calculated. The Maine Medical Center Institutional Review Board at has approved this study and waived theneed for patient consent.
Duration and Staging of AKI
The last pre-operative SCr value prior to surgery was compared to each post-operative SCr as a measure of AKI. SCr was measured daily until 48 hours post-surgery, followed by additional days of measurement per the attending provider’s discretion. All SCr laboratory measures were conducted as part of the hospitals standing protocol and no SCr measurements were conducted for research. AKI was defined as a ≥0.3 (mg/dL) or ≥50% increase in SCr from baseline for each post-operative day.[6] Duration of AKI was defined by the number of days AKI was present and categorized by: no AKI, AKI for 1–2, 3–6 and ≥7 days. Severity of AKI was classified by AKIN staging criteria.[6] We also classified AKI into transient and persistent categories based on renal recovery back to baseline at the time of discharge (SCr values returning to “no AKI” status).
Patient Follow-Up
Patients were followed during the index hospital admission for cardiac surgery until discharge. Baseline, procedural and clinical outcomes were collected at discharge. Long-term follow-up for mortality was obtained by linking the registry to the Social Security Death Master File (SSDMF).[7] Patients were matched by name, social security number, and date of birth.[8] Follow-up time was calculated from the date of the procedure to the date of death recorded by the SSDMF as previously described.[9, 10]
Statistical Analysis
Baseline patient and disease characteristics and clinical outcomes were summarized by percentages and means (± standard deviation). We used χ2 tests and tests of trend to assess similarities between categories of duration of AKI. Degrees of freedom for the χ2 tests depended on the number of groups. We used an alpha of 0.05. In Table 1, gender, hypertension, chronic obstructive pulmonary disease, vascular disease, diabetes, left main disease (≥50% stenosis), unstable angina, intra-aortic balloon pump, and prior CABG were tested using χ2 test with 1 degree of freedom; priority using χ2 test had 2 degrees of freedom and baseline estimated glomerular filtration rate using the Modification of Diet in Renal Disease equation (mL/min/1.73 m2).[11] All other continuous variables were tested using Cuzick’s nonparametric test for trend 16 across ordered groups of duration of AKI, which is an extension of the Wilcoxon rank-sum test. All variables in Table 2 were tested using the χ2 test, except for AKIN stages,(2) percent changes in peak SCr,(3) and length of stay where compared using test of trend.
Table 1.
Patient and Disease Characteristics
| Duration of AKI (Days) | |||||
|---|---|---|---|---|---|
| Variables | None (n = 2,932) |
1–2 Days (n = 900) |
3–6 Days (n = 554) |
≥7 Days (n = 445) |
p-value |
| Patient demographics | |||||
| Age, mean ± SD | 64.3 ± 11.0 | 67.0 ± 10.6 | 70.1 ± 9.8 | 72.2 ± 9.3 | <0.001 |
| Sex (% female) | 29.4 | 29.0 | 29.4 | 32.8 | 0.496 |
| BMI, mean ± SD | 28.9 ± 5.5 | 29.5 ± 6.1 | 29.8 ± 6.3 | 29.6 ± 6.2 | 0.013 |
| Preoperative characteristics | |||||
| Hypertension (%) | 62.1 | 68.6 | 78.2 | 72.1 | <0.001 |
| COPD (%) | 10.5 | 13.0 | 14.1 | 24.5 | <0.001 |
| Vascular disease (%) | 18.9 | 20.0 | 32.2 | 33.7 | <0.001 |
| Diabetes (%) | 29.1 | 32.1 | 42.1 | 38.7 | <0.001 |
| Preoperative hematocrit mean ± SD | 40.0 ± 4.8 | 39.4 ± 4.9 | 37.7 ± 5.2 | 37.1 ± 5.5 | < 0.001 |
| Preoperative creative, mean ± SD (mg/dL) | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.1 ± 0.4 | 1.2 ± 0.6 | <0.001 |
| Preoperative eGFR, mean ± SD (mL/min/m2) | 82.5 ± 25.0 | 80.0 ± 24.7 | 70.3 ± 32.2 | 67.0 ± 27.3 | <0.001 |
| Preoperative eGFR Categories | |||||
| ≥90 (mL/min/m2) | 35.7 | 31.0 | 20.5 | 15.9 | <0.001 |
| 60–89 | 47.9 | 49.6 | 41.2 | 39.1 | |
| 30–59 | 14.6 | 18.0 | 34.6 | 39.3 | |
| 15–29 | 1.5 | 1.1 | 3.7 | 5.0 | |
| <15 | 0.2 | 0.3 | 0.0 | 0.7 | |
| Cardiac profile | |||||
| Surgical priority (%) | |||||
| Elective | 31.1 | 30.8 | 29.8 | 17.1 | <0.001 |
| Urgent | 60.4 | 61.2 | 63.5 | 71.5 | |
| Emergent | 8.5 | 8.0 | 6.7 | 11.5 | |
| Left main disease (≥50% stenosis, %) | 25.6 | 24.8 | 23.8 | 34.8 | <0.001 |
| Unstable angina (%) | 56.3 | 55.2 | 58.7 | 47.1 | 0.001 |
| Intra-aortic balloon pump (%) | 7.3 | 5.8 | 6.7 | 13.5 | <0.001 |
| Number of diseased vessels, mean ± SD | 1.9 ± 1.1 | 1.9 ± 1.1 | 2.0 ± 1.1 | 2.1 ± 1.1 | <0.001 |
| Ejection fraction mean, ± SD | 51.9 ± 12.5 | 52.1 ± 12.6 | 50.5 ± 12.7 | 45.9 ± 14.5 | <0.001 |
| LVEDP, mean ± SD (mmHg) | 18.5 ± 7.8 | 19.9 ± 7.5 | 21.0 ± 8.2 | 21.9 ± 8.3 | <0.001 |
| Pumptime, mean ± SD (min) | 119 ± 46 | 121 ± 45 | 127 ± 48 | 146 ± 59 | <0.001 |
| Prior CABG (%) | 6.3 | 4.7 | 6.7 | 9.2 | 0.014 |
P-value: chi2 test, test of trend; SD: Standard deviation; BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; PVD: Periferal vasuclar disease; LVEDP: Left ventricular end diastolic pressure; CABG: Coronary artery bypass graft surgery. Postoperative eGFR calculated from the highest postoperative serum creatinine (mg/dL) using the MDRD equation.
Table 2.
Assessment of In-hospital Outcomes
| Duartion of AKI (Days) | |||||
|---|---|---|---|---|---|
| Variables | None (n = 2,932) |
1–2 Days (n = 900) |
3–6 Days (n = 554) |
≥7 Days (n = 445) |
p-value |
| Postoperative clinical outcomes | |||||
| Postoperative peak creatinine mean ± SD | 1.0 ± 0.8 | 1.4 ± 1.0 | 1.9 ± 1.6 | 2.4 ± 1.3 | <0.001 |
| AKI Duration mean ± SD (days) | 0.0 | 1.4 ± 0.5 | 4.1 ± 1.1 | 16.2 ± 15.3 | <0.001 |
| AKIN Criteria | |||||
| Stage 1 | 57.6 | 28.8 | 13.6 | <0.001 | |
| Stage 2 | 14.2 | 35.8 | 50.0 | ||
| Stage 3 | 7.2 | 20.9 | 71.9 | ||
| Percent change in peak creatinine | |||||
| <50% | 81.0 | 14.4 | 3.6 | 1.1 | <0.001 |
| 50–99% | 0.0 | 43.8 | 36.3 | 19.9 | |
| 100–199% | 0.0 | 15.9 | 34.9 | 49.2 | |
| ≥200% | 0.0 | 7.4 | 22.2 | 70.4 | |
| Length of postoperative stay, mean ± SD (days) | 6.5 ± 5.6 | 7.2 ± 5.8 | 9.3 ± 9.2 | 24.1 ± 21.8 | <0.001 |
| In-hospital acute dialysis (%) | 0.0 | 0.2 | 0.5 | 7.2 | <0.001 |
| Atrial fibrillation (%) | 24.3 | 29.7 | 35.7 | 55.8 | <0.001 |
| Low output syndrome (%) | 3.9 | 6.4 | 10.7 | 23.6 | <0.001 |
| Mediastintis (%) | 1.1 | 1.7 | 2.7 | 6.7 | <0.001 |
| Return to OR for bleeding (%) | 2.9 | 4.1 | 2.9 | 5.9 | 0.005 |
| Q-wave myocardial infarction (%) | 2.5 | 2.8 | 1.5 | 3.8 | 0.385 |
| Pneumonia (%) | 1.3 | 1.1 | 3.8 | 22.0 | <0.001 |
| In-hospital mortality (%) | 2.3 | 4.1 | 5.8 | 15.3 | <0.001 |
P: P-value for chi-square or test of trend; SD: Standard deviation; AKIN: Acute Kidney Injury Network; OR: Operating room; Renal failure: new onset of acute dialysis during post-operative admission.
Kaplan-Meier techniques were used to conduct the survival analysis and failure plots for time to AKI recovery; patients were stratified by the duration of AKI post-operatively. Cox’s proportional hazard models were used to calculate crude and adjusted hazard ratios (HR): with “No AKI” as the referent, adjusting for age, sex, prior CABG, COPD, emergency surgery, ejection fraction, and baseline eGFR due to biological plausibility and univariate associations with survival and duration of AKI. Adjusted HRs were reported with 95% confidence intervals and p-values. Propensity matched blocks were created AKI categories, matching on covariates listed above and Cox’s proportional hazard modeling was conducted across the propensity matched blocks. All analyses were conducted using Stata 9.0 (College Station, TX).
Results
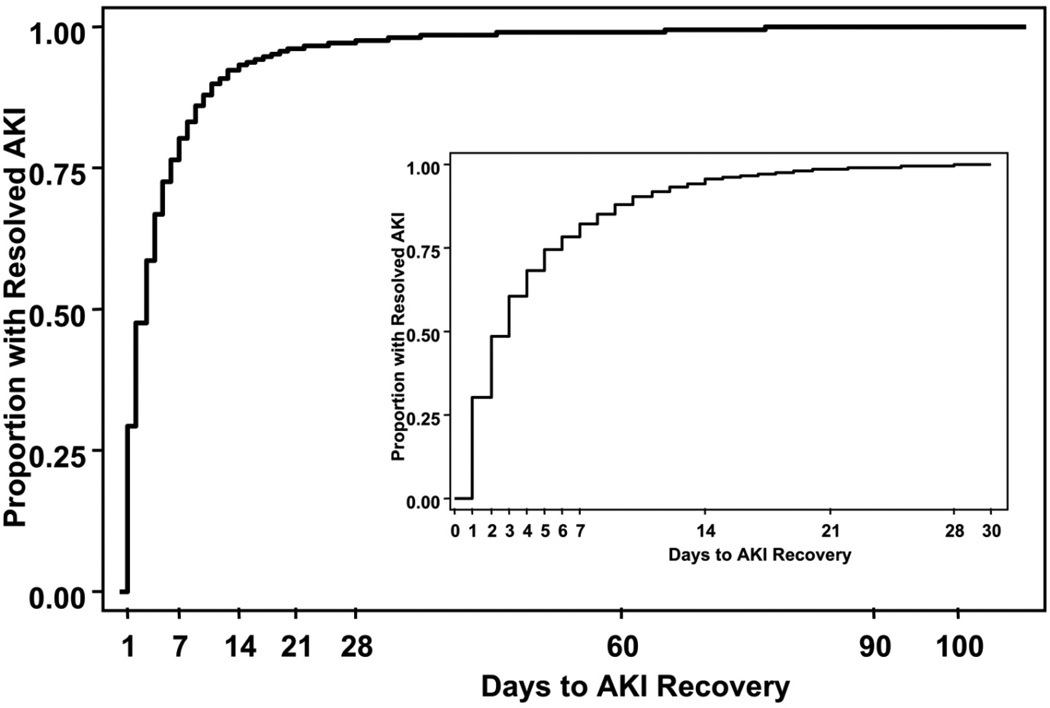
During the study period, thirty-nine percent (1,886/4,837) of patients developed AKI. We stratified patients by duration of AKI: no AKI (2,951), 1–2 days of AKI (896; 18.5%), 3–6 days (552; 10.8%), and ≥7 days (438; 9.1%). Patients developing AKI significantly differed with regard to baseline patient and disease characteristics (Table 1) with lower baseline eGFR, longer duration on cardiopulmonary bypass and other co-morbidities representing important risk factors for the development on AKI and duration of AKI. We displayed the time to AKI recovery (duration of AKI) in Figure 1; nearly 50 percent of patients with AKI recovered after 2 days, however the remaining AKI patients took as long as three months to recover renal function.
Figure 1. Duration of AKI.
The duration of AKI is plotted with the proportion of resolved AKI on the y-axis and days to AKI recovery on the abcissa. The insert is a magnification of the data for the first 30-days.
Clinical outcomes
AKIN stage was associated with duration of AKI, where stage 2 and 3 AKIN patients developed AKI for more days than AKIN stage 1 patients who experienced a more transient episode of AKI (Table 2). The duration of AKI provided additional predictive information for 5-year mortality risk over that of AKIN stage alone. In-hospital unfavorable outcomes were also more common among patients with a longer duration of AKI, except for a new onset of Q-wave myocardial infarction. Longer durations of AKI were more likely to result in acute dialysis (Table 2).
Long-term Mortality
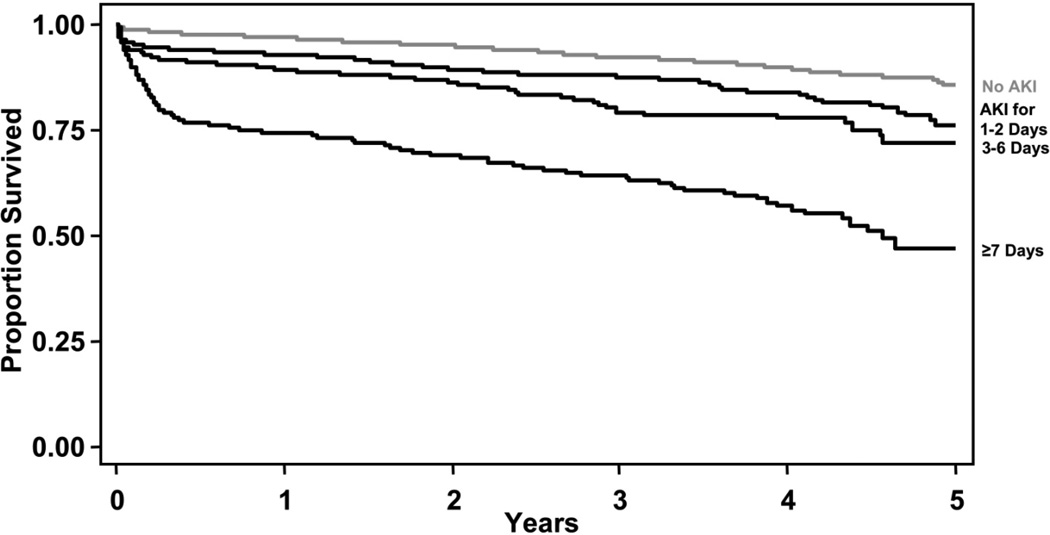
The Kaplan-Meier survival plot demonstrates that patients with longer durations of AKI die more often during the 5-years following cardiac surgery than patients with shorter durations of AKI (p < 0.001, Figure 2) with an average follow-up of 2.6 years, (median 2.6 years). The proportion of patients, adjusted hazard ratios (HR) and 95% confidence intervals for AKI duration (no AKI as referent) are reported in Table 3 and are as follows: 1–2 days (18%, HR 1.66; 1.32–2.09), 3–6 days (11%, HR 1.94; 1.51–2.49), ≥7 days (9%, HR 3.40; 2.73–4.25). This graded relationship of duration of AKI with long-term mortality persisted when patients who died during hospitalization were excluded from analysis (Table 3).
Figure 2. Survival by Duration of AKI.
The proportion of patients surviving from the time of cardiac surgery is plotted by the categories for the duration of AKI: no AKI (grey line), AKI for 1–2, 3–6, and ≥7 days (black lines, Log rank p-value <0.001)
Table 3.
Lon-term Mortality
| Adjusted HR (95% CI) | ||||
|---|---|---|---|---|
| Duration of AKI | Model 1 Age/Sex |
Model 2 Age/Sex+Covariates |
Model 3 Age/Sex+Covariates+ baseline eGFR |
Model 4 Propensity |
| None | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference |
| 1–2 Days | 1.75 (1.40–2.18) | 1.73 (1.38–2.17) | 1.66 (1.32–2.09) | 1.71 (1.37–2.13) |
| 3–6 Days | 2.19 (1.72–2.78) | 2.10 (1.64–2.69) | 1.94 (1.51–2.49) | 2.08 (1.32–3.30) |
| ≥7 Days | 4.71 (3.82–5.80) | 3.78 (3.04–4.70) | 3.40 (2.73–4.25) | 4.78 (3.08–7.44) |
| AKI duration among patients alive at discharge | ||||
| None | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference |
| 1–2 Days | 1.43 (1.11–1.84) | 1.37 (1.06–1.78) | 1.33 (1.03–1.73) | 1.38 (1.07–1.79) |
| 3–6 Days | 1.73 (1.30–2.30) | 1.49 (1.11–2.00) | 1.40 (1.04–1.89) | 2.36 (1.32–4.23) |
| ≥7 Days | 3.42 (2.66–4.40) | 2.61 (2.00–3.41) | 2.40 (1.83–3.15) | 5.03 (2.86–8.85) |
| Transient and Persistent AKI | ||||
| None | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference |
| Transient AKI 1–2 Days | 1.58 (1.26–1.99) | 1.57 (1.24–1.99) | 1.51 (1.19–1.91) | 1.54 (1.23–1.94) |
| Transient AKI 3–6 Days | 1.96 (1.52–2.53) | 1.89 (1.46–2.46) | 1.74 (1.34–2.26) | 1.91 (1.19–3.07) |
| Transient AKI ≥7 Days | 4.70 (3.79–5.84) | 3.79 (3.02–4.75) | 3.45 (2.75–4.34) | 4.94 (3.14–7.77) |
| Persistent AKI at Discharge | 7.08 (5.14–9.75) | 6.19 (4.43–8.65) | 5.75 (4.10–8.07) | 6.13 (3.68–10.23) |
P: P-value; 95% confidence interval; Adjusted HR: Adjusted hazard ratio from Cox proportional hazard model; Model 1: adjusted for age, sex; Model 2: adjusted for age, sex, prior CABG, chronic obstructive pulmonary disease, emergency surgery, and ejection fraction; Model 3: adjusted for age, sex, prior CABG, chronic obstructive pulmonary disease, emergency surgery, ejection fraction and baseline eGFR; Model 4: propensity matched analysis.
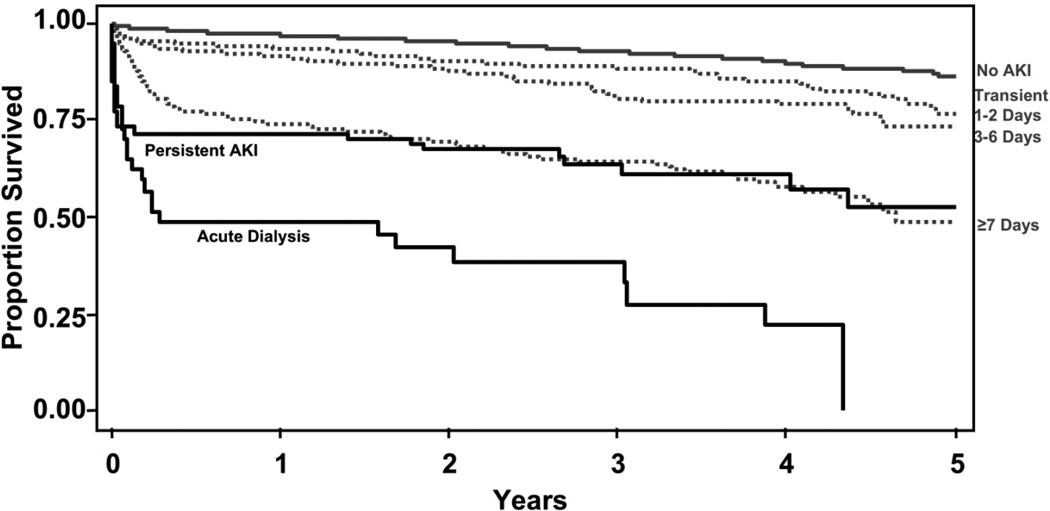
We further divided our AKI duration groups to compare transient AKI (AKI resolved by the time of discharge), persistent AKI (unresolved AKI at the time of discharge), and new onset of acute dialysis (Figure 3, Table 3). We discovered there was an immediate upfront mortality risk equal to that of acute dialysis among patients that were discharged with AKI unresolved. The adjusted HRs for patients in this subgroup analysis confirmed these findings, with no AKI as the referent (Table 3): transient AKI 1–2 days (HR 1.46; 1.15–1.85), transient AKI 3–6 days (HR 1.66; 1.27–2.17), transient AKI ≥7 days (HR 3.22; 2.54–4.08), persistent AKI (HR: 4.96; 3.43–7.17), and acute dialysis (HR: 8.53; 5.56–13.08). Propensity matched analysis confirmed the results (Table 3).
Figure 3. Survival by Transient and Persistent AKI.
The proportion of patients surviving from the time of cardiac surgery is plotted by no AKI (grey line), transient AKI (grey dotted lines: resolved AKI by the time of discharge lasting for 1–2, 3–6, and ≥7 days, respectively), persistent AKI (black line: unresolved AKI at the time of discharge), and acute dialysis (bottom black line, Log rank p-value <0.001).
Comment
We demonstrate the duration of AKI is associated with in-hospital and long-term mortality. The duration of AKI provides additional information over the AKIN stage alone and can provide additive risk information for in-hospital and long-term mortality risks for patients. This finding is novel for the AKI literature, as all consensus definitions of AKI and the majority of previous studies of AKI and outcomes have focused on the severity of AKI as defined by the magnitude in rise of SCr (or blood urea nitrogen), the degree of oliguria, or the need for hemodialysis.[3, 12–14] All of these parameters are measuring the same dimension of AKI (functional decline in GFR), but ignore the important dimension of time of AKI which can be attributed, in part, to structural and repair potential of the kidney. Since incorporation of the duration of AKI into the schema for classification of AKI is very easy and non-invasive for clinicians and researchers, and it provides prognostic value, and represents assessment of another dimension of AKI, future investigations of AKI should evaluate outcomes using both of these constructs. Furthermore, recent data suggest that incorporating time of creatinine elevation into AKI definitions may be more efficient and a less biased method of ascertainment of outcome in interventional trials for AKI as demonstrated by Pickering and colleagues using mathematical modeling of creatinine changes in AKI.[5] Before these types of new constructs for measurement of treatment efficacy of AKI interventions are routinely employed as endpoints in clinical trials for AKI, it first needs to be determined whether these time-based metrics of creatinine elevation in AKI are associated with important hard outcomes, such as long-term survival.
In our large, prospective study of consecutive patients undergoing cardiac surgery in the new millennium with daily SCr measurement following cardiac surgery, we have confirmed the early reports that transient and persistent AKI is associated with worse survival;[3, 15–17] furthermore, we are the first to identify that the duration of AKI during the index cardiac surgery admission is an important indicator of in-hospital and long-term risk, is a better predictor of long-term risk for death than traditional methods of classifying AKI, and thus should be evaluated in future studies as a possible addition to the AKIN criteria for AKI. Most importantly, we are the first to report on a particularly high-risk group of patients developing AKI after cardiac surgery: Patients discharged with unresolved AKI, or persistent AKI. We found an immediate mortality risk equal to that among patients receiving acute dialysis among patients developing AKI and discharged with unresolved AKI. This is an important patient population that needs more attention in the literature and in clinical practice as to how to effectively treat patients with persistent AKI before and after hospital discharge.
Prior Studies of AKI Recovery and Outcomes
Our findings add substantially to the merit of using the duration of AKI as a hard outcome for in hospital and long-term prognosis. While many studies have examined long-term outcomes after AKI, only a few have examined outcomes based on the recovery pattern of AKI. Liano et al. reported on 187 consecutive acute tubular necrosis patients and ten-year survival.[15] They demonstrated patients with only partial recovery of AKI had worse survival compared to patients that had fully recovered renal function (P=0.028). Loef et al. demonstrated that 8 year survival of 843 patients undergoing cardiac surgery was diminished after AKI, as defined by SCr elevations of ≥25% or <25%, regardless of whether SCr returned to baseline by the time of hospital discharge.[16] Hobson et al., reported long-term follow-up on a retrospective cohort of 2,973 surgical patients from 1992 and 2002 and reported long-term survival was significantly worse among patients with AKI (using RIFLE criteria).[3] They stratified by complete, partial, and no recovery of renal function and demonstrated that patients that recovered from a transient elevation in SCr had a sustained increase risk of long-term mortality compared to patients without AKI. Patients with partial and no recovery of renal function had even worse survival.
Clinical Construct and Mechanisms
The mechanism of duration of AKI represents a different process occurring at the level of the kidney. The classification of AKI by duration may be potentially intuitive as "pre-renal" azotemia, which is usually hemodynamic, does not involve true kidney injury, and thus should recover in 24–48 hours by optimizing volume or hemodynamics. However, in patients with true acute kidney injury (acute tubular necrosis), the duration of AKI may denote recovery potential of the person or continued on-going insults. In cardiac surgery, longer duration of AKI may likely denote poor recovery potential as one of the AKI insults may occur at a single time-point, intra-operatively, during cardiopulmonary bypass. Recovery from AKI involves replacement of necrotic or apoptotic renal tubular cells and requires several steps such as dedifferentiation, redifferentiation and possibly recruitment of local (renal) or bone-marrow stem cells.[18] It has been demonstrated that in older patients or in the presence of other organ co-morbidities, renal recovery may be prolonged and frequently incomplete.[19] Mild insults resulting in mild decrements in GFR will manifest varying durations of AKI depending on one’s individual capacity for tubular cell repair. Thus, in cases where the repair potential is impaired, the risk otherwise associated with mild or moderate functional renal failure, as denoted may the rise in SCr, may increase as a function of the duration of AKI, thereby adding to prognostic potential to AKIN stages.
Strengths and Limitations
There are several limitations of this study. First, this is a prospectively collected, retrospectively analyzed single-center study. Second, we reported on long-term survival but we did not have information on long-term incidence of end stage renal disease or mode of death. Third, we lacked post-discharge follow-up on kidney function on patients with persistent AKI. However, our study also has several strengths. This is a modern prospective cohort of consecutive patients with a broad mix of comorbidites, race and gender, which permits adequate generalizability of our findings. Patients were followed during a period of time when many evidenced-based processes, such as tight glucose control, conservative blood transfusion strategies, and low tidal volumes have become standard of care.[20–23] Furthermore, SCr labs were drawn daily until 48 hours post-surgery on all participants; after 48 hours, SCr labs were ordered per provider discretion until hospital discharge.
Conclusions
AKI, once thought to be benign when SCr returned to baseline, has significant long-term implications. Not only does AKI potentially drive the increased mortality, it is likely a surrogate for other end organ injuries. The fact that the duration of AKI differentiates AKI from volume responsive renal dysfunction and is proportional to the amount of renal injury and mortality compels us to have more impetus to change our processes of care specifically with regard to AKI.
The duration of AKI after cardiac surgery is directly proportional to long-term mortality. This AKI dose-dependent effect on long-term mortality helps to close the gap between association and causation, whereby AKI and AKI duration have important implications for patient care and can aid clinicians in evaluating the risk of in-hospital and post-discharge death. AKI duration can also be employed as a surrogate outcome for long-term mortality in intervention trials to prevent or treat AKI. The long-term implications of AKI compel us to have some urgency regarding seeking changes of processes of care specifically regarding renal protective strategies.
Acknowledgements
None.
Sources of Funding: Dr. Brown is supported by grant number K01HS018443 from the Agency for Healthcare Research and Quality. Dr. Parikh is supported by grant R01HL085757 from the National Institutes of Health. Dr. Coca is funded by the career development grant K23DK08013 from the National Institutes of Health, by the Hartford Foundation Center of Excellence in Aging at Yale Subspecialty Scholar Award, and by the American Society of Nephrology-ASP Junior Development Award in Geriatric Nephrology.
References
- 1.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 2.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 3.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 4.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 5.Pickering JW, Frampton CM, Endre ZH. Evaluation of trial outcomes in acute kidney injury by creatinine modeling. Clin J Am Soc Nephrol. 2009;4:1705–1715. doi: 10.2215/CJN.00820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wentworth DN, Neaton JD, Rasmussen WL. An Evaluation of the Social-Security-Administration Master Beneficiary Record File and the National-Death-Index in the Ascertainment of Vital Status. Am J Pub Health. 1983;73:1270–1274. doi: 10.2105/ajph.73.11.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JR, Cochran RP, MacKenzie TA, et al. Long-term survival after cardiac surgery is predicted by estimated glomerular filtration rate. Ann Thorac Surg. 2008;86:4–11. doi: 10.1016/j.athoracsur.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Dacey LJ, Liu JY, Braxton JH, et al. Long-term survival of dialysis patients after coronary bypass grafting. Ann Thorac Surg. 2002;74:458–462. doi: 10.1016/s0003-4975(02)03768-2. [DOI] [PubMed] [Google Scholar]
- 10.Brown JR, Cochran RP, Dacey LJ, et al. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation. 2006;114:I409–I413. doi: 10.1161/CIRCULATIONAHA.105.000596. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 13.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009 doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 14.Lopes JA, Fernandes P, Jorge S, et al. Acute kidney injury in intensive care unit patients: a comparison between the RIFLE and the Acute Kidney Injury Network classifications. Crit Care. 2008;12:R110. doi: 10.1186/cc6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liano F, Felipe C, Tenorio MT, et al. Long-term outcome of acute tubular necrosis: a contribution to its natural history. Kidney Int. 2007;71:679–686. doi: 10.1038/sj.ki.5002086. [DOI] [PubMed] [Google Scholar]
- 16.Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 17.Welten GM, Schouten O, Chonchol M, et al. Temporary worsening of renal function after aortic surgery is associated with higher long-term mortality. Am J Kidney Dis. 2007;50:219–228. doi: 10.1053/j.ajkd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Cantley LG. Adult stem cells in the repair of the injured renal tubule. Nat Clin Pract Nephrol. 2005;1:22–32. doi: 10.1038/ncpneph0021. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt R, Coca S, Kanbay M, et al. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 21.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 22.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 23.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352–360. doi: 10.1016/s0003-4975(99)00014-4. discussion 360-2. [DOI] [PubMed] [Google Scholar]