Abstract
Background
Development of Salmonella enterica serovar Typhimurium (S. Typhimurium) live attenuated vaccine carrier strain to prevent enteric infections has been a subject of intensive study. Several mutants of S. Typhimurium have been proposed as an effective live attenuated vaccine strain. Unfortunately, many such mutant strains failed to successfully complete the clinical trials as they were suboptimal in delivering effective safety and immunogenicity. However, it remained unclear, whether the existing live attenuated S. Typhimurium strains can further be attenuated with improved safety and immune efficacy or not.
Results
We deleted a specific non-SPI (Salmonella Pathogenicity Island) encoded virulence factor mig-14 (an antimicrobial peptide resistant protein) in ssaV deficient S. Typhimurium strain. The ssaV is an important SPI-II gene involved in Salmonella replication in macrophages and its mutant strain is considered as a potential live attenuated strain. However, fatal systemic infection was previously reported in immunocompromised mice like Nos2−/− and Il-10−/− when infected with ssaV deficient S. Typhimurium. Here we reported that attenuation of S. Typhimurium ssaV mutant in immunocompromised mice can further be improved by introducing additional deletion of gene mig-14. The ssaV, mig-14 double mutant was as efficient as ssaV mutant, with respect to host colonization and eliciting Salmonella-specific mucosal sIgA and serum IgG response in wild-type C57BL/6 mice. Interestingly, this double mutant did not show any systemic infection in immunocompromised mice.
Conclusions
This study suggests that ssaV, mig-14 double mutant strain can be effectively used as a potential vaccine candidate even in immunocompromised mice. Such attenuated vaccine strain could possibly used for expression of heterologous antigens and thus for development of a polyvalent vaccine strain.
Background
Enteric infections represent a major threat to human health worldwide affecting both children and adults in developing and industrialized countries. These infections are caused by a number of pathogens including Salmonella, Shigella, Campylobacter species, Aeromonas, Plesiomonas, Vibrio, Yersinia entercolitica, E. coli 0157:H7 and Rotavirus. Among these enteric pathogens, Salmonella enterica with more than 2500 serovars is considered as a key pathogen that can infect a wide range of host species and is the leading cause of acute gastroenteritis. The increased mortality, morbidity and limited availability of specific drugs against these infection demands an alternative to reduce the global disease burden. One such promising alternative is the development of live-attenuated vaccines. These vaccines are attenuated forms of the pathogen itself which can provide defense against the infection from the same pathogen. In case of Salmonella, a facultative intracellular pathogen, specific cell mediated immune response is critical to control and clear the pathogen from the host [1-4]. In order to stimulate cellular immunity with higher efficacy, live attenuated Salmonella are preferred over the inactivated or killed vaccine candidates [5-7]. Ideally, a live attenuated vaccine strain should be able to withstand the host stress, provide defense against the concerned pathogen and should successfully colonize the host lymphoid tissues while retaining its avirulent nature. Researchers have established mice models in order to efficiently screen the possible vaccine attributes of genetically modified Salmonella enterica strains or their derivatives [8-12]. However, many live attenuated strains are known to develop systemic infection when administered to immune deficient individuals [13-15]. In order to prevent the systemic infection in immune-compromised patients, it is very crucial to attain sufficient attenuation. Many attenuated Salmonella vaccine strains carrying deletion mutation either in the metabolic gene or in the virulence factors have been developed but with a little success in the clinical trials [16]. This study primarily focuses on the development of an improved live-attenuated S. Typhimurium strain. A number of S. Typhimurium mutants developed, are known to elicit optimal immune response but showed reduced survival efficacy [17-26]. Earlier studies have shown that only a few such mutants have been actually tested in a pilot study in order to investigate their protection efficacy [27-29]. When tested, such a few proposed vaccine strains resulted in developing diseases in the hosts of variable immune status [20,30-32]. Therefore, the development of a safer immunogenic live-attenuated S. Typhimurium strain is a need of an hour [33] and can be accomplished by development of a suitably attenuated strain with an avirulent property in immunocompromised individuals. Previous studies have shown that TTSS-2 deficient S. Typhimurium strains were highly attenuated and conferred protection from further challenges of wild-type S. Typhimurium by eliciting O-antigen specific serum IgG and secretory IgA in C57BL/6 mice [34-36]. In a recent study, the ssaV mutant of S. Typhimurium was found to be virulent in immune compromised C57BL/6 mice devoid of Nos2 and Il-10 gene [37]. These two mice strains were used as they lack key elements of the antibacterial defense like the inducible nitric oxide (NO) synthase, a reactive oxygen species generating enzyme and interleukin-10 gene [38]. In this study, we have also used CD40L KO mice to screen the attenuation of proposed vaccine strain. This particular mouse model is used as it is partially immunocompromised in terms of generation of different class of antibodies.
Virulence of TTSS-2 deficient S. Typhimurium in immunocompromised mice unveils the role of other factors favoring the replication and long-term survival of S. Typhimurium in host tissues. Mig-14, an antimicrobial peptide resistance protein, is one such important factor that supports the long-term persistence of Salmonella in the macrophages [39]. Mig-14 protein binds to the anti-microbial peptides like CRAMPS to protect Salmonella from antimicrobial peptides [40]. The presence of Mig-14 in the periplasmic localization inhibits the entry of antimicrobial peptides to the cytoplasm of the bacterium, eventually making macrophage a good niche for Salmonella to replicate and survive. This study proposes a diverse role for mig-14 in the survival of TTSS-2 deficient Salmonella in immunocompromised mice like Nos2−/−, Il-10−/−and CD40L−/− and explores the possible potential of S. Typhimurium ssaV and mig-14 double mutant as a safe vaccine carrier strain.
Methods
Bacterial strains and plasmids
Streptomycin resistant S. Typhimurium SB300 and Salmonella Enteritidis P125109 (S. Enteritidis) strains were taken as the wild-type controls [41,42]. Mutants MT5 (SB300; ΔssaV) and MT4 (SB300; ΔssaV, Δmig-14) were generated by lambda red-mediated recombinase process [43]. Briefly, the host bacterial strain to be mutated was transformed with plasmid pKD46 and induced with arabinose (10 mM). The kanamycin open reading frame was PCR-amplified from template plasmid pKD4 using gene specific knockout primers (Table 1). The cassette was introduced into host bacterial genome with the help of Exo, Bet and Gam proteins from induced pKD46 plasmid of host bacterial strain. The positive mutants were selected on LB agar plates supplemented with kanamycin (50 μg/ml) and mutation in the target gene was confirmed using gene specific confirmatory primers in combination with respective forward knock-out primer (Table 1). Later, the antibiotic cassette was flipped by plasmid pCP20 [43]. An ampicillin resistant plasmid (pM973) was used to maintain the ampicillin resistant trait in wild-type strain (SB300) while challenging vaccinated mice groups with wild-type S. Typhimurium [44]. The bacterial strains and the plasmids used in this study are listed in Table 2.
Table 1.
Primers used in the study
| Fw-ssaV |
AGT CGC AAT GCG TTC ATG GTT AG |
| Rw-ssaV |
TTC TTC ATT GTC CGC CAA CTC |
| KO-Fw-ssav |
AAT AAA ATT TCT GGA GTC GCA ATG CGT TCA TGG TTA GGT GAG GGA TGT GTA GGC TGG AGC TGC TT |
| KO-Rw-ssaV |
GCA TCA ATT CAT TCT TCA TTG TCC GCC AAC TCC TCT TCG CTA AGG ATA TGA ATA TCC TCC TTA GT |
| Conf-ssaV |
GCA AAG CTT TGC TGC CAT TAA TCC |
| Fw-mig14 |
GAG TTT TGG TGA AAA TAC AAG AAG |
| Rw-mig14 |
GTA TAG TGT AAG TGA ATT TCG AGT AAT TG |
| KO-Fw-mig14 |
AGC AAA AAA ATA ATA CAA AAT AGC ATT TTC AGT AAG CTA AGT CAG TGT GTA GGC TGG AGC TGC TT |
| KO-Rw-mig14 |
GAA AAA TCT GGA CGT AAA AAA CAT ATT TAC GTC CAG GCT TTC TTT ATA TGA ATA TCC TCC TTA GT |
| Conf-mig14 | CAT CAT CTG TTC CTG ACG CCA G |
Table 2.
Bacterial strains and plasmids used in the study
| Strains | Genetic information | Background | References |
|---|---|---|---|
| SB300 |
Salmonella Typhimurium, Smr |
Wild type |
[41] |
| M1525 |
Salmonella Enteritidis 125109 wild type; Smr |
Wild type |
[42] |
| MT4 |
S. Typhimurium ΔssaV,Δmig-14; Smr |
SB300 |
This study |
| MT5 |
S. Typhimurium ΔssaV; Smr |
SB300 |
This study |
|
Plasmids |
Relevant genotype (S) and/or phenotype (S) |
Resistance |
References |
| pM973 |
bla PssaH gfpmut2 plasmid with oripMB1 |
Ampr |
[44] |
| pKD46 |
Red recombinase expression plasmid; ParaB; oriR101 |
Ampr |
[43] |
| pKD4 |
Template plasmid; FRT-aphT-FRT |
Kmr |
[43] |
| pCP20 | FLP recombinase expression plasmid | Cmr, Ampr | [43] |
Bacterial growth condition
Luria-Bertani medium supplemented with 0.3 M sodium chloride (SPI-1 inducing medium) was used to grow all the bacterial strains (Table 2) at 37°C for 12 h. Strains were diluted 1:20 in fresh SPI-1 inducing medium and sub-cultured for another 4 h until the bacteria attained their early log phase. Bacterial cells were pelleted, washed in ice-cold phosphate buffered saline (PBS) and approximately 5 × 107 CFU were suspended in 50 μl cold PBS for use in the in vivo experiments. All the strains were tested for growth attenuation for 16 h in 10 ml of culture medium at 37°C with 150 rpm under aerated conditions.
Ethical statement
All the animal experiments were performed in strict accordance with guidelines laid by the Institutional Animal Ethics Committee (IAEC) of National Centre for Cell Science (NCCS) Pune, India; Permit Number: 7/1999/CPCSEA-09/03/1999.
Mouse lines
All experimental mice were specific pathogen free (SPF) C57BL/6 maintained in individually ventilated cages (IVC) (Tacket et al., 1992). Wild-type, Nos2−/− (B6.129P2- Nos2tm1Lau/J), Il-10−/− (B6.129P2-Il10tm1cgn/J) and CD40L−/− (B6.129S2-Cd40lgtm1Imx/J) mice were procured from Jackson Labs (Bar Harbor, ME) and bred in the C57BL/6 background at the animal facility of National Center for Cell Sciences (NCCS), Pune, India.
Mice infection experiment for assessment of strain attenuation
The infection experiments were performed in streptomycin pretreated SPF mice in IVC as described earlier [45,46]. C57BL/6, iNos−/−, Il10−/−and CD40L−/− mice were pretreated orally with 50 mg of streptomycin before infecting with wild-type and mutant strains. After 24 h, mice were infected with 5 × 107 CFU (oral gavage) of the corresponding bacterial strain (i.e. MT5, MT4 and SB300). The bacterial load in the cecum, mesenteric lymph nodes (mLNs), liver and spleen was determined by plating the respective tissue homogenates on MacConkey agar plates supplemented with appropriate antibiotics (Streptomycin, 50 μg/ml; kanamycin, 50 μg/ml; ampicillin, 100 μg/ml). For statistical analysis, samples without bacterial counts were adjusted to the minimum detection level (10 CFU/organ in the mLN, 20 CFU/organ in the spleen, 10/x CFU/g, where x represents the net weight of the cecum content or feces collected). Cecal pathology of the infected mice was scored to analyze the degree of inflammation [45].
Histopathological evaluation
Segments of the cecum, colon and ileum were embedded in Optimum Cutting Temperature solution O.C.T. (Sakura Finetek Inc., USA), snap-frozen in liquid nitrogen, and stored at −80°C. The 5 μm thick tissue sections were obtained on glass slides and stained with hematoxylin and eosin (H&E) stains after drying for at least 2 h at room temperature. The stained cryosections were evaluated on the basis of a previously described scoring system for the quantitative analysis of cecal inflammation [45,47]. The sections were scored on the basis of the pathological changes that include sub-mucosal edema (0–3), polymorphonuclear leukocyte infiltration (0–4), loss of goblet cells (0–3) and epithelial ulceration (0–3). The cumulative pathological scores ranged from 0 to 13 with arbitrary units covering the inflammation levels that included intact intestine without any sign of inflammation (pathoscore 0); minimal sign of inflammation (pathoscore 1–2), which is commonly found in the ceca of specific pathogen-free mice and generally not considered as a pathological feature; slight inflammation as a minimal sign of tissue pathology (pathoscore 3–4); moderate inflammation (pathoscore 5–8); and significant inflammation (pathoscore 9–13).
Vaccination and challenge experiment
For vaccination study, three groups of wild type C57BL/6 mice (n = 10; each group) were pretreated with streptomycin according to the protocol described earlier [34]. Mice groups (3 groups; n = 5 mice each group) were vaccinated with MT5, MT4 strains and PBS respectively; the mice group treated with PBS served as a negative control group [34,48]. Fecal samples from each mice group were collected weekly and plated on MacConkey agar plate for analysis of fecal shedding of the vaccine strain. At day 30 post vaccination (p.v.), the histopathology of cecal mucosa and bacterial loads of different tissues of vaccinated mice (n = 5; each group) were analyzed. Further, the gut wash and serum samples of vaccinated mice were collected to assess serum IgG and gut secretory IgA (sIgA) by Western blot. The remaining mice (n = 5) from each vaccinated group were treated with ampicillin (25 mg by gavage) and challenged after 24 h with wild-type S. Typhimurium (SB300; 200 CFU) harboring ampicillin resistant plasmid pM973. The colonization efficiency of the challenged strain was evaluated at various host sites at day 3 post challenge (p.c.).
Evaluation of serum and gut antibody response
To measure the mucosal immune response, serum IgG and secretory gut IgA responses were quantified by Western blot as described previously [34,48]. Serum and gut washes were collected at day 30 p.v from MT5 and MT4 immunized mice and the PBS treated control mice. The protein fractions of lysates from the overnight-grown S. Typhimurium wild-type strain (SB300), ssaV mutant (MT5), ssaV and mig-14 double mutant (MT4) and S. Enteritidis P125109 (M1525) wild-type strain were separated on polyacrylamide gels and transferred to nitrocellulose membrane. The membrane was treated with suitably diluted serum sample or gut washes followed by incubation with conjugated α-mouse IgG (for serum; Santa cruz) and α-mouse IgA (for gut wash; Santa cruz). The blots were developed by ECL kit (Thermo Scientific).
Statistical analysis
Statistical analyses were performed using the two-way ANOVA (GraphPad Prism 5). p < 0.05 was considered statistically significant.
Results and discussion
Additional mig-14 mutation in S. Typhimurium ssaV mutant shows significant attenuation in immunocompromised mice
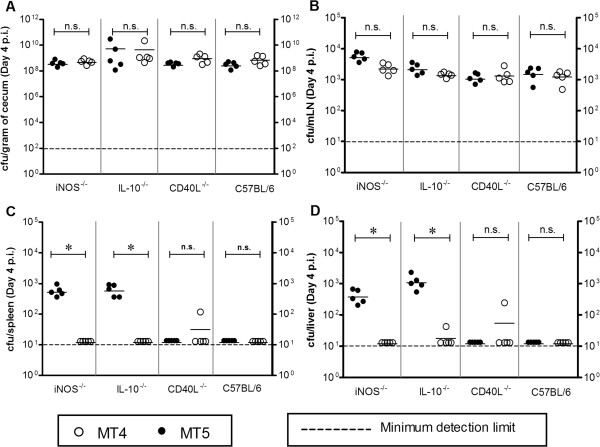
The attenuation of MT5 and MT4 strains in various immunocompromised mice was analyzed by normal infection experiment at day 4 p.i. In our initial observations, equivalent loads of MT5 and MT4 strains were detected in the cecal content of Nos2−/−, Il-10−/− mice (Figure 1A) whereas, MT4 showed reduced colonization in spleen and liver (Figure 1B, C and D) as compared to MT5. Similar experiment was carried out to assess the performance of MT4 in wt C57BL/6 and CD40L−/− mice. It was observed that neither MT4 nor MT5 colonized spleen and liver of CD40L−/− and wild-type C57BL/6 mice (Figure 1C-D). However, MT4 (ssaV, mig-14 mutant) colonized the mLN of wild-type mice as efficiently as MT5 (ssaV mutant) (Figure 1B). We also tested the attenuation profile in terms of competitive index of mig14::aphT single mutant against wild-type S. Typhimurium strain; it was appreciable that the mig14::aphT single mutant has reduced ability to colonize to systemic sites (Additional file 1: Figure S1 and Additional file 1: Figure S2); however, this reduced colonization in liver and spleen was not as sharp as in case of C57BL/6 mice infected with ssaV mutant MT5 (compare Additional file 1: Figure S2 with Figure 1C,D). Overall the data demonstrates that the deletion of mig-14 in the ssaV knockout background does not allow S. Typhimurium to colonize the systemic sites like liver and spleen in severely immunocompromised mice (Figure 1C and D).
Figure 1.
Analysis of MT4 attenuation in comparison to MT5 in Nos2−/−, Il-10−/−, CD40L−/−and wild-type C57BL/6 mice. Streptomycin pretreated mice were infected either with MT5 or MT4 (5x107 CFU by gavage; n = 5mice). Bacterial loads in cecum content (A), mLN (B), spleen (C) and liver (D) were assessed by plating at day 4 p.i.. n.s., statistically not significant; *, statistically significant (p < 0.05, Two-way ANOVA).
MT4 protects wild-type C57BL/6 mice when challenged with wild-type S. Typhimurium
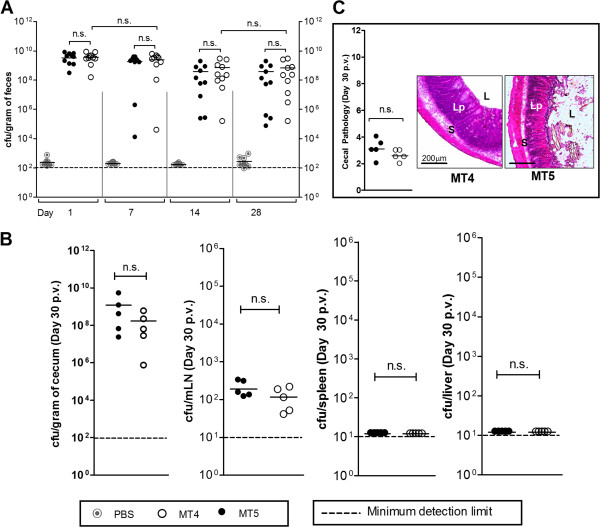
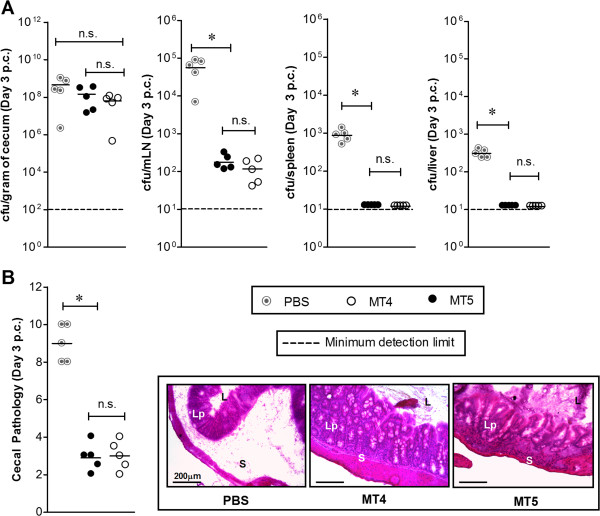
The immunogenic potential of MT4 in wild-type C57BL/6 mice was analyzed by previously established vaccination and challenge protocol using TTSS-2 deficient S. Typhimurium strain [34]. Three groups of wild-type C57BL/6 mice were vaccinated with MT4 (n = 10), MT5 (n = 10) and PBS (negative control; n = 10). The fecal shedding was analyzed as a measure of cecal colonization during vaccination period. Both, MT5 and MT4 strains reached a bacterial load of ~109 CFU/g (of cecal content) in the gut lumen at the day 1 p.v.; however, the bacterial loads slightly declined at day 14 and day 28 p.v. (Figure 2A). Half the number of vaccinated mice (MT5, n = 5; MT4, n = 5; PBS, n = 5) were sacrificed to analyze cecal inflammation and the colonization levels in different systemic sites at day 30 p.i. With both the strains, cecum colonization was maintained up to ~107-9 CFU/g. The bacterial load in mLN was lower as compared to the acute infection experiments (compare Figure 1B to 2B) whereas cecal mucosa did not show any sign of disease (Figure 2C). The remaining mice were analyzed for protection against a challenge with wild-type S. Typhimurium. At day 30 p.v., the remaining vaccinated mice (MT4, n = 5; MT5, n = 5; PBS, n = 5) were treated with 20 mg of ampicillin to remove regrown gut flora and any residual vaccine strain. Mice groups were then challenged with wild-type S. Typhimurium at day 31st (200 CFU by gavage). The wild-type S. Typhimurium was able to colonize the lumen efficiently and reached the carrying capacity by day 3 p.c. in all three immunized groups (Figure 3A). Mice in the PBS treated control group suffered from severe enteropathy (Figure 3B). In contrast, the mice immunized with MT5 and MT4 strains did not show any signs of mucosal inflammation (Figure 3B). Furthermore, spleen and liver colonization by wild-type S. Typhimurium was significantly reduced in both the vaccinated groups (p < 0.05; Figure 3A). Thus, the data indicates that MT4 strain conferred equivalent level of protection from Salmonella inflicted disease as MT5 strain.
Figure 2.
Vaccination experiment analyzing the attenuation of MT4 at day 30 p.v. For vaccination, C57BL/6 mice were treated with PBS (n = 10; grey solid circles), MT5 (5x107CFU; n = 10; black solid circle) and MT4 (5x107 CFU; n = 10; open circle). (A) Fecal shedding as analyzed by plating. PBS-controls: below detection limit (stripped line); (B) Colonization by the vaccine strains (MT5, n = 5 and MT4, n = 5) in cecal content, mLN, spleen and liver; (C) Cecal pathology at day 30 p.v.. n.s., not significant; *, statistically significant (p < 0.05).
Figure 3.
Analysis of colonization and cecal pathology of the vaccinated mice after wild-type S. Typhimurium challenge. Mice immunized with PBS, MT5 and MT4 (n = 5) were treated with ampicillin (25 mg by gavage), challenged with wild-type SB300 (ampr, smr) and sacrificed three days later (day 3 p.c.). Disease parameters like colonization at various host-tissues (A) and cecal pathology (B) were determined. n.s., not significant; *, statistically significant (p < 0.05).
Mice immunized with MT4 and MT5 showed equivalent response for both luminal IgA and serum specific IgG
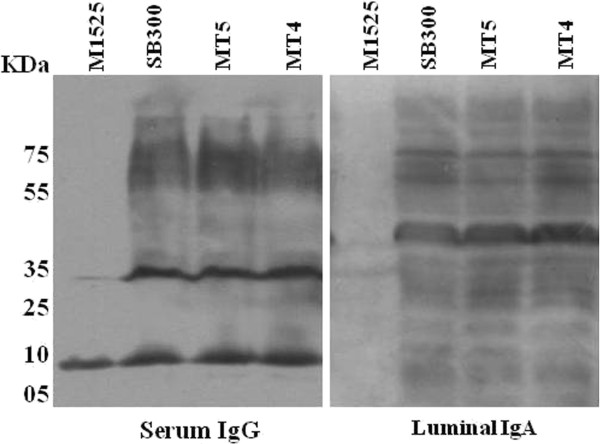
Earlier it has been established that immune-protection against S. Typhimurium is based on O-antigen specific luminal sIgA along with serum IgA, IgM and IgG responses [34]. To validate the immunogenic potential of MT4, the antibody titers of IgG from serum and IgA from gut wash samples of mice vaccinated with MT4 and MT5 strains were detected by western blotting at the end of the day 30 p.v. (Figure 4). This experiment relies on the specific antibody binding to specific antigens of the bacterium (wild-type S. Typhimurium) as compared to a bacterium of different serovar (wild-type S. Enteritidis). The intestinal wash and serum samples from mice vaccinated with either MT5 or MT4 exhibited equivalent antibody response of Salmonella specific serum IgG and luminal secretory IgA. We additionally tested the antibody response through flow cytometry analysis and the data supported the finding that MT4 or MT5 vaccination exhibits equivalent antibody response (Additional file 1: Figure S4). The T-cytotoxic and T-helper cells play a critical role in the clearance of Salmonella as well as in the production of specific antibodies during the late phase of infection. We analyzed the effect of MT5 and MT4 strains on T-cell population of the mesenteric lymph node. We quantified the CD4+ and CD8+ T-cell population recovered from the mLN of the vaccinated mice after day 30 p.v. The T-cell population were analyzed by flowcytometry and found to be almost equally populated in the vaccinated mice but significantly more in comparison to the PBS treated mice (Additional file 1: Figure S3). This gives a sign that, the MT4 strain has an ability to colonize and induce T-cell mediated innate and adaptive immune response in the wild-type C57BL/6 mice.
Figure 4.
Validation of antibody response (serum IgG and intestinal sIgA). Serum and gut wash from mice treated with PBS and vaccinated with MT4 and MT5 were collected, diluted to a highest dilution of 1:120 (serum) and 1:9 (gut wash). The presence of Salmonella specific IgG and secretory IgA were detected by Western blots. The representative Western blot analysis of the antibody responses was done by developing the blots of overnight grown cultures of MT5, MT4, SB300 (wild-type S. Typhimurium) and M1525 (S. Enteritidis; negative control) with the serum and gut wash of the immunized mice.
Conclusions
S. Typhimurium with a nonfunctional SPI-2 is considered as an avirulent and a potential vaccine strain [37]. In this study we have experimentally proved that S. Typhimurium diarrhea vaccine strain with nonfunctional SPI-2 system can be further attenuated without impeding the immunogenicity in immunocompromised hosts. We additionally mutated mig-14 in ssaV deficient S. Typhimurium strain. The ssaV, mig-14 double mutant was found to be highly attenuated in wild-type C57BL/6 mice and in immunocompromised mice like Nos2−/−, Il-10−/− and CD40L−/−. These transgenic immunocompromised mice were selected for this study because of their high susceptibility to different infections [33,49,50]. One of the characteristic features of Salmonella infections in humans is that few infected individuals can become chronic carriers. Such individuals comprise about 1–6% of the total infected population [19,24] acting as reservoirs, and restricting the pathogen within the human populations. Previous studies have established that the successive progression of host-adapted Salmonella species has led to an increased virulence because of their association with the host along with increased invasiveness and long-term persistence [51,52]. The virulence factors essential for long-term persistence of the pathogen in their respective hosts are therefore likely to be important for its evolutionary success.
Mig-14 is an important factor for Salmonella resistance to IFN-γ-mediated host responses and to different anti-microbial peptide during the establishment of infection as well as survival in the macrophages [16]. It has also been reported that mig-14 mutant can establish an infection but cannot persist for longer periods in the host system [53]. These reports support the contribution of Mig-14 in Salmonella long-term virulence. Although the mechanism of Mig-14 action is not completely established, the binding of Mig-14 deficient Salmonella to cathelin-related antimicrobial peptide (CRAMP) proves its active involvement in Salmonella antimicrobial peptide resistance [40]. Mechanistically, Mig-14 protein is a periplasmic protein which is tightly associated with the inner membrane of Salmonella[53]. The transmission electron microscopy study has revealed that the primary site of host CRAMP activity is the bacterial cytoplasm. Study of inner membrane localization of Mig-14 and cytoplasmic CRAMP activity, possibly suggests the role of Mig-14 in preventing penetration of CRAMP into the cytoplasm [40]. Taken together, these reports explain contribution of mig-14 towards pathogen survival by encountering host inflammatory responses and promoting both acute and persistent bacterial infection. Therefore, in the present study, mig-14 was taken as an important virulence factor to be knocked out from the existing live attenuated strain (MT5) with the goal to improve the attenuation attributes in immunocompromised mice.
In this study, we have assessed the degree of attenuation of S. Typhimurium ssaV mutant (MT5) and ssaV, mig-14 double mutant (MT4) in immunocompromised mice, by infecting these two strains to Nos2−/−, Il-10−/−and CD40L−/− C57BL/6 mice. The day 4 p.i. observation showed a high degree of systemic attenuation of MT4 (ssaV, mig-14) strain in Nos2−/−, Il-10−/− mice in comparison to the MT5 (ssaV) strain. On the other hand MT5 and MT4 strains were equally attenuated in CD40L−/− mice. Interestingly, MT4 strain also retained its capacity to colonize the mesenteric lymph node of Nos2−/−, Il-10−/−and CD40L−/− mice, demonstrating its ability to access the mLN but not the systemic sites. The in vivo data showed that the attenuation of MT4 in immunocompromised mice could be due to the absence of mig-14 in ssaV deficient S. Typhimurium. Furthermore, the MT4 and MT5 strains were used to vaccinate the wild-type C57BL/6 mice. Results showed that none of the mice developed cecal inflammation at day 30 p.v. However, both the strains (MT5 and MT4) equally colonized the gut lumen of vaccinated mice groups. Apart from this, at 30 day p. v., neither of the strain was found in the systemic organs which diminishes the possibility of late systemic dissemination and associated disease symptoms. Interestingly, apart from MT5, we also found a small population of MT4 strain in the mesenteric lymph node of the immunized mice, showing the potential of MT4 to stay in the lymphoid tissue for a longer period. In a challenge experiment, the vaccinated mice were protected when challenged with wild-type S. Typhimurium, however, the PBS treated mice developed significant inflammation and systemic dissemination of S. Typhimurium during subsequent Salmonella challenge.
In conclusion, the MT4 live-attenuated S. Typhimurium strain provides an efficient antibody mediated immune response which can protect even immunocompromised hosts from lethal infection of Salmonella. Specific antibody response to any protein antigens requires the involvement of both CD4+ and CD8+ T-cells along with the B-cells. The T-cell dependent antigens require the involvement of T-cells for the adaptive immune response. T helper (CD4+) cells play a vital role in stimulating the B-cells for the production of pathogen specific antibody via clonal propagation. Additionally, the activated CD4+ and CD8+ T-cells are the major producers of INF-γ which further activates the tissue and blood macrophages. As T-cell contributes to the cell mediated immune response, it is important to estimate the T-cell propagation during the course of Salmonella infection. In this study we have additionally estimated CD4+ and CD8+ T-cells from the mLN of the immunized mice. CD4+ and CD8+ T-cell population of the mice immunized with MT4 strain found to be comparable with the mice immunized with MT5 strain. Hence, it concludes that the MT4 strain retains its ability to induce the classical innate and adaptive immune response even after a strong attenuation. Therefore, we propose that incorporating additional “safety” features such as the deletion of mig-14 can be of a general interest for the design of new super live attenuated S. Typhimurium strain. This attenuated strain could also be used for developing the recombinant vaccine against other enteric pathogens.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NB, BS, MS designed the experiments; NP, VV, SS performed the experiments; NB, NS, MS wrote the manuscript; VV, NS, SD, MS reviewed and edited the manuscript; BS provided the animal facility; MS provided the chemicals and consumables for this study. All authors read and approved the final manuscript.
Supplementary Material
Evaluation of attenuation profile of mig14::aphT mutant in comparison to wild-type strain of Salmonella Typhimurium. Competitive index profile of mig-14::aphT mutant when compared against wild-type strain. n.s. = not significant; * = p < 0.05). Figure S2. Infection profile of mig14::aphT mutant in comparison to wild-type strain of Salmonella Typhimurium .Infection profile and systemic attenuation of mig14::aphT mutant. Bar indicates 200 μm. n.s. = not significant; * = p < 0.05). Figure S3. Flowcytometric analysis of T-cell population after Salmonella infection. The whole cells were isolated from the mLN of the vaccinated mice. The cells were then suspended in appropriate medium and processed for flow cytometric analysis (see materials and methods). The cells were detected by using specific conjugated antibodies against specific T-cells. Figure S4. Luminal and serum specific antibody responses in mice immunized with MT5 and MT4. Serum and gut wash from mice treated with PBS and vaccinated with MT4 and MT5 were collected, diluted to a highest dilution of 1:120 (serum) and 1:9 (gut wash). The presence of Salmonella specific IgG and secretory IgA were detected by bacterial flow cytometric (A) and Western blot (B). Each coloured line indicates data obtained from individual mice of respective group. The representative Western blot analysis of the antibody responses was done by developing the blots from the overnight cultures of MT5, MT4, SB300 (wt S. Typhimurium) and M1525 (S. Enteritidis; negative control) by using the sera and gut luminal sIgA of the immunized mice.
Contributor Information
Niladri Bhusan Pati, Email: niladribiotech@gmail.com.
Vikalp Vishwakarma, Email: vvikalpksbt@gmail.com.
Sathish kumar Selvaraj, Email: selvaraj.sathishk@gmail.com.
Sabyasachi Dash, Email: Sabyasachidash2012@gmail.com.
Bhaskar Saha, Email: sahab@nccs.res.in.
Neera Singh, Email: nrsingh2009@gmail.com.
Mrutyunjay Suar, Email: msbiotek@yahoo.com.
Acknowledgements
This work was supported by the grant from Department of Biotechnology, Govt. of India (Project No. BT/PR14489/Med/29/207/2010). We thank Himanshu Singh Chandel for his support during the experiments.
References
- Okamura M, Lillehoj HS, Raybourne RB, Babu US, Heckert RA. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-gamma production. Comp Immunol Microbiol Infect Dis. 2004;27(4):255–272. doi: 10.1016/j.cimid.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Thatte J, Rath S, Bal V. Analysis of immunization route-related variation in the immune response to heat-killed Salmonella typhimurium in mice. Infect Immun. 1995;63(1):99–103. doi: 10.1128/iai.63.1.99-103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penha Filho RA, Moura BS, de Almeida AM, Montassier HJ, Barrow PA, Berchieri Junior A. Humoral and cellular immune response generated by different vaccine programs before and after Salmonella Enteritidis challenge in chickens. Vaccine. 2012;30(52):7637–7643. doi: 10.1016/j.vaccine.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect Immun. 2011;79(7):2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EN, Snoeyenbos GH, Weinack OM, Smyser CF. Studies on the use of 9R strain of Salmonella gallinarum as a vaccine in chickens. Avian Dis. 1981;25(1):38–52. doi: 10.2307/1589825. [DOI] [PubMed] [Google Scholar]
- Roland K, Tinge S, Warner E, Sizemore D. Comparison of different attenuation strategies in development of a Salmonella hadar vaccine. Avian Dis. 2004;48(3):445–452. doi: 10.1637/7106. [DOI] [PubMed] [Google Scholar]
- Robertsson JA, Lindberg AA, Hoiseth S, Stocker BA. Salmonella typhimurium infection in calves: protection and survival of virulent challenge bacteria after immunization with live or inactivated vaccines. Infect Immun. 1983;41(2):742–750. doi: 10.1128/iai.41.2.742-750.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladoianu IR, Dubini F. Experimental model of oral antityphoid vaccination with live streptomycin-dependent Salmonella typhimurium in C57BL/6 mice. J Hyg (Lond) 1975;75(2):215–218. doi: 10.1017/S0022172400047240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totemeyer S, Kaiser P, Maskell DJ, Bryant CE. Sublethal infection of C57BL/6 mice with Salmonella enterica Serovar Typhimurium leads to an increase in levels of Toll-like receptor 1 (TLR1), TLR2, and TLR9 mRNA as well as a decrease in levels of TLR6 mRNA in infected organs. Infect Immun. 2005;73(3):1873–1878. doi: 10.1128/IAI.73.3.1873-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma V, Pati NB, Chandel HS, Sahoo SS, Saha B, Suar M. Evaluation of Salmonella enterica serovar Typhimurium TTSS-2 deficient fur mutant as safe live-attenuated vaccine candidate for immunocompromised mice. PLoS One. 2012;7(12):e52043. doi: 10.1371/journal.pone.0052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toobak H, Rasooli I, Talei D, Jahangiri A, Owlia P, Darvish Alipour Astaneh S. Immune response variations to Salmonella enterica serovar Typhi recombinant porin proteins in mice. Biologicals. 2013;41(4):224–230. doi: 10.1016/j.biologicals.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Chaudhuri RR, Peters SE, Pleasance SJ, Northen H, Willers C, Paterson GK, Cone DB, Allen AG, Owen PJ, Shalom G. et al. Comprehensive identification of Salmonella enterica serovar typhimurium genes required for infection of BALB/c mice. PLoS Pathog. 2009;5(7):e1000529. doi: 10.1371/journal.ppat.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheminay C, Hensel M. Rational design of Salmonella recombinant vaccines. Int J Med Microbiol. 2008;298(1–2):87–98. doi: 10.1016/j.ijmm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Gilks CF, Brindle RJ, Otieno LS, Simani PM, Newnham RS, Bhatt SM, Lule GN, Okelo GB, Watkins WM, Waiyaki PG. et al. Life-threatening bacteraemia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet. 1990;336(8714):545–549. doi: 10.1016/0140-6736(90)92096-Z. [DOI] [PubMed] [Google Scholar]
- Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree MJ, Walsh AL, Corkill JE, Hart CA, Gilks CF, Molyneux ME. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. Aids. 2002;16(12):1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- Raupach B, Kaufmann SH. Bacterial virulence, proinflammatory cytokines and host immunity: how to choose the appropriate Salmonella vaccine strain? Microbes Infect. 2001;3(14–15):1261–1269. doi: 10.1016/s1286-4579(01)01486-1. [DOI] [PubMed] [Google Scholar]
- Dunstan SJ, Simmons CP, Strugnell RA. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune responses against a heterologous antigen. Infect Immun. 1998;66(2):732–740. doi: 10.1128/iai.66.2.732-740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmory HS, Leary SE, Griffin KF, Williamson ED, Brown KA, Titball RW. The use of live attenuated bacteria as a delivery system for heterologous antigens. J Drug Target. 2003;11(8–10):471–479. doi: 10.1080/10611860410001670008. [DOI] [PubMed] [Google Scholar]
- Hohmann EL, Oletta CA, Miller SI. Evaluation of a phoP/phoQ-deleted, aroA-deleted live oral Salmonella typhi vaccine strain in human volunteers. Vaccine. 1996;14(1):19–24. doi: 10.1016/0264-410X(95)00173-X. [DOI] [PubMed] [Google Scholar]
- Tacket CO, Kelly SM, Schodel F, Losonsky G, Nataro JP, Edelman R, Levine MM, Curtiss R 3rd. Safety and immunogenicity in humans of an attenuated Salmonella typhi vaccine vector strain expressing plasmid-encoded hepatitis B antigens stabilized by the Asd-balanced lethal vector system. Infect Immun. 1997;65(8):3381–3385. doi: 10.1128/iai.65.8.3381-3385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SN, Strugnell RA, Dougan G. Live Salmonella as vaccines and carriers of foreign antigenic determinants. Vaccine. 1989;7(6):495–498. doi: 10.1016/0264-410X(89)90271-5. [DOI] [PubMed] [Google Scholar]
- Curtiss R 3rd, Wanda SY, Gunn BM, Zhang X, Tinge SA, Ananthnarayan V, Mo H, Wang S, Kong W. Salmonella enterica serovar typhimurium strains with regulated delayed attenuation in vivo. Infect Immun. 2009;77(3):1071–1082. doi: 10.1128/IAI.00693-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heithoff DM, Enioutina EY, Daynes RA, Sinsheimer RL, Low DA, Mahan MJ. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect Immun. 2001;69(11):6725–6730. doi: 10.1128/IAI.69.11.6725-6730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Suzuki M, Isshiki Y, Kodama C, Eguchi M, Kikuchi Y, Motokawa K, Takaya A, Tomoyasu T, Yamamoto T. Oral immunization with ATP-dependent protease-deficient mutants protects mice against subsequent oral challenge with virulent Salmonella enterica serovar typhimurium. Infect Immun. 2003;71(1):30–39. doi: 10.1128/IAI.71.1.30-39.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland WC, Stocker BA. Effect of different purine auxotrophic mutations on mouse-virulence of a Vi-positive strain of Salmonella dublin and of two strains of Salmonella typhimurium. Microb Pathog. 1987;3(2):129–141. doi: 10.1016/0882-4010(87)90071-4. [DOI] [PubMed] [Google Scholar]
- Miller SI, Loomis WP, Alpuche-Aranda C, Behlau I, Hohmann E. The PhoP virulence regulon and live oral Salmonella vaccines. Vaccine. 1993;11(2):122–125. doi: 10.1016/0264-410X(93)90006-J. [DOI] [PubMed] [Google Scholar]
- Angelakopoulos H, Hohmann EL. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun. 2000;68(4):2135–2141. doi: 10.1128/IAI.68.4.2135-2141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR. et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun. 2002;70(7):3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F. et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20(1):142–152. doi: 10.1200/JCO.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone DM, Tacket CO, Harris AM, Kay B, Losonsky G, Levine MM. Evaluation in volunteers of a candidate live oral attenuated Salmonella typhi vector vaccine. J Clin Invest. 1992;90(2):412–420. doi: 10.1172/JCI115876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilts DA, Riesenfeld-Orn I, Fulginiti JP, Ekwall E, Granert C, Nonenmacher J, Brey RN, Cryz SJ, Karlsson K, Bergman K. et al. Phase I clinical trials of aroA aroD and aroA aroD htrA attenuated S. typhi vaccines; effect of formulation on safety and immunogenicity. Vaccine. 2000;18(15):1473–1484. doi: 10.1016/S0264-410X(99)00424-7. [DOI] [PubMed] [Google Scholar]
- Kotton CN, Lankowski AJ, Scott N, Sisul D, Chen LM, Raschke K, Borders G, Boaz M, Spentzou A, Galan JE. et al. Safety and immunogenicity of attenuated Salmonella enterica serovar Typhimurium delivering an HIV-1 Gag antigen via the Salmonella Type III secretion system. Vaccine. 2006;24(37–39):6216–6224. doi: 10.1016/j.vaccine.2006.05.094. [DOI] [PubMed] [Google Scholar]
- Kwon YM, Cox MM, Calhoun LN. Salmonella-based vaccines for infectious diseases. Expert Rev Vaccines. 2007;6(2):147–152. doi: 10.1586/14760584.6.2.147. [DOI] [PubMed] [Google Scholar]
- Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M. et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010;6(9):e1001097. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel M, Shea JE, Gleeson C, Jones MD, Dalton E, Holden DW. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269(5222):400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- Shea JE, Beuzon CR, Gleeson C, Mundy R, Holden DW. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun. 1999;67(1):213–219. doi: 10.1128/iai.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periaswamy B, Maier L, Vishwakarma V, Slack E, Kremer M, Andrews-Polymenis HL, McClelland M, Grant AJ, Suar M, Hardt WD. Live attenuated S. Typhimurium vaccine with improved safety in immuno-compromised mice. PLoS One. 2012;7(9):e45433. doi: 10.1371/journal.pone.0045433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2(10):820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Valdivia RH, Cirillo DM, Lee AK, Bouley DM, Falkow S. mig-14 is a horizontally acquired, host-induced gene required for salmonella enterica lethal infection in the murine model of typhoid fever. Infect Immun. 2000;68(12):7126–7131. doi: 10.1128/IAI.68.12.7126-7131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky IE, Ghori N, Falkow S, Monack D. Mig-14 is an inner membrane-associated protein that promotes Salmonella typhimurium resistance to CRAMP, survival within activated macrophages and persistent infection. Mol Microbiol. 2005;55(3):954–972. doi: 10.1111/j.1365-2958.2004.04444.x. [DOI] [PubMed] [Google Scholar]
- Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Vishwakarma V, Periaswamy B, Bhusan Pati N, Slack E, Hardt WD, Suar M. A novel phage element of Salmonella enterica serovar Enteritidis P125109 contributes to accelerated type III secretion system 2-dependent early inflammation kinetics in a mouse colitis model. Infect Immun. 2012;80(9):3236–3246. doi: 10.1128/IAI.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Stecher B, Barthel M, Kremer M, Muller AJ, Heikenwalder M, Stallmach T, Hensel M, Pfeffer K, Akira S. et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174(3):1675–1685. doi: 10.4049/jimmunol.174.3.1675. [DOI] [PubMed] [Google Scholar]
- Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71(5):2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suar M, Jantsch J, Hapfelmeier S, Kremer M, Stallmach T, Barrow PA, Hardt WD. Virulence of broad- and narrow-host-range Salmonella enterica serovars in the streptomycin-pretreated mouse model. Infect Immun. 2006;74(1):632–644. doi: 10.1128/IAI.74.1.632-644.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suar M, Periaswamy B, Songhet P, Misselwitz B, Muller A, Kappeli R, Kremer M, Heikenwalder M, Hardt WD. Accelerated type III secretion system 2-dependent enteropathogenesis by a Salmonella enterica serovar enteritidis PT4/6 strain. Infect Immun. 2009;77(9):3569–3577. doi: 10.1128/IAI.00511-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endt K, Maier L, Kappeli R, Barthel M, Misselwitz B, Kremer M, Hardt WD. Peroral ciprofloxacin therapy impairs the generation of a protective immune response in a mouse model for Salmonella enterica serovar Typhimurium diarrhea, while parenteral ceftriaxone therapy does not. Antimicrob Agents Chemother. 2012;56(5):2295–2304. doi: 10.1128/AAC.05819-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews FJ, Katz F, Jones A, Smith S, Finn A. CD40 ligand deficiency presenting as unresponsive neutropenia. Arch Dis Child. 1996;74(5):458–459. doi: 10.1136/adc.74.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padigel UM, Alexander J, Farrell JP. The role of interleukin-10 in susceptibility of BALB/c mice to infection with Leishmania mexicana and Leishmania amazonensis. J Immunol. 2003;171(7):3705–3710. doi: 10.4049/jimmunol.171.7.3705. [DOI] [PubMed] [Google Scholar]
- Levine MM, Black RE, Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. J Infect Dis. 1982;146(6):724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- Hoffman TA, Ruiz CJ, Counts GW, Sachs JM, Nitzkin JL. Waterborne typhoid fever in Dade County, Florida. Clinical and therapeutic evaluation of 105 bacteremic patients. Am J Med. 1975;59(4):481–487. doi: 10.1016/0002-9343(75)90255-7. [DOI] [PubMed] [Google Scholar]
- Brodsky IE, Ernst RK, Miller SI, Falkow S. mig-14 is a Salmonella gene that plays a role in bacterial resistance to antimicrobial peptides. J Bacteriol. 2002;184(12):3203–3213. doi: 10.1128/JB.184.12.3203-3213.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of attenuation profile of mig14::aphT mutant in comparison to wild-type strain of Salmonella Typhimurium. Competitive index profile of mig-14::aphT mutant when compared against wild-type strain. n.s. = not significant; * = p < 0.05). Figure S2. Infection profile of mig14::aphT mutant in comparison to wild-type strain of Salmonella Typhimurium .Infection profile and systemic attenuation of mig14::aphT mutant. Bar indicates 200 μm. n.s. = not significant; * = p < 0.05). Figure S3. Flowcytometric analysis of T-cell population after Salmonella infection. The whole cells were isolated from the mLN of the vaccinated mice. The cells were then suspended in appropriate medium and processed for flow cytometric analysis (see materials and methods). The cells were detected by using specific conjugated antibodies against specific T-cells. Figure S4. Luminal and serum specific antibody responses in mice immunized with MT5 and MT4. Serum and gut wash from mice treated with PBS and vaccinated with MT4 and MT5 were collected, diluted to a highest dilution of 1:120 (serum) and 1:9 (gut wash). The presence of Salmonella specific IgG and secretory IgA were detected by bacterial flow cytometric (A) and Western blot (B). Each coloured line indicates data obtained from individual mice of respective group. The representative Western blot analysis of the antibody responses was done by developing the blots from the overnight cultures of MT5, MT4, SB300 (wt S. Typhimurium) and M1525 (S. Enteritidis; negative control) by using the sera and gut luminal sIgA of the immunized mice.