Abstract
Purpose
RTOG 9704 demonstrated a prognostic role for postoperative CA 19-9 in patients with resectable pancreatic carcinoma following surgery. Our study aimed to investigate whether CA 19-9 provided similar prognostic information in patients with locally advanced unresectable pancreatic cancer (LAPC) treated with chemoradiotherapy (CRT) and to determine whether such endpoints should therefore be reported in future randomized trials.
Methods and materials
Between December 1998 and October 2009, 253 patients with LAPC were treated with 5-fluourouracil-based concurrent CRT at our institution. Median radiation dose was 50.4 Gy. Only patients with a bilirubin of less than 2 mg/dL at the time the CA 19-9 was evaluated were included in the analysis to avoid the confounding effect of hyperbilirubinemia. Of the eligible patients, 54 had pre and post CRT CA 19-9 values available. The median age was 68 years and 52% were female. Categorized versions of the first post-CRT CA 19-9 were tested in 50 point increments beginning at <50 to >1,000 and percent change in pre to post-CRT CA 19-9 using cut points of 10% increments from <0% (increased) to >90%. Survival was measured from the date of first post CRT CA 19-9 level until death or last follow-up. Univariate and multivariate statistical methodologies were used to determine significant prognostic factors for overall survival.
Results
Median CA 19-9 prior to CRT was 363 U/mL and post CRT median was 85.5 U/mL. Following CRT, patients with a decrease of >90% from their baseline CA 19-9 level had a significantly improved median survival than those that did not (16.2 vs. 7.5 months, P=0.01). The median survival of patients with a CA 19-9 level lower than the median post CRT value was 10.3 months, compared with 7.1 months for those with a CA 19-9 level greater than the median (P=0.03). Post CRT CA 19-9 less than 50 U/mL and histologic grade I-II also showed prognostic significance (both P=0.03). In multivariate analysis, post CRT CA 19-9 less than the median level of 85.5 U/mL was an independent prognostic factor for overall survival (HR 0.34; 95% CI, 0.13-0.85, P=0.02).
Conclusions
Our results indicate that post treatment CA 19-9 is predictive for overall survival in patient with LAPC following CRT. We recommend that pre and post treatment CA 19-9 levels be obtained in patients receiving CRT and that these values be considered for prognostic nomograms and future clinical trials.
Key Words: CA19-9, pancreatic cancer, chemoradiotherapy (CRT)
Introduction
Most patients with adenocarcinoma of the pancreas present with metastatic disease or locally advanced unresectable pancreatic cancer (LAPC) that are defined as surgically unresectable at the time of diagnosis. With only about 1% of patients is still alive 5 years from the time of diagnosis, these patients have a very poor prognosis. Current therapeutic approaches for patients with LAPC include these of chemoradiotherapy (CRT) or chemotherapy (1,2). Tumor-associated antigens, including carcinoembyronic antigen (CEA), pancreatic anti-oncofetal antigen, tissue polypeptide, cancer antigen (CA) 125, and carbohydrate antigen (CA) 19-9 have been linked to pancreatic adenocarcinoma. CA 19-9 is a sialylated Lewis a blood group antigen most commonly expressed in pancreatic cancer as well as benign hepatobiliary disease.
Several studies have demonstrated a relation between the kinetics of CA 19-9 levels in patients with resectable pancreatic carcinoma undergoing surgery. Low postoperative serum CA 19-9 levels and a decrease in serial levels following surgery have been shown to correlate with survival (3). RTOG 9704 demonstrated a prognostic role for postoperative CA 19-9 levels in patients with resectable pancreatic carcinoma following surgery (4). The National Comprehensive Cancer Network recommends measurement of serum CA 19-9 level following surgery prior to the administration of adjuvant therapy. Although initial CA 19-9 levels have been shown to correlate with survival in patients with LAPC or metastatic disease, there is conflicting evidence regarding the predictive value of peri-treatment CA 19-9 levels in patients with LAPC treated with radiotherapy or chemotherapy (5-8). In patients who receive chemoradiation for LAPC, data is limited regarding the prognostic significance of peri-treatment CA 19-9 (9-11).
Our study aimed to investigate whether CA 19-9 provides prognostic information in patients with LAPC treated with CRT and to determine whether such endpoints should be reported in future randomized trials. This could help to identify patients who may likely benefit from various therapeutic strategies.
Methods
Patients
From December 1998 to October 2009, 253 consecutive patients with pancreatic adenocarcinoma treated at Roswell Park Cancer Institute (RPCI) were identified. All patient data were entered retrospectively by a single investigator after approval from the hospital institutional review board. Of the 253 patients, 159 underwent treatment with CRT or chemotherapy alone. Patients with metastatic disease at presentation and those who underwent surgery for definitive resection were excluded. Patients with islet-cell tumors and mucinous cystadenocarcinoma were also excluded from the analysis.
The variables evaluated included age, gender, race, Eastern Cooperative Oncology Group performance status, weight loss >10%, chemotherapy regimen, grade 3-4 toxicity, tumor diameter, and tumor location, T stage, nodal status, histologic grade, hemoglobin at diagnosis, pre and post CRT CA 19-9 and percent change from pre and post CRT. Stage was determined according to the American Joint Committee on Cancer staging system 6th edition (12). Patient data was obtained through the tumor registry and review of medical records and abstracted by a single investigator.
To avoid false-positive elevation of serum CA19-9 due to hepatobiliary diseases, chronic pancreatitis, obstruction of the common bile duct, all CA l9-9 levels were matched to a concomitant bilirubin to ensure biliary obstruction was not affecting the interpretation of CA 19-9 concentration. Patients with a serum bilirubin more than 2 mg/dL at the time of CA 19-9 measurement were excluded. The median pre-CRT CA 19-9 and post-CRT values were obtained. This was tested in 50 point increments beginning at <50 to ≥1,000. Percent change in pre to post-CRT CA 19-9 levels were calculated as follows: [(pre-CRT CA 19-9)-(Post-CRT CA 19-9)]/(pre-CRT CA 19-9) and were tested using cut points of 10% increments were from <0% (increased) to ≥90%.
Statistical analysis
Survival was measured from the date of first post CRT CA 19-9 level until death or last follow-up to ensure meaningful interpretation for the variable when evaluating a decrease in value. Progression free survival was calculated from date of first post CRT CA 19-9 level until recurrence as evidenced by radiographic imaging. Initial (univariate) log-rank tests were performed to determine the predictive value of categorized versions of the first post-CRT CA 19-9. Univariate and multivariate statistical methodologies were used to determine significant prognostic factors for overall survival.
The Kaplan-Meier method was used to obtain overall survival and recurrence free survival estimates while survival was compared between groups using the log-rank test. P values for multiple comparison were adjusted using the method developed by Lausen and Schumacher (13). Values for continuous variables are given as median (range). Values for categorical data are specified as frequency (percent). Statistical Analysis was performed using SAS Statistical analysis software version 9.2 (SAS Institute Inc, Cary, NC, USA). A nominal significance level of 0.05 was used.
Results
Patient and treatment characteristics
Of 116 patients, 84 underwent CRT and 32 received chemotherapy alone. Of the 84 patients that underwent CRT, 54 patients had available pre and post CRT CA 19-9 levels and a bilirubin of less than 2 mg/dL at the time the CA 19-9 was measured. The characteristics of the patients are shown in Table 1.
Table 1. Patient and treatment characteristics.
| Characteristics | Number (%) n=54 | |
|---|---|---|
| Age | <65 | 23 (42.6) |
| ≥65 | 31 (57.4) | |
| Sex | Male | 26 (48.2) |
| Female | 28 (51.9) | |
| Race | White | 50 (92.6) |
| Nonwhite | 4 (7.4) | |
| ECOG | 0-1 | 51 (94.4) |
| 2 | 3 (5.6) | |
| Weight loss >10% | Yes | 33 (64.7) |
| No | 18 (35.3) | |
| Chemo regimen | Gem | 46 (85.2) |
| Non-Gem | 8 (14.8) | |
| T stage | T4 | 34 (63.0) |
| T3 | 20 (37.0) | |
| Node status | Negative | 32 (59.3) |
| Positive | 22 (40.7) | |
| Grade 3-4 toxicity | Yes | 9 (16.7) |
| No | 45 (83.3) | |
| Tumor >30 mm | Yes | 37 (69.8) |
| No | 16 (30.2) | |
| Tumor location | Head | 31 (57.4) |
| Body/Tail | 10 (18.5) | |
| Overlap | 11 (20.4) | |
| Other | 2 (3.7) | |
The median follow up was 7.15 months (range, 3.0-10.6 months). The median pre-CRT Ca 19-9 level was 363.7 and the median post CRT CA 19-9 level was 85.5. Median time from the end of RT to post CRT CA 19-9 was 35.89 days (range, 0.00-168.81 days). CA 19-9 values ranging from 50-1,000 were tested in 50 point increments and % change was tested in 10% increments (Tables 2,3).
Table 2. First post-CRT CA 19-9 level in increments of 50.
| Variable | Median survival | P-value | No. of patients | Median RFS | P-value | No. of patients |
|---|---|---|---|---|---|---|
| <50 | 11.0710 (7.1945,14.4875) | 0.0661 | 23 | 8.5085 (4.4678,14.36) | 0.30735 | 23 |
| ≥50 | 7.0959 (4.4678,8.5742) | 31 | 5.4534 (4.1721,7.8844) | 31 | ||
| <100 | 8.5742 (7.1945,14.3890) | 0.12986 | 29 | 7.6873 (4.7963,12.2208) | 0.42710 | 29 |
| ≥100 | 7.0959 (4.1721,8.7714) | 25 | 5.4534 (2.8252,7.8844) | 25 | ||
| <150 | 8.5742 (7.0959,14.3561) | 0.19159 | 31 | 7.6873 (5.0920,11.0710) | 0.51395 | 31 |
| ≥150 | 5.5191 (4.1721,8.7714) | 23 | 5.4534 (2.8252,8.7714) | 23 | ||
| <200 | 8.5414 (7.0959,14.3561) | 0.30101 | 32 | 7.1945 (5.0920,11.0710) | 0.66898 | 32 |
| ≥200 | 7.4901 (3.6137,9.1327) | 22 | 5.0591 (2.7267,8.7714) | 22 | ||
| <250 | 8.5414 (7.0302,12.2208) | 0.42058 | 34 | 7.0959 (4.7963,8.5742) | 1.0000 | 34 |
| ≥250 | 7.4901 (2.8252,9.1327) | 20 | 5.4534 (2.7267,9.1327) | 20 | ||
| <300 | 8.5414 (7.0302,12.2208) | 0.42058 | 34 | 7.0959 (4.7963,8.5742) | 1.0000 | 34 |
| ≥300 | 7.4901 (2.8252,9.1327) | 20 | 5.4534 (2.7267,9.1327) | 20 | ||
| <350 | 8.5742 (7.0959,12.2208) | 0.25902 | 36 | 7.1945 (5.0920,9.4284) | 0.91659 | 36 |
| ≥350 | 5.4534 (2.8252,8.7714) | 18 | 5.0591(2.7267,8.7714) | 18 | ||
| <400 | 8.5742 (7.0959,12.2208) | 0.25902 | 36 | 7.1945 (5.0920,9.4284) | 0.91659 | 36 |
| ≥400 | 5.4534 (2.8252,8.7714) | 18 | 5.0591 (2.7267,8.7714) | 18 | ||
| <450 | 8.5414 (7.0302,11.4652) | 0.89806 | 38 | 7.0959 (4.7963,8.5742) | 1.0000 | 38 |
| ≥450 | 7.4901 (2.8252,9.1327) | 16 | 5.4534 (2.0039,9.1327) | 16 | ||
| <500 | 8.5414 (7.0302,11.4652) | 0.89806 | 38 | 7.0959 (4.7963,8.5742) | 1.0000 | 38 |
| ≥500 | 7.4901 (2.8252,9.1327) | 16 | 5.4534 (2.0039,9.1327) | 16 | ||
| <550 | 8.5414 (7.0302,11.4652) | 0.89806 | 38 | 7.0959 (4.7963,8.5742) | 1.0000 | 38 |
| ≥550 | 7.4901 (2.8252,9.1327) | 16 | 5.4534 (2.0039,9.1327) | 16 | ||
| <600 | 8.5414 (7.0302,11.4652) | 0.89806 | 38 | 7.0959 (4.7963,8.5742) | 1.0000 | 38 |
| ≥600 | 7.4901 (2.8252,9.1327) | 16 | 5.4534 (2.0039,9.1327) | 16 | ||
| <650 | 8.5085 (7.0302,11.0710) | 1.0000 | 39 | 7.0959 (4.4678,8.5742) | 1.0000 | 39 |
| ≥650 | 7.5230 (2.8252,9.1327) | 15 | 7.4901 (2.0039,9.1327) | 15 | ||
| <700 | 8.5085 (7.0302,11.0710) | 1.0000 | 39 | 7.0959 (4.4678,8.5742) | 1.0000 | 39 |
| ≥700 | 7.5230 (2.8252,9.1327) | 15 | 7.4901 (2.0039,9.1327) | 15 | ||
| <750 | 8.5414 (7.0302,11.0710) | 1.0000 | 40 | 7.0959 (4.7963,9.1327) | 1.0000 | 40 |
| ≥750 | 7.4901 (2.8252,8.7714) | 14 | 5.4534 (2.0039,8.7714) | 14 | ||
| <800 | 8.5414 (7.0302,11.0710) | 1.0000 | 40 | 7.0959 (4.7963,9.1327) | 1.0000 | 40 |
| ≥800 | 7.4901 (2.8252,8.7714) | 14 | 5.4534 (2.0039,8.7714) | 14 | ||
| <850 | 8.5414 (7.0302,11.0710) | 1.0000 | 40 | 7.0959 (4.7963,9.1327) | 1.0000 | 40 |
| ≥850 | 7.4901 (2.8252,8.7714) | 14 | 5.4534 (2.0039,8.7714) | 14 | ||
| <900 | 8.5085 (7.0302,11.0710) | 1.0000 | 41 | 7.0959 (4.4678,8.5742) | 1.0000 | 41 |
| ≥900 | 7.5230 (4.1721,10.5453) | 13 | 7.4901(2.0039,10.5453) | 13 | ||
| <950 | 8.5085 (7.0302,11.0710) | 1.0000 | 41 | 7.0959 (4.4678,8.5742) | 1.0000 | 41 |
| ≥950 | 7.5230 (4.1721,10.5453) | 13 | 7.4901 (2.0039,10.5453) | 13 | ||
| <1,000 | 8.5085 (5.7162,10.3154) | 1.0000 | 42 | 7.0302 (4.4678,8.5742) | 1.0000 | 42 |
| ≥1,000 | 7.5230 (4.172,10.5453) | 12 | 7.4901 (1.2484,10.5453) | 12 |
Table 3. Percent change in pre to post CRT CA 19-9 level.
| Variable | Median survival | P-value | No. of patients | Median RFS | P-value | No. of patients |
|---|---|---|---|---|---|---|
| <0% (increased) | 5.0591 (2.726,17.477) | 1.0000 | 12 | 4.4678 (2.7267,5.0591) | 0.28719 | 12 |
| ≥0% (decreased) | 7.8844 (7.095,10.315) | 42 | 7.6873 (5.519,9.132) | 42 | ||
| <10% | 5.0591 (2.726,17.477) | 1.0000 | 13 | 4.4678 (2.726,7.194) | 0.26456 | 13 |
| ≥10% | 8.5085 (7.095,10.315) | 41 | 7.6873 (5.453,9.428) | 41 | ||
| <20% | 7.1945 (3.613,14.487) | 1.0000 | 15 | 4.4678 (2.825,8.771) | 1.0000 | 15 |
| ≥20% | 7.8844 (7.030,10.315) | 39 | 7.4901 (5.453,9.132) | 39 | ||
| <30% | 7.8844 (4.467,14.487) | 1.0000 | 19 | 5.0591 (3.613,9.428) | 1.0000 | 19 |
| ≥30% | 7.6873 (5.716,10.315) | 35 | 7.0959 (5.092,8.574) | 35 | ||
| <40% | 7.8844 (4.467,9.428) | 1.0000 | 22 | 5.0920 (3.613,8.771) | 0.90931 | 22 |
| ≥40% | 7.6873 (7.030,11.071) | 32 | 7.4901 (4.796,11.071) | 32 | ||
| <50% | 7.8844 (4.467,10.315) | 1.0000 | 25 | 5.0920 (3.613,8.508) | 0.42786 | 25 |
| ≥50% | 7.6873 (7.030,11.465) | 29 | 7.6873 (4.796,11.071) | 29 | ||
| <60% | 7.1945 (5.059,9.428) | 1.0000 | 27 | 5.0920 (3.613,7.884) | 0.15978 | 27 |
| ≥60% | 8.5414 (7.030,11.465) | 27 | 8.5414 (4.796,11.465) | 27 | ||
| <70% | 7.6873 (5.519,9.428) | 0.87356 | 33 | 7.0959 (4.467,8.508) | 0.29637 | 33 |
| ≥70% | 8.5742 (4.172,12.220) | 21 | 8.5742 (2.825,12.220) | 21 | ||
| <80% | 7.6873 (5.519,9.428) | 1.0000 | 38 | 5.9790 (4.467,8.508) | 0.46802 | 38 |
| ≥80% | 8.5742 (2.003,12.220) | 16 | 8.5742 (0.788,12.220) | 16 | ||
| <90% | 7.5230 (5.519,8.771) | 0.017853 | 48 | 5.9790 (4.467,7.687) | 0.0066 | 48 |
| ≥90% | 16.2615 (8.574,52.825) | 6 | 16.2615 (8.574,52.825) | 6 |
Patient characteristics including age, sex, race, performance status, weight loss >10% were tested and not statistically significant on univariate analysis. Tumor and treatment factors including chemo regimen, T stage, node status, grade 3-4 toxicity, tumor >30 mm, and tumor location were tested and not statistically significant. On univariate analysis, post CRT CA 19-9 <50, postCRT CA 19-9 <85.5, percent change ≥90%, and histologic grade all showed prognostic significance (Table 4).
Table 4. Univariate Analysis of prognostic factors associated with survival in patients with locally advanced pancreatic carcinoma.
| Variable | N | Median survival (months) | 1-year survival rate (%) | Relative risk (CI) | P value |
|---|---|---|---|---|---|
| Age (yrs) | |||||
| <65 | 23 | 7.5 | 32.6 | 0.76 (0.41-1.41) | 0.3803 |
| ≥65 | 31 | 8.51 | 19.6 | ||
| Gender | |||||
| Male | 26 | 7.1 | 18.0 | 1.63 (0.89-2.99) | 0.1135 |
| Female | 28 | 9.4 | 32.4 | ||
| Race | |||||
| White | 50 | 7.6 | 22.0 | 1.94 (0.59-6.34) | 0.2633 |
| Non-White | 4 | 12.2 | 66.7 | ||
| ECOG | |||||
| 0-1 | 51 | 7.8 | 25.5 | 0.93 (0.12-6.88) | 0.9425 |
| 2 | 3 | -- | 0 | ||
| Weight loss >10% | |||||
| Yes | 33 | 7.0 | 14.8 | 1.94 (0.97-3.86) | 0.0566 |
| No | 18 | 9.1 | 28.6 | ||
| Chemotherapy regimen | |||||
| Gemcitabine based | 46 | 7.6 | 22.2 | 1.57 (0.66-3.76) | 0.3023 |
| Non-Gemcitabine based | 8 | 8.5 | 37.5 | ||
| Grade 3-4 toxicity | |||||
| Yes | 9 | 10.3 | 44.4 | 0.84 (0.39-1.76) | 0.0638 |
| No | 45 | 7.6 | 20.01 | ||
| Tumor >30 mm | |||||
| Yes | 37 | 7.7 | 30.1 | 0.71 (0.35-1.44) | 0.3453 |
| No | 16 | 7.6 | 9.23 | ||
| Tumor location | 0.6763 | ||||
| Head | 31 | 7.2 | 24.7 | Ref; | |
| Body/tail | 10 | 11.5 | 38.1 | 0.67 (0.28-1.59); | |
| Overlap | 11 | 7.5 | 18.2 | 1.21 (0.59-2.50); | |
| Other | 2 | -- | 0 | 0.84 (0.11-6.32) | |
| T stage | 0.78 (0.48-1.48) | 0.4630 | |||
| T4 | 34 | 7.9 | 30.7 | ||
| T3 | 20 | 7.6 | 13.7 | ||
| Node status | 0.89 (0.48-1.63) | 0.7049 | |||
| Negative | 32 | 8.5 | 29.0 | ||
| Positive | 22 | 7.7 | 20.2 | ||
| Pre-treatment CA 19-9 >1,000 | |||||
| Yes | 16 | 7.5 | 7.5 | 1.70 (0.88-3.26) | 0.1066 |
| No | 38 | 8.5 | 32.8 | ||
| PostCRT CA 19-9 | |||||
| <50 | 23 | 11.1 | 45.7 | 0.50 (0.26-0.94) | 0.0287 |
| >50 | 31 | 7.1 | 8.3 | ||
| PostCRT CA 19-9 | |||||
| <85.5 | 27 | 10.3 | 43.9 | 0.50 (0.26-0.92) | 0.0242 |
| >85.5 | 27 | 7.1 | 8.6 | ||
| Percent change | |||||
| <90% | 48 | 7.5 | 18.0 | 4.45 (1.33-14.79) | 0.0084 |
| >90% | 6 | 16.3 | 80.0 | ||
| Histologic grade | |||||
| I-II | 31 | 10.3 | 40.5 | 0.37 (0.15-0.90) | 0.0288 |
| III-IV | 9 | 7.5 | 0 | ||
| Hemoglobin at diagnosis | |||||
| >12 | 44 | 7.9 | 27.7 | 0.70 (0.31-1.56) | 0.3832 |
| <12 | 10 | 7.6 | 12.7 |
The median survival of patients with a postCRT CA 19-9 level <85.5 U/mL was 10.3 months compared with 7.1 months in patients with higher levels (P=0.0242) (Figure 1). The median survival of patients with a decrease in CA 19-9 of >90% post CRT was 16.3 months compared with 7.5 months in those with a <90% post CRT CA 19-9 change (P=0.0179) (Figure 2). The median survival of patients with a post CRT CA 19-9 levels <50 U/mL was 11.1 months compared with 7.1 months in patients with levels ≥50 U/mL (P=0.0287)
Figure 1.
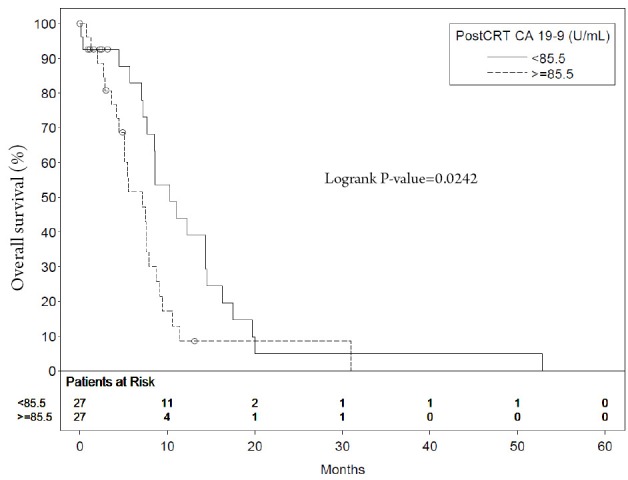
Median survival of patients with postCRT CA 19-9 level <85.5 U/mL compared with those with higher levels
Figure 2.
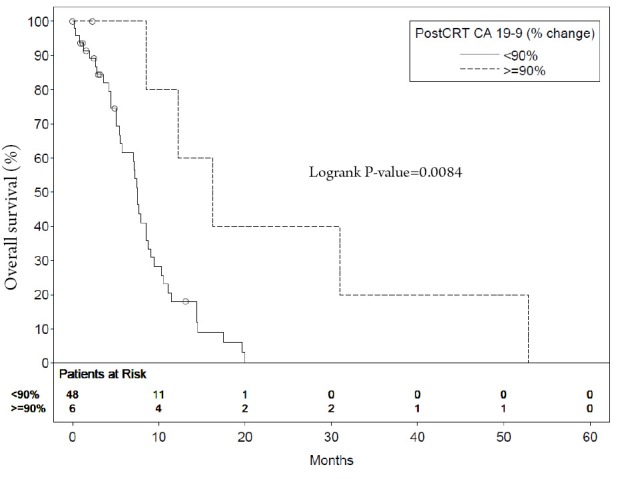
Median survival of patients with postCRT CA 19-9 level <90% increased compared with those with higher levels
On multivariate analysis, post CRT CA 19-9 <85.5 U/mL was an independent prognostic factor for overall survival (HR 0.34, 95% CI, 0.13-0.85, P=0.0216) (Table 5).
Table 5. Multivariate analysis for overall survival.
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Post-CRT CA 19-9 (<50 vs. ≥50) | 0.41(0.14-1.22) | 0.1081 |
| Post-CRT CA 19-9 (<85.5 vs. ≥85.5) | 0.34 (0.13-0.85) | 0.0216 |
| Percent change (<90% vs. ≥90%) | 3.56(0.81-15.66) | 0.0935 |
Discussion
The majority of patients with pancreatic cancer present with unresectable disease and appropriate selection of patients for CRT continues to be a challenge and the treatment of LAPC continues to evolve. Analysis of prognostic factors may be useful in determining which patients would benefit from intensification of therapy and designing future clinical trials.
CA 19-9 is the most common and important tumor marker used in for patients with pancreatic cancer. There have been many studies evaluating CA 19-9 as prognostic for resectable pancreatic cancer. RTOG 9704 demonstrated a prognostic role for postoperative CA 19-9 in patients with resectable pancreatic carcinoma following surgery. With a post-resection CA 19-9 higher than 90 U/mL, patients had a highly significant increased risk of death (HR, 3.34; P<0.0001) compared with those with a value less than or equal to that cutoff. This was the most important predictor of death in this cohort of patients. The results of this analysis of postoperative CA 19-9 level are important because they clearly identify a subgroup of patients who have a much higher risk of death after surgery with curative intent.
In patients receiving systemic chemotherapy for metastatic disease as well as LAPC, CA 19-9 levels have also been shown to be of prognostic significance in terms of overall survival. Tsavaris et al. demonstrated through multivariate analysis CA 19-9 levels of >30 times the normal limit had a significant independent effect on survival (5). Serum CA 19-9 alterations have been defined in a number of ways. In a study by Takahashi et al., they developed a new classification utilizing pretreatment CA 19-9 and proportional alteration of CA 19-9 2 months after the initiation of treatment (14) Their categories were defined as: I (increased), MD (modestly decreased), and SD (substantially decreased). In a study by Halm et al., a decrease of CA 19-9 during chemotherapy with gemcitabine predicted overall survival time in patients with advanced pancreatic cancer (8). In their study, they found that a decrease in CA 19-9 of >20% had the greatest prognostic impact.
There is limited data identifying CA 19-9 as a prognostic factor in patients with LAPC treated with concurrent CRT as the primary therapy (10-11). In a study by Micke et al. patients with LAPC were treated with hyperfractionated accelerated radiotherapy to a total dose of 44.8 Gy combined with 5-fluorouracil and folinic acid. Patients with a pretreatment CA 19-9 less than the median of 420 U/mL had a better median survival versus those with levels greater than the median (12.3 vs. 7.1 months, P=0.0056) (10). The median post-treatment CA 19-9 level for all patients was 293 U/mL and also exhibited prognostic significance. The median survival of patients with a CA 19-9 less than the post-treatment median was 13.5 months compared with 7.2 months for those with a CA 19-9 level greater than the median (P=0.003). Patients with no decline in CA 19-9 had a significantly lower tumor response rate and a significantly worse overall survival (6 months compared to 13.9 months, P=0.0002). On multivariate analysis, pretreatment CA 19-9 values greater than and less than the median value of 420 U/mL, post-treatment CA 19-9 values, and a tumor marker decrease during therapy were significantly independent prognostic factors for overall survival. In another concurrent CRT with conventional fractionation as the primary treatment in sixty-nine patients with LAPC, Koom et al. documented that the powerful cutoff points were pretreatment CA 19-9 level of 1,200 U/mL, post-treatment CA 19-9 level of 100 U/mL, and CA 19-9 decline of 40% (11). Their data support the theory that post-treatment CA19-9 levels and CA19-9 decline are significant prognostic factors.
These results are very similar to our findings in the present study. On univariate analysis, we found that post CRT CA 19-9 <50 U/mL, post CRT CA 19-9 <85.5 U/mL, percent change ≥90%, and histologic grade all showed prognostic significance predictor of survival. The median survival of patients with a CA 19-9 less than the post-treatment median was 10.3 months compared with those with a CA 19-9 level greater than the median value of 85.5 U/mL (P=0.0242). Our results were confirmed on multivariate analysis showing that a post treatment CA 19-9 level less than the median value of 85.5 U/mL was an independent prognostic factor for overall survival.
A strength of our study was that the first post-CRT CA 19-9 levels was tested in 50 point increments and percent change in pre and post treatment CA 19-9 was tested in 10% increments. This allowed us to detect subtle incremental changes that would otherwise not have been detected if a different method was used. In addition, all patients with a serum bilirubin more than 2 mg/dL at the time of CA 19-9 measurement were excluded to account for altered biliary excretion, for which bilirubin is a reasonable marker. This has been documented to occur at levels 1.5× the upper limit of normal or at a level of approximately 2.0 mg/dL (15).
The retrospective nature and sample size are limitations of our study. Patients with CA 19-9 levels within normal limits were not tested for the Lewis antigen. Lewisa-b- and are unable to increase their serum CA 19-9 levels and were not excluded from our analysis (16). However, only approximately 5% of the population are Lewisa-b- so this was unlikely to have a significant effect on our patient population In this study, we analyzed CA 19-9 as a prognostic factor and determined its utility in developing treatment strategies and designing future clinical trials. We analyzed whether peri-chemoradiation CA 19-9 values in the setting of normal bilirubin could predict post treatment survival. Additionally, the optimal time to evaluate CA 19-9 has not been fully investigated in patients receiving definitive CRT, chemotherapy alone, as well as postoperative setting. In our study, median time from the end of concurrent CRT to post CRT CA 19-9 was 36 days (range, 0.00-168.81 days). In RTOG 9704, the median time from surgery to the blood draw for postoperative CA 19-9 determination was 45 days (range, 11 to 57 days) as a secondary end point of its phase III study (4). To correct for the variability in the time between CRT and evaluation of the first post CRT CA19-9 value, we chose to measure survival as a time-varying covariate from the time of post CRT CA19-9 measurement rather than from CRT. Further study is warranted to determine the best time for CA 19-9 measurement to predict survival.
Patients who develop early metastasis are unlikely to benefit from radiation, and identifying this population prior to radiation would be ideal. An attractive strategy to facilitate patient selection for CRT is through a trial of systemic therapy. The time interval between the onset of chemotherapy and CRT provides an observation period of approximately 2 to 3 months. Restaging at the end of this period may identify the emergence of overt metastatic disease. In a study by The Groupe Cooperateur Multidisciplinaire en Oncologie (GERCOR) LAP07, 181 patients were reviewed who were treated with 5-fluorouracil (5-FU) or gemcitabine based chemotherapy for four months. Those without evidence of disease progression were given additional chemotherapy or chemoradiation. Overall survival was improved in patients who went on to receive chemoradiation (17). An accurate surrogate marker for disease progression such as CA 19-9 could further identifying those patients that would most benefit from intensification of therapy. Substantially rising CA 19-9 levels during the induction period may be a harbinger of occult metastatic disease which would allow more careful selection of patients who would most likely benefit from local therapy. The half-life of serum CA 19-9 levels are approximately 1 day but can vary from less than 1 day to 3 days. The median lead time for CA 19-9 elevation before detection of a clinical relapse was 23 weeks (range, 2-48 weeks) (10). Thus, there is a need to optimize the timing of serum measurement that must be validated in a prospective clinical trial.
We demonstrated the prognostic impact of the post CRT CA 19-9 levels. Patients with a post CRT CA 19-9 level greater than 85.5 U/mL had significantly worse overall survival in multivariate analysis. These patients may not benefit from intensification of therapy and could be considered for alternative management scheme as those with lower levels of CA 19-9 would benefit from a more aggressive therapeutic approach.
Conclusions
We suggest that CA 19-9 levels be obtained pre and post chemoradiotherapy. Our results indicate that post CRT CA 19-9 levels may have predictive value for prognosis of patients with locally advanced unresectable pancreatic cancer receiving concurrent CRT. These findings should be validated in future randomized trials and considered for prognostic nomograms.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Yang GY, Wagner TD, Fuss M, et al. Multimodality approaches for pancreatic cancer. CA Cancer J Clin 2005;55:352-67 [DOI] [PubMed] [Google Scholar]
- 2.Hsueh CT. Pancreatic cancer: current standards, research updates and future directions. J Gastrointest Oncol 2011;2:123-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 2006;24:2897-902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger AC, Garcia M, Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 2008;26:5918-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsavaris N, Kosmas C, Papadoniou N, et al. CEA and CA-19.9 serum tumor markers as prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. J Chemother 2009;21:673-80 [DOI] [PubMed] [Google Scholar]
- 6.Katz A, Hanlon A, Lanciano R, et al. Prognostic value of CA 19-9 levels in patients with carcinoma of the pancreas treated with radiotherapy. Int J Radiat Oncol Biol Phys 1998;41:393-6 [DOI] [PubMed] [Google Scholar]
- 7.Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008;9:132-8 [DOI] [PubMed] [Google Scholar]
- 8.Halm U, Schumann T, Schiefke I, et al. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer 2000;82:1013-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 2012;3:105-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micke O, Bruns F, Kurowski R, et al. Predictive value of carbohydrate antigen 19-9 in pancreatic cancer treated with radiochemotherapy. Int J Radiat Oncol Biol Phys 2003;57:90-7 [DOI] [PubMed] [Google Scholar]
- 11.Koom WS, Seong J, Kim YB, et al. CA 19-9 as a predictor for response and survival in advanced pancreatic cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1148-54 [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, et al. eds. The American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer-Verlag, 2002. [Google Scholar]
- 13.Bloch DA. Comparing two diagnostic tests against the same “gold standard” in the same sample. Biometrics 1997;53:73-85 [PubMed] [Google Scholar]
- 14.Takahashi H, Ohigashi H, Ishikawa O, et al. Serum CA19-9 alterations during preoperative gemcitabine-based chemoradiation therapy for resectable invasive ductal carcinoma of the pancreas as an indicator for therapeutic selection and survival. Ann Surg 2010;251:461-9 [DOI] [PubMed] [Google Scholar]
- 15.Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg 2003;138:951-5; discussion 955-6 [DOI] [PubMed] [Google Scholar]
- 16.Magnani JL, Steplewski Z, Koprowski H, et al. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res 1983;43:5489-92 [PubMed] [Google Scholar]
- 17.Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326-31 [DOI] [PubMed] [Google Scholar]


