Abstract
The 90-kDa heat shock protein (Hsp90) has become an important therapeutic target with ongoing evaluation in a number of malignancies. Although Hsp90 inhibitors have a high therapeutic index with limited effects on normal cells, they have been described to inhibit dendritic cell function. However, its effect on human immune effector cells may have significant clinical implications, but remains unexplored. In this study, we have evaluated the effects of Hsp90 inhibition on human T lymphocyte and NK cells, including their Ag expression, activation, proliferation, and functional activities. These studies demonstrate that Hsp90 inhibition irreversibly downregulates cell surface expression of critical Ags (CD3, CD4, CD8), the costimulatory molecule (CD28, CD40L), and αβ receptors on T lymphocytes, as well as activating receptors (CD2, CD11a, CD94, NKp30, NKp44, NKp46, KARp50.3) on NK cells. Hsp90 inhibition significantly reduced CD4 protein expression on T lymphocytes at both the cell surface and intracellular level, which was shown to be associated with aberrant regulation of Src-kinase p56Lck. Downregulation of the Ags triggered by Hsp90 inhibition on CD3+ T lymphocytes, both in CD4+ and CD8+ T cell subsets, was associated with a disruption in their cellular activation, proliferation, and/or IFN-γ production, when the inhibition occurred either in activated or inactivated cells. In addition, downregulation of key activating receptors on NK cells following Hsp90 inhibition resulted in decreased cytotoxicity against tumor cells. Therefore, these observations demonstrate the need to closely monitor immune function in patients being treated with a Hsp90 inhibitor and may provide a potential therapeutic application in autoimmune diseases.
Hsp90 (90-kDa heat shock protein) is a ubiquitously expressed conserved molecular chaperone, which mediates the maturation and stabilization of various cellular proteins. It influences the activity of many client proteins that function as critical regulators of cellular growth, differentiation, and apoptotic pathways (1–5). Hsp90 client proteins include oncogenic proteins in human malignancies acting via multiple signal transduction pathways: steroid receptors, epidermal growth factor receptor family members, MET, Raf-1 kinase, AKT, Bcr-abl, mutant p53, CDK4, and many other molecules (6–8). Inhibition of Hsp90 causes the simultaneous degradation of multiple oncogenic proteins and thereby affects signal transduction pathways important for cancer cell proliferation and survival; therefore, Hsp90 inhibitors have attracted a great deal of attention as promising anticancer drugs (9–11).
Geldanamycin, the first known Hsp90 inhibitor, modulates the proteasomal degradation of Hsp90 client proteins and displays a potent antitumor activity in vitro, but has been shown to be too hepatotoxic for clinical use (12, 13). The anasamysin geldanamycin derivative, 17-allylaminogeldanamycin (tanespimycin, KOS-953), is less toxic and also induces the degradation of proteins that require the Hsp90 chaperone for conformational maturation, disrupts association with client proteins, and has shown potent antitumor activity in preclinical and clinical studies (14, 15). Additionally, several other types of geldanamycin derivatives, including 17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride (alvespimycin, KOS-1022) and IPI-504 (retaspimycin), are in evaluation as promising therapeutic candidates in various cancer trials (4, 16, 17). Besides geldanamycin derivatives, numerous other classes of Hsp90 inhibitors have recently been developed, including the macrolide radicicol and purine-scaffold derivatives, pyrazoles and shepherdins, which bind to the N-terminal high-affinity ATP-binding domain of Hsp90. Additionally, other drugs such as cisplatin and novobiocin as well as histone deacetylase or proteasome inhibitors have recently been shown to modify Hsp90 posttranslationally through binding to the C-terminal dimerization domain (18–24). The first in-class Hsp90 inhibitor 17-allylaminogeldanamycin entered into Phase I clinical trials in 1999, and there are now 18 natural and synthetic Hsp90 inhibitors in a variety of chemical scaffolds targeting different types of cancer in clinical trials worldwide (15, 25, 26).
The anticancer effects of Hsp90 inhibitor have been well studied in malignant cells, but their impact on human effector cells has not been well characterized. In these studies, we demonstrate the potential modulation of immune responses in patients treated with Hsp90 inhibitor, focusing on changes in the phenotype and function of human T lymphocytes and NK cells. Our studies reveal that Hsp90 inhibition leads to a significant and irreversible decrease in expression of critical Ags on human T lymphocytes at protein and mRNA levels. The downregulation of these Ags was associated with impairment of cellular activation, proliferation, and IFN-γ secretion by T lymphocytes stimulated with a different type of Ag. Additionally, after Hsp90 inhibition, NK cells displayed decreased activating receptor expression at protein and mRNA levels, which correlated with a downregulation in their cytotoxic activity against tumor cells. Therefore, these results suggest that Hsp90 inhibition disrupts both the Ag expression profile and functional activities of both T lymphocytes and NK cells, and highlights the relevance of potential impact of Hsp90 inhibitors on effector cell function in cancer patients during clinical trials.
Materials and Methods
Cell lines
MM cell lines, McCAR and ARP, obtained from the American Type Culture Collection (Manassas, VA), were cultured in RPMI 1640 medium (Life Technologies-Life Technologies, Rockville, MD) supplemented with 10% FCS (BioWhittaker, Walkersville, MD).
Reagents
Recombinant cytokines, including human IL-2, IL-4, IFN-α, and TNF-α, were purchased from R&D Systems (Minneapolis, MN), and human GM-CSF was obtained from Immunex (Seattle, WA). Mouse anti-human CD2, CD3, CD4, CD8, CD11a, CD25, CD28, CD69, CD80, CD83, CD86, CD154, HLA-DPQR, IFN-γ, and TCR αβ mAbs conjugated with FITC, PE, PerCP, or allophycocyanin were purchased from BD Biosciences (San Diego, CA). Mouse anti-human CD94, NKp30, NKp44, NKp46, KARp50.3, and KIRp70 mAbs labeled with FITC, PE, PerCP, or allophycocyanin were purchased from Beckman Coulter Immunotech (Miami, FL). Hsp90 inhibitor (geldanamycin) was obtained from National Cancer Institute (Bethesda, MD).
Generation of human monocyte-derived dendritic cells
PBMCs were isolated by standard density gradient centrifugation over Ficoll-Paque Plus (Amersham Pharmacia Biotech AB, Uppsala, Sweden) from leukopaks obtained from normal donors. In brief, dendritic cells (DC) were generated from monocytes isolated as the adherent cell fraction following overnight incubation of PBMC in tissue culture flasks at 37°C and 5% CO2. The monocytes were cultured in the presence of 1000 U/ml GM-CSF and 1000 U/ml IL-4 in RPMI 1640 medium supplemented with 10% FCS and 2 mM l-glutamine (Life Technologies-Life Technologies) for 7 d to obtain immature DC. Fresh media plus GM-CSF and IL-4 was added to the cultures every other day. On day 7, the cultures were supplemented with IFN-α (1000 U/ml) plus TNF-α (10 ng/ml) to induce the maturation of the DC. After an additional 3 d of incubation, mature DC (mDC) were harvested and evaluated for their phenotype (CD80, CD83, CD86, HLA-DPQR) by flow cytometry.
Isolation human T lymphocytes and NK cells
Human T lymphocytes or NK cells were isolated from leukopaks using a Pan T cell or NK cell isolation kit (Miltenyi Biotec, Auburn, CA), respectively. In brief, CD3+ T cell enrichment was accomplished by an indirect magnetic labeling system for the isolation of T cells from human leukopaks. Non-T cells, that is, B cells, NK cells, DC, monocytes, granulocytes, and erythroid cells, were labeled with a mixture of biotin-conjugated Abs against CD14, CD16, CD19, CD36, CD56, CD123, and glycophorin A. These cells were subsequently incubated with antibiotin microbeads and magnetically depleted. In addition, CD4+ Th or CD8+ Tc cells were purified using an indirect magnetic isolation method. Alternatively, NK cell enrichment was achieved using an indirect magnetic labeling system for the isolation of NK cells from the leukopaks. The purity of the enriched CD3+, CD4+, or CD8+ T lymphocytes and NK cells was found to be >90% (mean ± SE) by flow cytometry.
Treatment with geldanamycin
Titration and kinetic studies for geldanamycin treatment were examined by flow cytometry. Downregulation of cell surface CD4 and CD8 Ags measured by mean fluorescence intensity (MFI) was observed in dose-and time-dependent manners (Supplemental Fig. 1). These results supported the dose and time length of geldanamycin (1 μM concentration for 24 h) used in these studies.
Cell survival and apoptosis by treatment of cells with geldanamycin
The effect of the Hsp90 inhibitor treatment on induction of apoptosis was examined by flow cytometry using the annexin V/propidium iodide (PI)-apoptosis kit (Invitrogen, Carlsbad, CA) as per the manufacturer’s instructions. Cell viability measurement was also performed manually using a hemacytometer and light microscopy using trypan blue dye exclusion.
Examination of cell surface Ags on T lymphocytes or NK cells
Expression of surface proteins was analyzed by staining with fluorochrome-conjugated mouse anti-human mAbs specific to CD3, CD4, CD8, CD25, CD28, CD40L, or TCR αβ on purified T lymphocytes, and CD2, CD11a, CD94, NKp30, NKp44, NKp46, KARp50.3, or KIRp70 on purified NK cells. Following staining, the cells were analyzed using a FACSCalibur flow cytometer and CellQuest v2.1 software (BD Biosciences) after gating on the respective purified cell population. To examine the recovery of the cell surface protein expression, Hsp90 inhibitor–treated cells were extensively washed and cultured in fresh complete media. The level of cell surface expression was re-evaluated on day 3 of incubation.
Analyses of intracellular protein expression
Enriched CD3+ T cells, either untreated or treated with Hsp90 inhibitor, were incubated with CD28/CD49d mAb mixture, and the intracellular expression of CD4 or CD8 Ag was evaluated by flow cytometry. For the intracellular expression of IFN-γ, CD3+ T cells were stimulated with 1 μg/ml staphylococcal enterotoxin B (SEB; Sigma-Aldrich) for 6 h in the presence of brefeldin A (1 μg/ml; Sigma-Aldrich) to block protein secretion. Cells were washed three times in FACS buffer (0.1% BSA/PBS/ 0.05% sodium azide), fixed in 2% paraformaldehyde, permeabilized in buffer containing 0.1% saponin, and stained with mAbs specific to CD4, CD8, and CD69 at 4°C for 30 min, followed by flow cytometry analysis.
[3H]Thymidine incorporation assay
The proliferation of T lymphocytes was assessed in an allogeneic MLR. Allogeneic mDC (5 × 103 cells/well; stimulator cells) were irradiated at 10 Gy and cocultured with untreated or Hsp90 inhibitor–treated T lymphocytes (5 × 104 cells/well, 1 × 105 cells/well; responder cells) in 96-well U-bottom microtiter plates. Control cell cultures established in the absence of stimulators or responders were prepared to monitor background proliferation. The cells were cultured in AIM-V media (Life Technologies-Life Technologies) supplemented with 10% human AB serum (BioWhittaker) and 50 U/ml IL-2. After 5 d of culture, the cells were pulsed with 1 μCi [3H]thymidine for 18 h and harvested on day 6 to measure T cell proliferation. The results were evaluated for 3H cpm for triplicate wells within each experiment. The overall proliferation (mean CPM ± SE) of T lymphocytes was calculated from three separate experiments using CD3+ T cells obtained from different individual donors.
Membrane labeling of T cells with CFSE in mitogen stimulation assay
Untreated or Hsp90 inhibitor–treated CD3+ T lymphocytes were washed three times and resuspended at 1 × 106 cells/ml in AIM-V media supplemented with 10% human AB serum and 50 U/ml IL-2. The cells were labeled with CFSE (Molecular Probes, Eugene, OR) at a final concentration of 5 μM and incubated for 10 min at 37°C in a CO2 incubator protected from light. Five volumes of ice-cold PBS with 2% FCS were added to the cells to quench the reaction, incubated for 5 min on ice, centrifuged, and resuspended in fresh PBS with 2% FCS after a total of three washings. CFSE-labeled T lymphocytes were adjusted to 2 × 106 cells/ml with AIM-V media containing 1 μg/ml Con A (Sigma-Aldrich), and then incubated in a 24-well plate at 37°C and 5% CO2. The Con A-stimulated CFSE-labeled T lymphocytes were examined by flow cytometry on day 3 or 4.
Cytotoxicity assay
The cytotoxic activity of NK cells was measured using a calcein-release assay. Briefly, target cells were incubated in serum-free culture medium containing 10 mM calcein-AM (Molecular Probes) for 30 min at 37°C, washed three times in cold PBS with 5% FCS, and incubated with effector cells at various E:T cell ratios in 96-well U-bottom microtiter plates (triplicates/sample) for 3 h at 37°C and 5% CO2. After incubation, the cells were pelleted by centrifugation at 1000 rpm for 5 min, and 100 μl of the supernatant was transferred from each well to 96-well flat-bottom plates; calcein release was then measured using a VICTOR2-1420 multilabel counter (PerkinElmer, Wellesley, MA). Maximum release was obtained from detergent-released target cell counts and spontaneous release from target cell counts in the absence of effector cells. Cellular cytotoxicity was calculated as follows: percentage of specific lysis = ([experimental release – spontaneous release]/[maximum release – spontaneous release]).
Oligonucleotide microarray analysis of gene expression
Total RNA was extracted from untreated or Hsp90 inhibitor (1 μM for 24 h)–treated T lymphocytes using a RNeasy kit (Qiagen, Valencia, CA). RNA concentration was determined by absorbance at 260 nm, and 15 μg high-quality total RNA was used in a cDNA synthesis reaction using the Megascript T7 kit (Ambion, Austin, TX). The resulting cDNA was subsequently used to generate biotin-labeled antisense cRNA for hybridization with Human Genome HG-U133A Affymetrix gene chips. Scanning of image output files and analysis of gene expression data sets were followed by filtering of upregulated or downregulated transcripts based on conventional criteria for statistical significance, as well as by hierarchical and functional clustering algorithms (27–29). In statistical analysis, expression values and fold change values were calculated for the groups (treated versus untreated samples) of CD4+ T cells, CD8+ T cells, and NK cells. Expression value >100 and fold change >1.2 were taken as threshold and used to report upregulated and downregulated genes, because they are more likely to correspond to biologically relevant changes rather than expression or technical noise. Microarray raw data files and normalized expression values are available in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE42253.
Results
Geldanamycin-induced Hsp90 inhibition irreversibly downregulates expression of critical T cell Ags at both the cell surface and intracellular levels
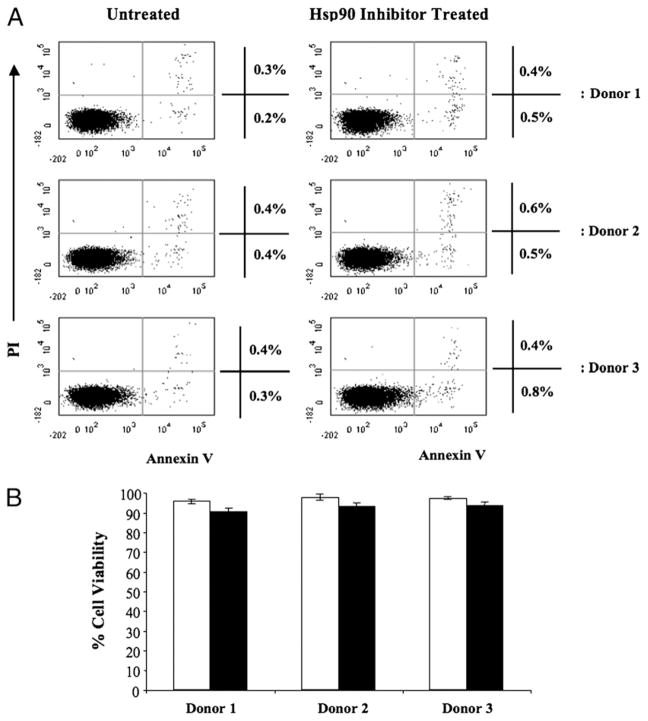
We first evaluated the effect of geldanamycin (1 μM concentration for 24 h) on T cell survival using annexin V/PI staining and flow cytometry. Hsp90 inhibition did not induce apoptosis (annexin V-FITC+, PI−) in enriched CD3+ T lymphocytes from three separate normal donors (Fig. 1A). We also confirmed >90% viability in treated cells by light microscopy using trypan blue dye exclusion staining, which was similar to the untreated control cells (Fig. 1B).
FIGURE 1.
No apoptosis induction or decrease in human T lymphocyte viability after treatment with geldanamycin. Human CD3+ T lymphocytes isolated from normal donors were treated with 1 μM geldanamycin for 24 h, washed, and evaluated for cellular apoptosis/necrosis and viability. (A) Untreated (left panel) or Hsp90 inhibitor–treated (right panel) lymphocytes were stained with annexin VFITC and PI and analyzed by flow cytometry. Geldanamycin treatment did not induce apoptosis (annexin VFITC+, PI−). Results are shown from enriched CD3+ T lymphocytes isolated from three normal individuals (donor 1, 2, or 3). (B) T lymphocytes, either untreated (□) or treated (■) with Hsp90 inhibitor, were analyzed by trypan blue dye exclusion using a hemacytometer and light microscopy. Hsp90 inhibitor–treated T lymphocytes showed no significant difference in viable cell numbers as compared with the untreated control cells (mean ± SE, n = 3).
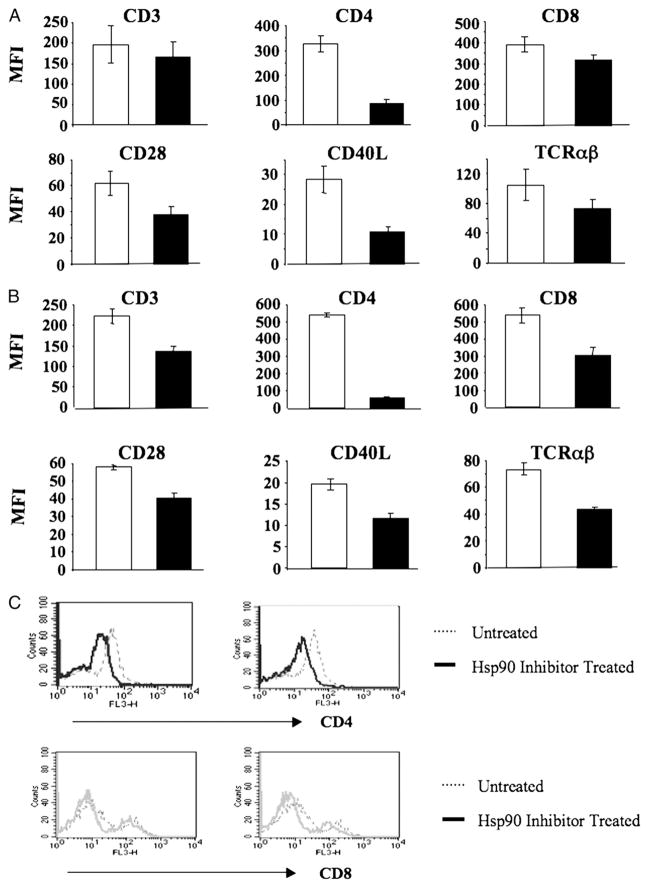
Expression of critical cell surface Ags on enriched CD3+ T lymphocytes following Hsp90 inhibition was evaluated by flow cytometric analyses after gating for the enriched T cell population. Hsp90 inhibitor–treated T cells (1 μM geldanamycin for 24 h) showed a significant (p < 0.05) decrease in the expression of CD3, CD4, and CD8 T cell Ags, CD28 and CD40L (CD154) costimulatory molecules, and the TCR αβ as compared with untreated control T cells obtained from the same donors (Fig. 2A). Recovery of downregulated T cell surface Ags was next evaluated after washing of the cells extensively to remove free Hsp90 inhibitor, followed by 3 d of culture in fresh media. Whereas overall cell viability remained high (>80%) in the cultures on day 3 following removal of the inhibitor, the downregulated surface Ags CD3, CD4, CD8, CD28, CD40L, and TCR αβ were not recovered on T lymphocytes treated with Hsp90 inhibitor (Fig. 2B). These results demonstrate that inhibition of Hsp90 irreversibly interferes with the expression of critical cell surface Ags on CD3+ T lymphocytes. In addition, intracellular protein expression of the CD4 and CD8 Ags, evaluated using enriched CD3+ T cells, was also decreased after Hsp90 inhibition (Fig. 2C). Taken together, CD4 expression on T cells showed the most significant down-regulation after Hsp90 inhibition at both the cell surface and intracellular levels.
FIGURE 2.
Hsp90 inhibition irreversibly downregulates key Ags on and in human T lymphocytes. Human CD3+ T lymphocytes isolated from normal donors were treated with 1 μM geldanamycin for 24 h, washed, and evaluated for cell surface or intracellular expression of key Ags. (A) T lymphocytes, either untreated (□) or treated (■) with Hsp90 inhibitor, were analyzed for expression of key cell surface Ags by flow cytometry. Hsp90 inhibition results in the downregulation of key cell surface Ags on T lymphocytes. Results are shown as the MFI (mean ± SE) of three separate experiments using T cells obtained from different individual donors. (B) Hsp90 inhibitor–treated T lymphocytes were washed three times and recultured in a fresh media for an additional 3 d prior to the analyses. Surface Ag expression (MFI: mean ± SE, n = 3) was not recovered on the T lymphocytes treated with the Hsp90 inhibitor (■) as compared with the untreated (□) cells. (C) Hsp90 inhibitor–treated T lymphocytes were analyzed for their intracellular protein expression by flow cytometry. Both CD4 and CD8 expression was decreased in T lymphocytes by Hsp90 inhibition compared with untreated control cells to a great extent on CD4+ T lymphocytes (upper panel) than CD8+ T lymphocytes (lower panel).
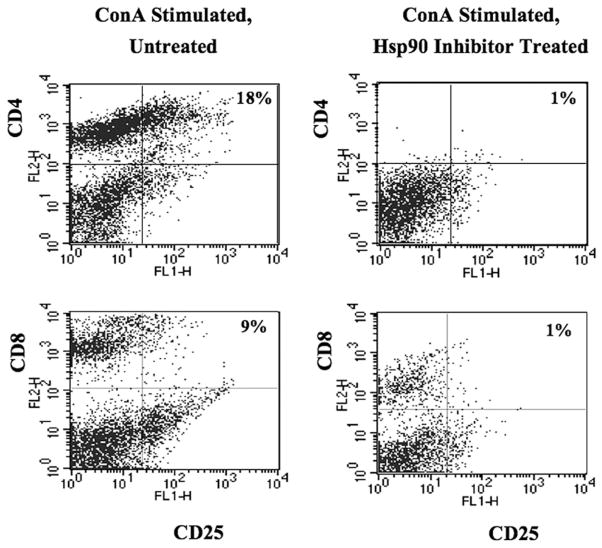
Geldanamycin downregulates CD25 on activated T lymphocytes
The effect of Hsp90 inhibition was next evaluated on activated CD4+ or CD8+ T lymphocytes. For the analyses, CD3+ T lymphocytes were stimulated with a lymphocyte mitogen (Con A) and then treated with the Hsp90 inhibitor. Modification of IL-2R α-chain CD25 was examined on T lymphocytes by flow cytometry. Complete abrogation of CD4 Ag was detected on activated T cells following Hsp90 inhibition (Fig. 3); therefore, the double-positive population was dramatically reduced, including CD4+/ CD25+ T cell subset (untreated versus treated; 18% versus 1%). In contrast to CD4, CD8 Ag was still present after Hsp90 inhibition on the activated T cells, although the expression level was reduced. The CD8+ T cells expressing CD25 decreased from 9 to 1% after Hsp90 inhibition (Fig. 3). Thus, these results demonstrate the Hsp90 inhibition downregulates a critical cell surface molecule, CD25 on activated T cells, which may impair their ability to respond to exogenous IL-2.
FIGURE 3.
The level of CD25 is decreased on activated T lymphocytes by Hsp90 inhibition. Enriched human CD3+ T lymphocytes were activated by stimulation with Con A (1 μg/ml) prior to Hsp90 inhibition, and expression of the IL-2Rα (CD25) was then analyzed on T lymphocytes by flow cytometry. Expression of CD4 cell surface Ag was completely abrogated by Hsp90 inhibition. The CD4+ and CD8+ T cell population expressing IL-2Rα (CD25+/CD4+, CD25+/CD8+ cells) was dramatically reduced by Hsp90 inhibition.
Geldanamycin inhibits proliferation of T lymphocytes
Next, we evaluated the impact of Hsp90 inhibition on T cell proliferation in response to either allogeneic mDC or a mitogen. T cells were initially treated with the Hsp90 inhibitor geldanamycin for 24 h, washed, and then cultured with irradiated (10 Gy) allogeneic mDC. T cell proliferation measured by [3H]thymidine incorporation was significantly (*p < 0.05) reduced in Hsp90 inhibitor–treated T cell cultures as compared with the untreated control cultures at responder (T lymphocytes):stimulator (mDC) ratios of 10:1 or 20:1 (Fig. 4A). Next, we evaluated T cell proliferation in CFSE assays in response to the mitogen Con A (1 μg/ml). The proliferating cell population (gated in M1) was identified by a decrease in CFSE intensity (CFSE low). Untreated T cells stimulated with Con A showed increased cell proliferation (22% on day 3, 41% on day 4) as compared with the control unstimulated T cells (3% on day 3, 7% on day 4) (Fig. 4B). In contrast, Con A-induced T cell proliferation was significantly decreased in the Hsp90 inhibitor–treated T cell cultures (0% on day 3, 3% on day 4). Thus, these results demonstrate that both MHC–TCR-mediated (allogeneic mDC stimulation) and non-MHC–TCR-mediated (Con A stimulation) CD3+ T cell proliferation was significantly decreased by Hsp90 inhibition. We further examined the effects of Hsp90 inhibition on CD4+ or CD8+ T cell subset proliferation using the CFSE assay. Proliferation of CD4+ T cells and CD8+ T cells (gated in Q1) was significantly reduced in Hsp90-inhibited T cell cultures as compared with the control T cells after stimulation with Con A (Fig. 4C). Overall, Hsp90 inhibition reduced CD4+ T cell proliferation from 28 to 0% and CD8+ T cell proliferation from 13 to 0%, as measured on day 3 of culture. Therefore, these results demonstrate that the proliferation of CD4+ T cells and CD8+ T cells in response to mitogen stimulation was significantly reduced by Hsp90 inhibition.
FIGURE 4.
Hsp90 inhibition decreases T cell proliferation induced by allogeneic mDC or Con A. (A) Untreated (□) or Hsp90 inhibitor–treated (■) CD3+ T lymphocytes (5 × 104 cells/well, 1 × 105 cells/well) were cultured with allogeneic mDC (5 × 103 cells/well) in triplicate wells at 37°C for 5 d, pulsed overnight with 1 μCi/well [3H]thymidine, harvested, and analyzed for T cell proliferation. Hsp90 inhibition induced a significant (*p < 0.05) reduction in T cell proliferation in response to allogeneic mDC at both responder:stimulator ratios tested (10:1, 20:1). The values represent the mean cpm ± SE of three separate experiments using T cells obtained from different individual donors. (B) Untreated or Hsp90 inhibitor–treated CD3+ T lymphocytes were washed, labeled with CFSE, and stimulated with 1 μg/ml Con A for 3 or 4 d prior to measuring T cell proliferation by flow cytometry. The proliferating cell population (M1) was identified by a decrease in CFSE intensity. Con A stimulation (middle panel) induced cell proliferation as compared with unstimulated (left panel), untreated T cells. However, Hsp90 inhibition (right panel) significantly decreased T cell proliferation induced by Con A. (C) Con A–induced CD4- or CD8-specific T cell proliferation was evaluated in Hsp90 inhibitor–treated T lymphocytes on day 3 of culture. The specific proliferating CD4+ or CD8+ T cell populations (Q1) are identified by a decrease in CFSE intensity. Hsp90 inhibition (right panels) decreased both CD4+ and CD8+ T cell proliferation induced by Con A as compared with control T cells (left panels).
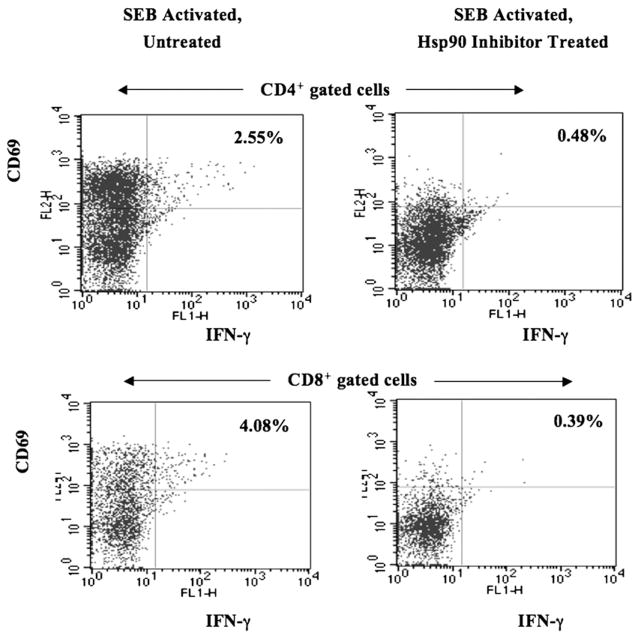
Geldanamycin abrogates IFN-γ production and cellular activation of CD4+ or CD8+ T cells
The impact of Hsp90 inhibition on T lymphocyte function was further investigated by measuring IFN-γ production and cellular activation. In contrast to the previous results presented in Fig. 4, enriched CD3+ T cells were initially activated with the super-antigen SEB (1 μg/ml) and then treated with Hsp90 inhibitor to measure the effects of Hsp90 inhibition on activated T cells. Our results demonstrate that Hsp90 inhibition induced a significant decrease in IFN-γ production and cell activation in both CD4+ T lymphocytes (untreated versus Hsp90 inhibitor treated; 2.55% versus 0.48% IFN-γ+/CD69+ cells) and CD8+ T lymphocytes (untreated versus Hsp90 inhibitor treated; 4.08% versus 0.39% IFN-γ+/CD69+ cells) (Fig. 5). These results offer evidence that Hsp90 inhibition interrupts cytokine production and cellular activation in both activated CD4+ and CD8+ T lymphocyte subsets.
FIGURE 5.
Hsp90 inhibition decreases SEB-induced T lymphocyte IFN-γ production and cellular activation. Human CD3+ T lymphocytes stimulated with SEB (1 μg/ml) were treated with geldanamycin and then evaluated for intracellular IFN-γ production and cell activation by flow cytometry. Hsp90 inhibition resulted in a dramatic decrease in IFN-γ production and activation (CD69+) in both CD4+ and CD8+ T lymphocyte subsets.
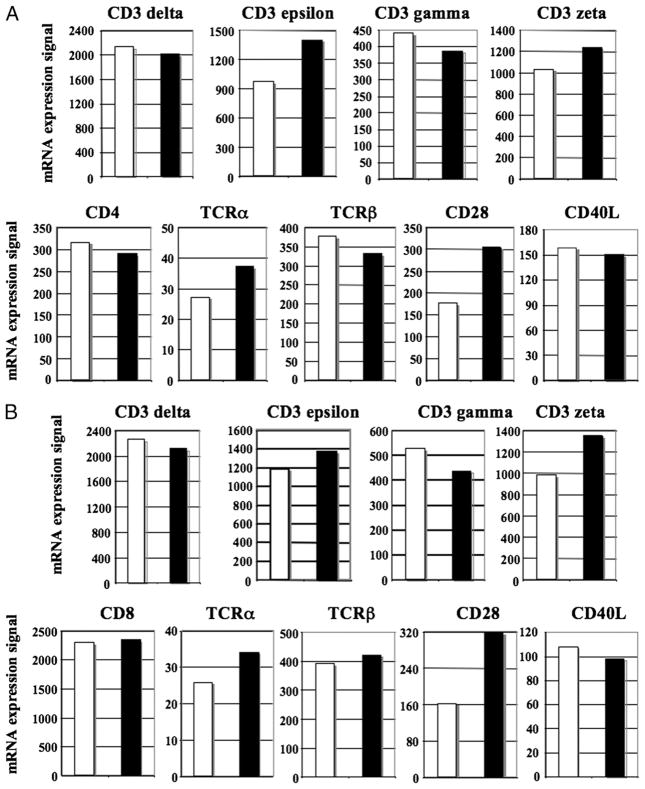
Geldanamycin regulates mRNA expression in T lymphocytes
Next, we evaluated mRNA expression profiles of key molecules in purified human CD4+ or CD8+ T lymphocytes treated with Hsp90 inhibitor (1 μM for 24 h) using HG-U133A gene arrays. In contrast to the consistent and significant downregulation of all the Ags tested at the protein level, the impact of Hsp90 inhibition on mRNA expression was varied, but limited among the Ags for both CD4+ and CD8+ T lymphocytes. Compared with untreated cells, Hsp90 inhibitor–treated CD4+ T lymphocytes showed only modest upregulation of only CD3ε (fold change: 1.44) and CD28 (fold change: 1.72) mRNA expression (Fig. 6A). Hsp90 inhibition also has the limited effect on mRNA expression in CD8+ T lymphocytes (Fig. 6B), including downregulation of CD3γ (fold change: −1.21) and upregulation of CD3ζ (fold change: 1.38) and CD28 (fold change: 1.98), but insignificant changes in other genes, including CD8 in CD8+ T lymphocytes.
FIGURE 6.
Transcript level of key molecules is regulated in CD4+ or CD8+ T lymphocytes by Hsp90 inhibition. Gene expression profiling was performed using Affymetrix HG-U133A gene arrays on either purified CD4+ or CD8+ T lymphocytes. The data are presented as an arbitrary unit for untreated (□) and Hsp90-treated (■) for CD4+ T lymphocytes (A) and CD8+ T lymphocytes (B).
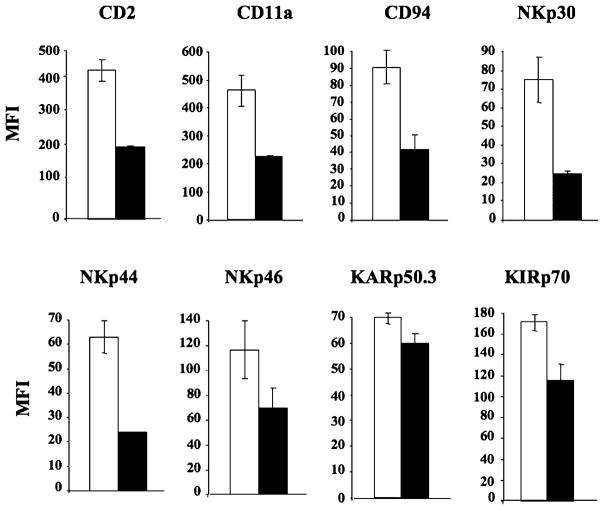
Geldanamycin downregulates expression of activating receptors on NK cells
NK cells isolated from three normal individuals were evaluated by flow cytometry for the expression of activating receptors following Hsp90 inhibition with geldanamycin (1 μM for 24 h). Hsp90 inhibition resulted in decreased expression of the NK cell-activating receptors, including CD2, CD11a, CD94, NKp30, NKp44, NKp46, KARp50.3, and KIRp70 (Fig. 7). Among these receptors, CD2, CD11a, CD94, NKp30, and NKp44 showed a significant (p < 0.05) downregulation in their MFI on NK cells after HsP90 inhibition as compared with the untreated control cells.
FIGURE 7.
Hsp90 inhibition downregulates key activating receptors on human NK cells. Control untreated (□) or Hsp90 inhibitor–treated (■) NK cells were analyzed for cell surface expression of key activating receptors by flow cytometry. Hsp90 inhibition resulted in a significant downregulation in the cell surface expression of CD2, CD11a, CD94, NKp30, NKp44, NKp46, KARp50.3, and KIRp70 receptors on NK cells. Results are shown as MFI (mean ± SE) of three separate experiments using purified NK cells obtained from different individual donors.
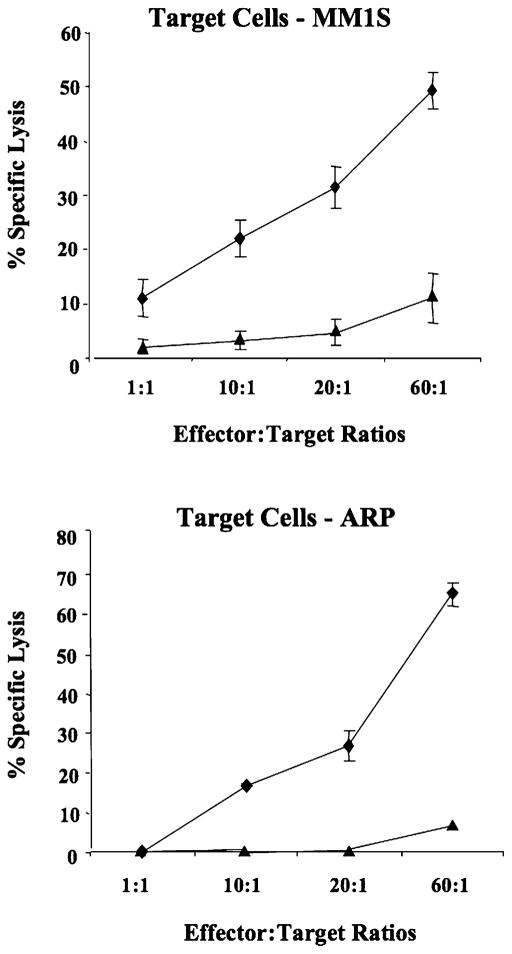
Geldanamycin decreases the cytotoxic activity of NK cells
Finally, we evaluated the effect of Hsp90 inhibitor on the cytotoxic activity of NK cells against cancer cell lines. Untreated control NK cells demonstrated effective lysis of NK-sensitive multiple myeloma cell lines MM1S (11–48%) and ARP (0–64%) at various E: T cell ratios. However, the cytotoxic activity of NK cells was significantly decreased following Hsp90 inhibition against both MM cell lines, including MM1S (2–10%) and ARP (0–5%) (Fig. 8). Thus, these results demonstrate that Hsp90 inhibition induced a dramatic decrease in cytotoxic activity of NK cells against multiple myeloma cells, which correlated with a reduction in the expression of activating receptors on NK cells.
FIGURE 8.
NK cell cytotoxicity is reduced by Hsp90 inhibition. The cytotoxic activity of untreated control (◆) or geldanamycin-treated (▲) NK cells was measured against multiple myeloma cell lines using a calcein-release assay. Target cells (MM1S, ARP) labeled with calcein-AM were incubated with effector cells (untreated NK cells, Hsp90 inhibitor–treated NK cells) at various E:T cell ratios. After 4-h incubation, the calcein release was measured in the supernatant. Maximum release was obtained from detergent-released target cell counts, and spontaneous release was obtained from target cell counts in the absence of effector cells. Cellular cytotoxicity was calculated, as described in Materials and Methods. Hsp90 inhibitor–treated NK cells showed a significant reduction in cytotoxic activity against both MM1S and ARP multiple myeloma cell lines as compared with untreated NK cells. The values represent the mean ± SE of three separate experiments using purified NK cells obtained from different individual donors.
Geldanamycin modifies mRNA expression of activation Ags in NK cells
We further evaluated mRNA expression profiles of the key activation molecules in purified NK cells following Hsp90 inhibition. In contrast to the consistent downregulation of cell surface Ags at the protein level seen by flow cytometry in NK cells after Hsp90 inhibition, we observed variable modifications in mRNA expression (Fig. 9). Specifically, Hsp90 inhibitor–treated cells showed a downregulation in mRNA expression for CD2 (fold change: −2.36) and CD94 (fold change: −2.23), although a minor upregulation of mRNA expression was observed for CD11a (fold change: 1.23) and NKG2D (fold change: 1.24), as compared with untreated NK cell control. The majority of these Ags had no significant change in their mRNA expression.
FIGURE 9.
The mRNA transcript levels of activating receptor molecules are modified in NK cells by Hsp90 inhibition. Gene expression profile of purified human NK cells was analyzed using Affymetrix HG-U133A gene arrays. The data are presented as an arbitrary unit for untreated (□) and Hsp90-treated (■) NK cells.
Discussion
Chaperones are ubiquitous and highly conserved proteins that passively prevent aggregation of damaged proteins or use active ATP-driven conformational processes to unfold their target proteins (30–32). Chaperones are also involved in quality control of the MHC–class I complex, T and B cell receptors, and many other key proteins required for immune signaling (33–37). Among the chaperone proteins, Hsp90 has emerged as an exciting target for cancer therapy due to its critical function in the conformation, stability, activity, regulation, and cellular localization of oncogenic client proteins that contribute to tumor cell survival. Numerous Hsp90 client proteins, including transmembrane tyrosine kinases, MET, and insulin-like growth factor-1 receptor, and mutated signaling proteins regulate multiple signal transduction pathways in human malignancies (38–42). Therefore, targeting Hsp90 offers the unique prospect of simultaneously inhibiting multiple signaling pathways required for the development and maintenance of malignant cancer cells. In addition, Hsp90 is known to be overexpressed in many types of cancer; moreover, Hsp90 isolated from tumor cells has a 20–200 times higher binding affinity for their inhibitors than does Hsp90 isolated from normal cells (43–45). This difference might be due to the fact that tumor cells, as compared with normal cells, exhibit a stressed phenotype, which is associated with an enhanced dependency on the cyto-protective action of Hsp90, providing further rationale to develop Hsp90 inhibitor drugs to treat cancer patients.
The majority of Hsp90 inhibitors to date act by docking in the N-terminal nucleotide binding site, thereby inhibiting the intrinsic ATPase activity and blocking the formation of the mature complex. Many of these Hsp90 inhibitors cause selective degradation of important signaling proteins involved in cell proliferation, cell cycle regulation, and apoptosis in a wide range of tumor models (46, 47). Previously, we have demonstrated in human tumors in vitro and in murine models that Hsp90 inhibitors result in the destabilization and eventual degradation of Hsp90 client proteins, thereby leading to apoptotic cell death (48).
The objective of current studies was to evaluate the effects of Hsp90 inhibition on the survival, activation, proliferation, and functional activities of human immune effector cells, including T lymphocytes and NK cells, and to estimate its potential impact on clinical outcomes in cancer patients treated with the specific inhibitor. Previous reports by other investigators have shown that geldanamycin at a concentration of either 1.78 μM (34, 35) or 3.6 μM (36) decreased T cell proliferation, IL-2R expression, and IL-2 secretion through blockage kinase-mediated signaling events (i.e., lck, Raf-1, ERK-2). Additionally, in a previous study using a panel of both drug-sensitive and drug-resistant MM cell lines, our group has demonstrated that the optimal dose and duration of exposure for anti-MM activity was 1 μM geldanamycin for 24 h (48). In these studies, we demonstrate that geldanamycin at a lower concentration and duration (1 μM, 24 h) than previously reported decreased T cell function and provided new information regarding blockage of NK cell function. In contrast to malignant cells, the Hsp90 inhibitor did not induce significant apoptosis or reduce viability of normal T lymphocytes (Fig. 1) under the same dose and treatment duration, which is consistent with our previous observations on inhibition of DC function (49). These observations are also consistent with others showing that Hsp90 extracted from malignant cells has significantly higher ATPase activity and binding affinity to ATP compared with various normal cells, which can lead to a significantly higher lysis of malignant cells than normal cells (43, 50–52). In addition, these studies further demonstrate that Hsp90 inhibition significantly decreases expression of key cell surface Ags on normal human T lymphocytes or NK cells. The downregulation of these critical markers was irreversible, because removal of Hsp90 inhibitor from the cells followed by long-term incubation in fresh media did not result in the recovery of Ag expression. Besides surface Ags, intracellular protein expression was also decreased in T lymphocytes following Hsp90 inhibition. These results are consistent with our previous study that demonstrated an irreversible downregulation of key cell surface Ags (CD40, CD80, CD83, CD86, HLA-A, -B, -C, and HLA-DP, -DQ, -DR) and intracellular proteins (CD40, CD83) on human DC after Hsp90 inhibition (49).
We hypothesize that the decrease in protein expression following Hsp90 inhibition may be mediated by several potential mechanisms, including modulation of transcription levels (2, 53), blockage of protein degradation required for their conformational maturation (11, 54), and/or modification of proper protein folding, which affects their structure and function (20, 55). To address this question at least in part, we performed gene array analyses to evaluate the modification of mRNA expression in CD4+ or CD8+ T cell subsets and in NK cells following Hsp90 inhibition. Our results demonstrate that Hsp90 inhibitor induced only a very modest modification of transcription, is varied among the different Ags, and is distinct from the consistent Ag downregulation we observed at the protein level. Thus, we postulate that effect of Hsp90 inhibitor on human T lymphocytes and NK cells may be on change mainly in protein folding and conformational changes, resulting in its lack of cell surface expression, anchoring, or eventual proteasomal degradation.
Lck is a member of the Src family of nonreceptor tyrosine kinases and is expressed primarily in T lymphocytes and thymocytes. The protein is predominantly associated with the cytosolic side of the plasma membrane (56, 57), a localization consistent with the importance of Lck in early signaling events through the TCR (58). Bijlmakers and Marsh (59) demonstrated that Lck associates with intracellular CD4 early after synthesis and with cell surface CD4 at later times. This group as well as other investigators demonstrated the essential role of Hsp90 for the synthesis and subsequent membrane association of Src-kinase p56Lck; thus, failure of this regulation would lead to a significant degradation of CD4 Ag (60–62). Our observations are consistent with these previous studies, by demonstrating a dramatic decrease of CD4 protein expression after Hsp90 inhibition, at both the cell surface as well as intracellular levels. Furthermore, our functional study showed that the downregulation of critical Ags on T cells by Hsp90 inhibition resulted in decreased cell activation and proliferation in response to either an alloantigen or mitogen. T cells in cancer patients can be somewhat activated due to the higher level of cytokines or chemokines released from different types of cells. To estimate the potential effect of Hsp90 inhibition in cancer patients, we first activated T lymphocytes with the superantigen SEB, followed by Hsp90 inhibition, and monitored the effects on the T cell functional activities (Fig. 5). In the analyses, we observed a downregulation of surface Ag expression and a decrease in activated T cell function, as demonstrated by reduced IFN-γ production and CD69 expression after Hsp90 inhibition (2–4% of IFN-γ+/CD69+ in T lymphocytes), which was consistent with a previous report using normal donors’ T cells (63). Finally, our observation using human T cells was consistent with other reports demonstrating abrogation of a mitogen-induced mouse splenocyte proliferation upon exposure to geldanamycin (36).
Unlike T lymphocytes, which are involved in adaptive immunity, NK cells are a key component of innate immunity and are involved not only in the elimination of tumor cells, but also in the regulation of the immune response through secretion of cytokines and che-mokines that can activate other important cellular components. Development of the antitumor activities of NK cells is controlled by multiple mechanisms, including direct cytotoxic activity against tumor cells (64, 65). Because activating NK cell receptors render tumor cells potentially susceptible to NK cell attack (66, 67), we investigated the effects of Hsp90 inhibition on the expression of activating receptors as well as their functional activity. Our studies show that Hsp90 inhibition leads to downregulation of CD2, CD11a, CD94, NKp30, NKp44, NKp46, KARp50.3, and KIRp70 activating receptors on NK cells. Corresponding to the reduction of their expression, a significant inhibition of cytotoxic activity was detected in the Hsp90 inhibitor–treated NK cells against NK-sensitive tumor cell lines. These results are consistent with our previous investigation, which demonstrated that Hsp90-mediated protein processing and folding are critical for the expression of critical proteins and function of DC (49).
In summary, our data offer a unique insight on the potential of Hsp90 inhibitor to modulate immune function. We demonstrate that Hsp90 inhibition could lead to a significant modulation of effector cells, including T lymphocytes and NK cells, with important clinical implication. On the basis of these findings, we highlight the concern that Hsp90 inhibition may result in immune suppression and suggest the importance of close monitoring of immune cells for their functional activities in patients being treated with a Hsp90 inhibitor. Finally, our results provide the rationale to investigate the effects of a Hsp90 inhibitor in preventing graft versus host disease as well as treating autoimmune diseases.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant RO1-124929 and a VA merit review grant (to N.C.M.); National Institutes of Health Grants P50-100007, PO1-78378, and PO1-155258 (to N.C.M. and K.C.A.); and National Institutes of Health Grant RO1-50947 (to K.C.A.).
Abbreviations used in this article
- DC
dendritic cell
- Hsp
heat shock protein
- mDC
mature DC
- MFI
mean fluorescence intensity
- PI
propidium iodide
- SEB
staphylococcal enterotoxin B
Footnotes
The microarray raw data files and normalized expression files presented in this article have been submitted to the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE42253.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Taipale MD, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 2.Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- 3.Robert J. Evolution of heat shock protein and immunity. Dev Comp Immunol. 2003;27:449–464. doi: 10.1016/s0145-305x(02)00160-x. [DOI] [PubMed] [Google Scholar]
- 4.Drysdale MJ, Brough PA, Massey A, Jensen MR, Schoepfer J. Targeting Hsp90 for the treatment of cancer. Curr Opin Drug Discov Devel. 2006;9:483–495. [PubMed] [Google Scholar]
- 5.Sreedhar AS, Nardai G, Csermely P. Enhancement of complement-induced cell lysis: a novel mechanism for the anticancer effects of Hsp90 inhibitors. Immunol Lett. 2004;92:157–161. doi: 10.1016/j.imlet.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Giménez Ortiz A, Montalar Salcedo J. Heat shock proteins as targets in oncology. Clin Transl Oncol. 2010;12:166–173. doi: 10.1007/s12094-010-0486-8. [DOI] [PubMed] [Google Scholar]
- 7.Prodromou C. Strategies for stalling malignancy: targeting cancer’s addiction to Hsp90. Curr Top Med Chem. 2009;9:1352–1368. doi: 10.2174/156802609789895656. [DOI] [PubMed] [Google Scholar]
- 8.Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9:4483–4493. [PubMed] [Google Scholar]
- 9.Fukuyo Y, Hunt CR, Horikoshi N. Geldanamycin and its anti-cancer activities. Cancer Lett. 2010;290:24–35. doi: 10.1016/j.canlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Banerji U, Judson I, Workman P. The clinical applications of heat shock protein inhibitors in cancer: present and future. Curr Cancer Drug Targets. 2003;3:385–390. doi: 10.2174/1568009033481813. [DOI] [PubMed] [Google Scholar]
- 11.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 12.Miller P, DiOrio C, Moyer M, Schnur RC, Bruskin A, Cullen W, Moyer JD. Depletion of the erbB-2 gene product p185 by benzoquinoid ansamycins. Cancer Res. 1994;54:2724–2730. [PubMed] [Google Scholar]
- 13.Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36:305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 14.Williams CR, Tabios R, Linehan WM, Neckers L. Intratumor injection of the Hsp90 inhibitor 17AAG decreases tumor growth and induces apoptosis in a prostate cancer xenograft model. J Urol. 2007;178:1528–1532. doi: 10.1016/j.juro.2007.05.120. [DOI] [PubMed] [Google Scholar]
- 15.Richardson PG, Chanan-Khan AA, Lonial S, Krishnan AY, Carroll MP, Alsina M, Albitar M, Berman D, Messina M, Anderson KC. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. Br J Haematol. 2011;153:729–740. doi: 10.1111/j.1365-2141.2011.08664.x. [DOI] [PubMed] [Google Scholar]
- 16.Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, McDougall J, Wylie AA, Robison K, Caliri K, et al. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene. 2011;30:2581–2586. doi: 10.1038/onc.2010.625. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan RK, Egorin MJ, Erlichman C, Remick SC, Ramalingam SS, Naret C, Holleran JL, TenEyck CJ, Ivy SP, Belani CP. Phase I pharmacokinetic and pharmacodynamic study of 17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor of heat-shock protein 90, in patients with advanced solid tumors. J Clin Oncol. 2010;28:1520–1526. doi: 10.1200/JCO.2009.25.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mollapour M, Tsutsumi S, Kim YS, Trepel J, Neckers L. Casein kinase 2 phosphorylation of Hsp90 threonine 22 modulates chaperone function and drug sensitivity. Oncotarget. 2011;2:407–417. doi: 10.18632/oncotarget.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usmani SZ, Bona RD, Chiosis G, Li Z. The anti-myeloma activity of a novel purine scaffold HSP90 inhibitor PU-H71 is via inhibition of both HSP90A and HSP90B1. J Hematol Oncol. 2010;3:40–47. doi: 10.1186/1756-8722-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usmani SZ, Bona RD, Li Z. 17 AAG for HSP90 inhibition in cancer: from bench to bedside. Curr Mol Med. 2009;9:654–664. doi: 10.2174/156652409788488757. [DOI] [PubMed] [Google Scholar]
- 21.Reikvam H, Ersvaer E, Bruserud O. Heat shock protein 90: a potential target in the treatment of human acute myelogenous leukemia. Curr Cancer Drug Targets. 2009;9:761–776. doi: 10.2174/156800909789271486. [DOI] [PubMed] [Google Scholar]
- 22.Moser C, Lang SA, Stoeltzing O. Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 2009;29:2031–2042. [PubMed] [Google Scholar]
- 23.Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol. 2008;8:370–374. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Janadi A, Chandana SR, Conley BA. Histone deacetylation: an attractive target for cancer therapy? Drugs R D. 2008;9:369–383. doi: 10.2165/0126839-200809060-00003. [DOI] [PubMed] [Google Scholar]
- 25.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, Arkenau HT, Moreno-Farre J, Banerji U, Roels B, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, Gray JE, Iafrate AJ, Katayama R, Hafeez N, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28:4953–4960. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JM, Zeitlin PL, Cebotaru L, Guggino SE, Guggino WB. Gene expression profile analysis of 4-phenylbutyrate treatment of IB3-1 bronchial epithelial cell line demonstrates a major influence on heat-shock proteins. Physiol Genomics. 2004;16:204–211. doi: 10.1152/physiolgenomics.00160.2003. [DOI] [PubMed] [Google Scholar]
- 28.de Ruijter AJ, Meinsma RJ, Bosma P, Kemp S, Caron HN, van Kuilenburg AB. Gene expression profiling in response to the histone deacetylase inhibitor BL1521 in neuroblastoma. Exp Cell Res. 2005;309:451–467. doi: 10.1016/j.yexcr.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Kohlmann A, Schoch C, Schnittger S, Dugas M, Hiddemann W, Kern W, Haferlach T. Molecular characterization of acute leukemias by use of microarray technology. Genes Chromosomes Cancer. 2003;37:396–405. doi: 10.1002/gcc.10225. [DOI] [PubMed] [Google Scholar]
- 30.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 31.Baker TA, Sauer RT. ATP-dependent proteases of bacteria: recognition logic and operating principles. Trends Biochem Sci. 2006;31:647–653. doi: 10.1016/j.tibs.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macario AJ, Conway de Macario E. Chaperonopathies by defect, excess, or mistake. Ann N Y Acad Sci. 2007;1113:178–191. doi: 10.1196/annals.1391.009. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. 2002;14:45–51. doi: 10.1016/s0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 34.Schnaider T, Somogyi J, Csermely P, Szamel M. The Hsp90-specific inhibitor, geldanamycin, blocks CD28-mediated activation of human T lymphocytes. Life Sci. 1998;63:949–954. doi: 10.1016/s0024-3205(98)00352-x. [DOI] [PubMed] [Google Scholar]
- 35.Schnaider T, Somogyi J, Csermely P, Szamel M. The Hsp90-specific inhibitor geldanamycin selectively disrupts kinase-mediated signaling events of T-lymphocyte activation. Cell Stress Chaperones. 2000;5:52–61. doi: 10.1043/1355-8145(2000)005<0052:THSIGS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yorgin PD, Hartson SD, Fellah AM, Scroggins BT, Huang W, Katsanis E, Couchman JM, Matts RL, Whitesell L. Effects of geldanamycin, a heat-shock protein 90-binding agent, on T cell function and T cell nonreceptor protein tyrosine kinases. J Immunol. 2000;164:2915–2923. doi: 10.4049/jimmunol.164.6.2915. [DOI] [PubMed] [Google Scholar]
- 37.Piatelli MJ, Doughty C, Chiles TC. Requirement for a hsp90 chaperone-dependent MEK1/2-ERK pathway for B cell antigen receptor-induced cyclin D2 expression in mature B lymphocytes. J Biol Chem. 2002;277:12144–12150. doi: 10.1074/jbc.M200102200. [DOI] [PubMed] [Google Scholar]
- 38.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 39.Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother Pharmacol. 1998;42:273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- 40.Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fumo G, Akin C, Metcalfe DD, Neckers L. 17-Allylamino-17-demethoxygeldanamycin (17-AAG) is effective in down-regulating mutated, constitutively activated KIT protein in human mast cells. Blood. 2004;103:1078–1084. doi: 10.1182/blood-2003-07-2477. [DOI] [PubMed] [Google Scholar]
- 42.Solit DB, Zheng FF, Drobnjak M, Münster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–993. [PubMed] [Google Scholar]
- 43.Stühmer T, Zöllinger A, Siegmund D, Chatterjee M, Grella E, Knop S, Kortüm M, Unzicker C, Jensen MR, Quadt C, et al. Signalling profile and antitumour activity of the novel Hsp90 inhibitor NVP-AUY922 in multiple myeloma. Leukemia. 2008;22:1604–1612. doi: 10.1038/leu.2008.111. [DOI] [PubMed] [Google Scholar]
- 44.Powers MV, Workman P. Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr Relat Cancer. 2006;13(Suppl 1):S125–S135. doi: 10.1677/erc.1.01324. [DOI] [PubMed] [Google Scholar]
- 45.Kim LS, Kim JH. Heat shock protein as molecular targets for breast cancer therapeutics. J Breast Cancer. 2011;14:167–174. doi: 10.4048/jbc.2011.14.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eder IE, Haag P, Bartsch G, Klocker H. Targeting the androgen receptor in hormone-refractory prostate cancer: new concepts. Future Oncol. 2005;1:93–101. doi: 10.1517/14796694.1.1.91. [DOI] [PubMed] [Google Scholar]
- 47.Minami Y, Kiyoi H, Yamamoto Y, Yamamoto K, Ueda R, Saito H, Naoe T. Selective apoptosis of tandemly duplicated FLT3-transformed leukemia cells by Hsp90 inhibitors. Leukemia. 2002;16:1535–1540. doi: 10.1038/sj.leu.2402558. [DOI] [PubMed] [Google Scholar]
- 48.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, Morgan G, Akiyama M, Shringarpure R, Munshi NC, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107:1092–1100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bae J, Mitsiades C, Tai YT, Bertheau R, Shammas M, Batchu RB, Li C, Catley L, Prabhala R, Anderson KC, Munshi NC. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J Immunol. 2007;178:7730–7737. doi: 10.4049/jimmunol.178.12.7730. [DOI] [PubMed] [Google Scholar]
- 50.Workman P. Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Mol Med. 2004;10:47–51. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Gallegos Ruiz MI, Floor K, Roepman P, Rodriguez JA, Meijer GA, Mooi WJ, Jassem E, Niklinski J, Muley T, van Zandwijk N, et al. Integration of gene dosage and gene expression in non-small cell lung cancer, identification of HSP90 as potential target. PLoS One. 2008;3:e0001722. doi: 10.1371/journal.pone.0001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaarur N, V, Gabai L, Porco JA, Jr, Calderwood S, Sherman MY. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res. 2006;66:1783–1791. doi: 10.1158/0008-5472.CAN-05-3692. [DOI] [PubMed] [Google Scholar]
- 53.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hahn JS. The Hsp90 chaperone machinery: from structure to drug development. BMB Rep. 2009;42:623–630. doi: 10.5483/bmbrep.2009.42.10.623. [DOI] [PubMed] [Google Scholar]
- 56.Ley SC, Marsh M, Bebbington CR, Proudfoot K, Jordan P. Distinct intracellular localization of Lck and Fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol. 1994;125:639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bijlmakers MJ, Isobe-Nakamura M, Ruddock LJ, Marsh M. Intrinsic signals in the unique domain target p56(lck) to the plasma membrane independently of CD4. J Cell Biol. 1997;137:1029–1040. doi: 10.1083/jcb.137.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 59.Bijlmakers MJ, Marsh M. Trafficking of an acylated cytosolic protein: newly synthesized p56(lck) travels to the plasma membrane via the exocytic pathway. J Cell Biol. 1999;145:457–468. doi: 10.1083/jcb.145.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bijlmakers MJ, Marsh M. Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck) Mol Biol Cell. 2000;11:1585–1595. doi: 10.1091/mbc.11.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartson SD, Ottinger EA, Huang W, Barany G, Burn P, Matts RL. Modular folding and evidence for phosphorylation-induced stabilization of an hsp90-dependent kinase. J Biol Chem. 1998;273:8475–8482. doi: 10.1074/jbc.273.14.8475. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96:109–114. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanna-Wakim R, Yasukawa LL, Sung P, Fang M, Sullivan B, Rinki M, DeHovitz R, Arvin AM, Gans HA. Age-related increase in the frequency of CD4(+) T cells that produce interferon-gamma in response to staphylococcal enterotoxin B during childhood. J Infect Dis. 2009;200:1921–1927. doi: 10.1086/648375. [DOI] [PubMed] [Google Scholar]
- 64.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L, Casado JG, Morgado S, Terme M, Ullrich E, Delahaye NF. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 65.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 66.Textor S, Dürst M, Jansen L, Accardi R, Tommasino M, Trunk MJ, Porgador A, Watzl C, Gissmann L, Cerwenka A. Activating NK cell receptor ligands are differentially expressed during progression to cervical cancer. Int J Cancer. 2008;123:2343–2353. doi: 10.1002/ijc.23733. [DOI] [PubMed] [Google Scholar]
- 67.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.