Abstract
Chagas disease is a leading cause of heart disease affecting approximately 10 million people in Latin America and elsewhere worldwide. The two major drugs available for the treatment of Chagas disease have limited efficacy in Trypanosoma cruzi-infected adults with indeterminate (patients who have seroconverted but do not yet show signs or symptoms) and determinate (patients who have both seroconverted and have clinical disease) status; they require prolonged treatment courses and are poorly tolerated and expensive. As an alternative to chemotherapy, an injectable therapeutic Chagas disease vaccine is under development to prevent or delay Chagasic cardiomyopathy in patients with indeterminate or determinate status. The bivalent vaccine will be comprised of two recombinant T. cruzi antigens, Tc24 and TSA-1, formulated on alum together with the Toll-like receptor 4 agonist, E6020. Proof-of-concept for the efficacy of these antigens was obtained in preclinical testing at the Autonomous University of Yucatan. Here the authors discuss the potential for a therapeutic Chagas vaccine as well as the progress made towards such a vaccine, and the authors articulate a roadmap for the development of the vaccine as planned by the nonprofit Sabin Vaccine Institute Product Development Partnership and Texas Children’s Hospital Center for Vaccine Development in collaboration with an international consortium of academic and industrial partners in Mexico, Germany, Japan, and the USA.
Keywords: Chagas disease, Mesoamerica, Mexico, Tc24, therapeutic vaccine, Trypanosoma cruzi, trypomastigote antigen, TSA-1, vaccine
Disease burden
Chagas disease, also known as American trypanosomiasis, is one of the world’s most important neglected tropical diseases and a leading cause of poverty in Latin America, resulting in economic losses of US$1.2 billion annually [1-4]. An estimated 10 million people are infected with Trypanosoma cruzi worldwide with more than 99% of the cases occurring in Latin America, especially in the poorest countries in the region [1,2]. Therefore, Chagas disease affects approximately 10% of Latin America’s ‘bottom 100 million’ – that is, the region’s poorest people who live in poverty [4]. Based on disability-adjusted life-years (DALYs) the disease burden of Chagas disease is five times greater than malaria, and is approximately one-fifth of that of HIV/AIDS in the Latin American and Caribbean region [1]. Most of the disability and deaths from Chagas disease result from chronic Chagas cardiomyopathy that develops in approximately 20–30% of individuals infected with T. cruzi [5]. Megaviscera (megaesophagus and megacolon) are also important clinical sequelae of chronic T. cruzi infection [5].
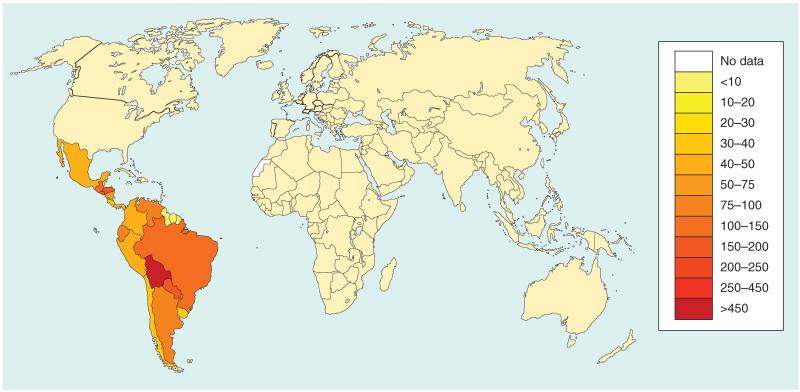
Chagas disease primarily affects people living in poverty, as the ‘kissing bug’ vector has the ability to live in poor-quality dwellings [1]. Furthermore, impoverished populations lack access to essential medicines and vector control practices [1-3]. Today, the greatest number of new cases of Chagas disease occurs in Bolivia [1], but the disease has either emerged or re-emerged in Colombia and in the region of Mesoamerica, which includes Mexico and Central America [6-8]. In Mexico, between 2 and 6 million people are infected, with the highest overall prevalence in the poorest southern states of Chiapas, Oaxaca, Puebla, Veracruz and Yucatan [7], and in at least one area of central Veracruz the disease is hyperendemic with seroprevalence rates approaching 50% [8]. Chagas disease has also emerged in the USA and Europe [9-13]. In the USA there are an estimated 300,000 cases, although some estimates indicate that there may be as many as 1 million cases, with the largest number in Texas and other states bordering Mexico [6,9-11]. Overall, there is a phenomenon known as the ‘globalization of Chagas’ that reflects the importation of this disease into North America, Europe, Japan and Australia as a consequence of immigration (Figure 1)[5,14].
Figure 1. Age-standardized disability-adjusted life-year rates from Chagas disease by country (per 100,000 inhabitants).
Data taken from [103].
Although vector-borne transmission remains the most common mechanism for acquiring T. cruzi infection, mother-to-child transmission (MTCT), transfusion- and organ transplantation-associated infections, and the ingestion of contaminated foods (especially in the Amazon region) have also emerged as an important transmission routes [5]. Regarding MTCT, pregnancy can increase T. cruzi parasitemia, resulting in vertical transmission rates as high as 5–10% to cause congenital Chagas disease [4,15-17]. Each year thousands of cases of congenital Chagas disease are believed to occur in Latin America, including an estimated 2000 T. cruzi-infected newborns in North America alone [4,18]. Aside from congenital infection, T. cruzi in pregnancy is associated with several other adverse birth outcomes for both mother and child [17], and independent of pregnancy, some large studies have revealed a higher prevalence of Chagas disease and chronic Chagasic cardiomyopathy in women relative to men [19]. Thus, Chagas disease has emerged as an important maternal–child global health disparity.
Disease progression
Following initial exposure to the T. cruzi parasite (typically through autoinoculation in the skin by the feces from the kissing bug vector), patients develop acute infection lasting 1–2 months [5]. This phase of the infection is typically asymptomatic or associated with fever, hepatosplenomegaly and edema [5]. Most of the acute infections are self-limited and become asymptomatic, even without antiparasitic treatment [5]. Importantly, virtually all of the acutely infected individuals seroconvert to T. cruzi following the acute phase. Of these seroconverters, approximately 60–70% do not develop clinical symptoms and are considered of indeterminate status, while 30–40% are initially indeterminate and progress to develop chronic disease (determinate status) characterized by cardiac and/or gastrointestinal signs and symptoms [5]. Currently, there are no available biomarkers to predict which patients will develop such chronic disease manifestations. The cardiac complications (occurring in 20–30% of patients) are the most severe and are characterized by arrhythmias, aneurysms, thromboembolic events and heart failure [5]. According to Rassi et al., sudden death as a result of ventricular tachycardia and fibrillation accounts for most of the deaths, with the remaining deaths linked to heart failure and thromboembolic events [5].
Current gaps in treatment
It is generally accepted that end-organ and tissue damage depends on the persistence of the parasite and resultant cardiac and gastrointestinal disease [5,20,21]. The goal of treatment for Chagas disease therefore depends on both the specific antitrypanosomal chemotherapy and targeting the associated cardiac and gastrointestinal manifestations [5]. Currently, anti-trypanosomal treatment is frequently provided for all children with infection and for all individuals with chronic disease up to the age of 50 years without advanced heart disease. Treatment is contraindicated in pregnancy and with advanced kidney and liver disease [5]. Benznidazole and nifurtimox are the two drugs of choice [5]. A 60-day treatment regimen is required for benznidazole, while a 60–90-day treatment regimen is required for nifurtimox [5]. Such prolonged treatment courses present a logistic and economic burden in vulnerable populations where access to healthcare providers is limited, as is the ability to assure that a patient continues regular treatment throughout the full period.
Overall, the current gaps in treatment can be summarized as follows [4]:
Lack of efficacy: only two randomized controlled trials on antitrypanosomal treatments have been published [22], and whether there is true benefit from these drugs in the treatment of Chagas disease of indeterminate status or for Chagasic cardiomyopathy remains controversial [23]. A recent systematic review and meta-analysis has strongly questioned the efficacy of Chagas disease treatment in late chronic infection [24]. Currently, a large Latin American multicenter trial, BENEFIT, is underway to compare benznidazole with placebo in 3000 patients [25]. Of added concern are the findings that these drugs do not eradicate the parasite among heart transplant recipients, and they do not usually result in seroreversion [26]. Such studies suggest that antitrypanosomal therapy does not eradicate the parasite in the host.
Intolerance and unacceptable side effects of current therapy. Antitrypanosomal treatments with benznidazole or nifurtimox have been criticized for their side effects, as well as efficacy [26-28]. Serious side effects have been noted in up to 30–50% of treated individuals, including peripheral neuropathy and bone marrow suppression in addition to digestive intolerance, urticaria and petechial rashes and hepatitis. Furthermore, both drugs are mutagens [26-28]. Between 12 and 18% of patients who undergo treatment have to suspend their therapy prematurely because of side effects [28]. The major drugs are also contraindicated in pregnancy, adding to the maternal–child health disparity of Chagas disease [4]. Overall, the 2010 Latin American Guidelines for Chagas cardiomyopathy indicate that unrestricted treatment for patients with chronic Chagas disease should not be regarded as standard therapy [26].
Cost of current therapy: there is also an issue of prohibitive costs. A 2008 analysis from Colombia indicates that the treatment of a chronic Chagas disease patient can cost between US$46.4 and US$7981 per year depending on the level of care used [29]. Combining cost and utilization estimates, the expected cost of treatment per patient-year is US$1028, with lifetime costs averaging US$11,619 per patient [29]. Estimates for Mexico are even higher, with the cost ranging from US$3000 to US$14,580 per patient-year, depending on the level of care considered [30]. As Chagas disease is a neglected tropical disease and therefore a disease of the poor [4], an estimated 22% of patients never seek care and many more have limited access to health care facilities [29].
In summary, there is an urgent need for new therapeutics for use in chronic Chagas disease together with expanded preventative efforts that include vector control programs [5,22,31,32].
Recently, a number of comparisons have been made between patients with indeterminate and determinate Chagas disease status and those living with HIV/AIDS, especially AIDS patients who lived during the first two decades of the global AIDS pandemic [33]. The comparisons include the chronicity of both diseases, high rates of MTCT and transfusion-associated infections, as well as the costs of treatment, lack of access to available drugs and the poor efficacy of the drugs [33]. Advances in new drug development for Chagas disease have been recently summarized [31,32].
Comparative advantage of a Chagas vaccine
An alternative approach for developing and testing new small molecular drug targets and candidates would be to develop and test a therapeutic vaccine, which could be administered as an immunotherapy either to individuals with chronic Chagas disease or those with indeterminate status who may go on to develop cardiomyopathy. The advantages of a therapeutic Chagas disease vaccine compared with benznidazole alone (the current major competing product in clinical use) could include the following:
Reductions in toxicities, thereby allowing its expanded use in indeterminate and determinate patients;
Higher efficacies at preventing cardiac complications;
Higher rates of seroreversion to T. cruzi antigens not contained in the vaccine;
Potential use in pregnancy to prevent congenital Chagas disease.
An effective therapeutic vaccine for human Chagas disease could prevent cardiac complications among the estimated 40,000 new cases of Chagas disease that occur in Latin America annually [22], avert over 600,000 DALYs annually that result from cardiomyopathy and gastrointestinal disease [1], and prevent 10,000 deaths or more annually (Box 1) [22].
Box 1. The public health impact of a Chagas disease vaccine.
An effective vaccine for Chagas disease could prevent cardiac complications among the estimated 40,000 new cases of Chagas disease that occur in Latin America annually [22], avert over 600,000 DALYs annually that result from cardiomyopathy and gastrointestinal disease [1], and prevent 10,000 deaths or more annually [22].
DALY: Disability-adjusted life-year
Three recent comprehensive reviews have summarized work on the development of a Chagas vaccine, with an emphasis on the development of a preventive vaccine [34-36]. Here the authors summarize recent efforts by a product development partnership (DP) to begin the development of a new therapeutic vaccine, with an emphasis on its development in Mexico and its use for Latin America and elsewhere.
Global access: economics & cost–effectiveness
An analysis of the economic value of a preventive Chagas disease vaccine in Latin America was reported by the Public Health Computational and Operations Research (PHICOR) group at the University of Pittsburgh (PA, USA) [3]. Results of their analysis indicated that vaccination would likely be beneficial across a wide range of infection risk (as low as 1%) with vaccination being economically dominant (i.e., less costly and more effective than not vaccinating) when vaccination cost ≤$5 and vaccine efficacy was ≥50%, highly cost-effective (i.e., ≤1 times the gross domestic product per capita) when vaccination cost ≤$20 and vaccine efficacy was ≥50%, and cost-effective (i.e., 1–3 times the gross domestic product per capita) as long as vaccination cost ≤$30 and vaccine efficacy was ≥50% [3]. More recently, we developed an economic model evaluating the potential cost–effectiveness and return on investment of a therapeutic Chagas disease vaccine for use in Mexico [37]. This analysis, which evaluated the likely benefit of a vaccine that delays or prevents the onset of cardiomyopathy, suggests that such a vaccine would be cost-effective and often “economically dominant” (i.e., providing both cost savings and health benefits) across a range of protection duration, adverse risks and dosing regimens [37]. Although a vaccine that delays the onset of cardiac outcomes by 10 years or more may remain cost-effective, a positive return on investment may only result if the vaccine prevents (rather than delays) the onset of cardiomyopathy [37].
Target product profile
A proposed target product profile for a therapeutic vaccine is outlined in Table 1. Briefly, the product would be a therapeutic vaccine to prevent (desired target) or delay (minimally acceptable target) the onset of Chagasic cardiomyopathy in patients with indeterminate Chagas disease (determined by antibody seropositivity using a licensed diagnostic kit) or in patients with early-stage evidence of clinical Chagas disease (as determined by antibody seropositivity, together with cardiac clinical manifestations, ECG or echocardiographic alterations) [4]. The vaccine would be administered by intramuscular injection and could be used for both children and adults, although adults alone would represent a minimally acceptable target. Ideally a therapeutic vaccine would require at most only two injections and would exhibit an 80% efficacy at preventing or delaying cardiac pathology and possibly also megaviscera-based sequelae. Downstream, the vaccine might also be used during pregnancy to prevent MTCT [4]. The desired and minimally acceptable target prices were selected based on previously reported studies from Colombia and Mexico on the costs of treating a Chagas disease patient with anti-parasitic chemotherapy [29,30].
Table 1.
Chagas vaccine initiative: proposed target product profile.
| Item | Desired target | Minimally acceptable target |
|---|---|---|
| Indication | A therapeutic vaccine to prevent the onset of Chagasic cardiomyopathy in patients with indeterminate Chagas disease (as determined by antibody seropositivity using a licensed diagnostic kit) or in determinate patients with early-stage evidence of clinical Chagas disease (as determined by antibody seropositivity, together with cardiac clinical manifestations; i.e., ECG or echocardiographic alterations) |
A therapeutic vaccine to delay the onset of Chagasic cardiomyopathy in patients with indeterminate Chagas disease (as determined by antibody seropositivity using a licensed diagnostic kit) or in determinate patients with early-stage evidence of clinical Chagas disease (as determined by antibody seropositivity, together with cardiac clinical manifestations; i.e., ECG or echocardiographic alterations) |
| Target population | Children (>2 years) and adults | Adults >16 years |
| Route of administration |
Intramuscular injection | Intramuscular injection |
| Product presentation | Single-dose vials. 1.0 ml volume of delivery | Single-dose vials. 0.5 ml volume of delivery |
| Dosage schedule | Maximum of two immunizations regardless of age, with the second injection given 1–2 months after the first immunization |
Maximum of four immunizations according to a 0, 1–2 months, 4–12 months and 5-year schedule |
| Warnings and precautions/ pregnancy and lactation |
Mild-to-moderate local injection site reactions such as erythema, edema and pain, the character, frequency and severity of which is similar to licensed recombinant protein vaccines. Less than 0.001% risk of urticaria and other systemic allergic reactions. Incidence of SAEs no more than licensed comparator vaccines |
Moderate local injection site reactions such as erythema, edema and pain. Less than 0.1% risk of urticaria. Temporary cardiac inflammation (carditis) as determined by ECG changes lasting no more than 2–3 weeks following administration of any one dose. Beyond the ECG changes, the incidence of SAEs no more than licensed comparator vaccines |
| Expected efficacy | 80% efficacy at preventing the onset of cardiac complications |
80% efficacy of delaying the onset of cardiac complications by ≥10 years |
| Coadministration | All doses may be coadministered with currently available trypanocidal drugs, as well as heart medicines |
Essential heart medicines |
| Shelf-life | 5 years | 1 year |
| Storage | Refrigeration between 2 and 8°C. Cannot be frozen. Can be out of refrigeration (at temperatures up to 25°C) for up to 72 h |
Refrigeration between 2 and 8°C. Cannot be frozen. Temperature monitor required |
| Product registration and WHO prequalifi cation |
US FDA, in addition to licensure by COFEPRIS, the Mexican National Regulatory Authority. WHO prequalification of manufacturing facility at Birmex (Mexico City, Mexico) |
Licensure by COFEPRIS, the Mexican National Regulatory Authority |
| Target price | $46, the minimal cost of treating a patient with Chagas |
$200, one-fifth of the expected cost of treatment per patient per year |
| SAE: Serious adverse event. |
Proposed first-generation therapeutic Chagas disease vaccine
Antigen selection
The authors recently proposed to accelerate the development of a bivalent vaccine for the immunotherapy of human Chagas disease [4,36]. The vaccine would be comprised of two T. cruzi recombinant protein antigens that were selected based on the evidence of protection in laboratory mice and dogs as DNA vaccines [4,36,38-44]. These two antigens include a T. cruzi 24 kDa trypomastigote excretory–secretory protein known as Tc24 and a T. cruzi trypomastigote surface transialidase known as TSA-1 [4]. The antigens are formulated on alum, together with an aqueous formulation of a novel Toll-like receptor 4 (TLR 4) agonist (see below [45,46]). Evidence that these antigens work as a therapeutic vaccine is based on immunizations in T. cruzi-infected mice and dogs, with evidence that protection is linked to T. cruzi-specific CD8+ immune responses (Box 2) [4,38-44]. These studies were conducted in the Laboratory of Parasitology of the Autonomous University of Yucatan (UADY; Merida, Mexico) under the direction of Eric Dumonteil, where since 2004 both Tc24 and TSA-1 have undergone preclinical testing as preventative and therapeutic vaccines for Chagas disease. Previous to these studies, TSA-1 was one of the first T. cruzi antigens to exhibit protective immune responses in laboratory conditions [47,48]. Subsequently, testing at UADY has confirmed the protective efficacy of both Tc24 and TSA-1 as DNA vaccines [36,38-44]. Plasmids encoding either of these two antigens were shown to protect BALB/c mice infected with an otherwise lethal dose of parasites following two immunizations postinfection, as evidenced by reduced parasitemia and cardiac inflammation and >70% survival (although sterilizing immunity was not achieved) [38]. The vaccine was efficacious even when it was delayed until 10 and 15 days postinfection [38]. DNA vaccine immunotherapy was also evaluated at 70 days postinfection in ICR (CD-1) mice infected with a low dose (500 T. cruzi parasites) in order to simulate a chronic infection and this also resulted in improved survival and reduced cardiac tissue inflammation [38]. The protection from TSA-1 DNA immunization was associated with the induction of CD8+ T-cell activity and IFN-γ production [39], with additional evidence showing that the protection was antigen-specific and required plasmids encoding either Tc24 or TSA-1 but not other antigens tested [40]. Combining Tc24 and TSA-1 resulted in significantly lower parasitemia and inflammatory cell density in the heart compared with controls in acutely infected BALB/c and C57BL/6 mice, and reduced cardiac tissue inflammation in chronically infected ICR mice [36,44]. In dogs, therapeutic administration of two doses of DNA vaccines encoding TSA-1 and Tc24 during the acute phase resulted in a decrease in cardiac arrhythmias [42], while when used as a preventive DNA vaccine the two antigens reduced the T. cruzi parasite load in the heart (density of amastigote nests), as well as the number of cardiac arrhythmias, and ultimately mortality [36].
Box 2. Rationale for selection of Tc24 and TSA-1 antigens.
Ability to protect BALB/c and C57BL/6 mice as a therapeutic Trypanosoma cruzi vaccine
Ability to protect ICR mice as a therapeutic vaccine in chronic T. cruzi infection
Protection evidenced by reduced parasitemia and reduced cardiac inflammation
Protection demonstrated to be antigen-specific for Tc24 and TSA-1
Protection demonstrated to depend on CD8+ T cells and production of IFN-γ
Antigens accessible to T cells and antibodies against the surface and excretory/secretory products of T. cruzi
Therefore, a therapeutic target for a human vaccine would be similar reductions in parasite burden and inflammation in the heart in order to prevent or delay Chagasic cardiomyopathy. Tc24 and TSA-1 were down-selected from a larger pool of candidate antigens, including rSA85-1.1, Tc52, ASP9 (ASP-2 like clone 9) and TS, on the basis of their performance as immunotherapeutic DNA vaccines in laboratory mice [35,36,38,40]. Performance metrics included increased IFN-γ and CD8+ cellular responses, decreased parasitemia, decreased T. cruzi parasite burden in the heart, increased survival and decreased inflammation (reviewed in [36]). Although in laboratory animals IFN-γ did not result in increased pathogenicity, the possibility that this cytokine could be immunopathogenic in humans will be considered and monitored. It is possible that additional candidate therapeutic vaccines may also undergo evaluation, including the multicomponent DNA-prime/DNA-boost TcVac1 and TcVac2, which have shown some promise recently as a preventive vaccine in dogs [49,50]. However, based on the evidence of therapeutic efficacy of Tc24 and TSA-1 vaccines in reducing T. cruzi-induced cardiac disease in mice and dogs, the authors are pursuing Tc24 and TSA-1 as lead candidate antigens. Although it has been more than 20 years since it was discovered that plasmid DNA injections induce Th1-type immunity in mice and other laboratory animals, to date the ability of such first-generation DNA vaccines to replicate similar responses in humans has met with mixed success at best. While second-generation DNA vaccines are being developed, especially for chronic noncommunicable diseases such as cancer [51], the authors believe that this approach is still not ready to be considered for the development of a therapeutic Chagas disease vaccine. Therefore, our focus is to attempt to reproduce the effect of DNA vaccines by immunizing with their recombinant protein counterparts. The authors have already shown proof-of-principle for expression of Tc24 using bacterial and yeast expression systems, and are currently evaluating clones selected from expression systems for TSA-1.
Formulation & adjuvants
Based on successful studies conducted in laboratory mice of DNA immunizations with two of the lead vaccine candidates, Tc24 and TSA-1, it was shown that therapeutic vaccination resulted in parasite-specific IFN-γ-producing CD4+ and CD8+ T cells in the spleen and in infected cardiac tissue [36,44]. These results were abrogated in CD8-deficient mice but not as much in CD4-deficient mice, suggesting that CD8+ T cells play a major role in the control of this infection by a therapeutic vaccine [36,44]. Therefore, achieving Th1-type immunity would be an important goal of human therapeutic vaccination. However, purified recombinant protein vaccines are sometimes limited by their weak immunogenicity and the observation that they may not always stimulate adequate Th1-type immune responses [52,53]. For this first-generation therapeutic recombinant Chagas vaccine, efforts will be made to enhance Th1 immunity by formulating the protein antigens on alum and co-injecting with a second adjuvant, E6020. E6020 consists of an aqueous formulation of a well-characterized synthetic lipid A derivative, which is a TLR 4 agonist [45,46]. Previously it was shown that the addition of E6020 can preferentially induce Th1 immunity [46]. Ultimately, the requirement for this second adjuvant will be determined pending preclinical studies and early clinical trials. As an alternative or parallel approach, the authors will investigate polymer-based delivery systems for the delivery of antigens and immunopotentiating adjuvants to antigen-presenting or dendritic cells [4]. Polymeric micro- or nano-particles can enhance uptake of antigen and adjuvant to dendritic cells, while simultaneously protecting antigens against degradation [53]. In addition, particulate delivery systems have been shown to cross-present antigen in order to generate cytotoxic T lymphocytes against intracellular pathogens. Our group has identified a promising microparticle technology based on a new pH-sensitive biodegradable polymer. The pH-sensitive microparticles are designed for rapid intralysosomal degradation and release of antigen and TLR agonists. Protein antigen formulated in this manner elicited robust antigen-specific CD8+ T-cell responses with dose-sparing of antigen and TLR3 agonist [54]. Other polymer-based systems include viscous polysaccharide solutions, which can create an extracellular depot of protein antigen and immunostimulatory molecules and have been shown to induce Th1-type immune responses in animal models [55]. Thus, as an advance the authors can re-engineer the bivalent vaccine candidate using microparticles or other polymer-based technologies to deliver protein antigen and immunopotentiating agents. As a back-up strategy, it may also be possible to employ viral vectors including adenovirus and modified vaccinia Ankara encoding Tc24/TSA-1 [4,56-58].
Product development of the vaccine
The studies described above provide a strong preclinical foundation for accelerating the development of a therapeutic human vaccine for Chagas disease. The authors are currently working to produce a vaccine for initial clinical testing in Mexico and elsewhere in Latin America where Chagas disease is highly endemic. To actually produce a vaccine under current good manufacturing practices (cGMP) suitable for Phase I clinical testing requires the implementation of a number of key steps, including:
The development of a process for the high-yield and low-cost expression, fermentation and purification of the two component recombinant antigens of the Chagas disease vaccine – that is, Tc24 and TSA-1 – followed by formulation on alum (Alhydrogel® [Brenntag Biosector, Frederikssund, Denmark] or aluminum phosphate) together with E6020, a synthetic TLR4 agonist;
The development and qualification of product-specific assays (including potency assays), followed by the process and formulation optimization, which incorporates biophysical profiling;
Technology transfer for the cGMP manufacture of both drug substance and drug product;
Formal release of the drug product based upon qualified assays and a formal stability program, which includes vaccine potency;
A preinvestigational new drug meeting followed by completion of a good laboratory practice (GLP) toxicology study;
Investigational New Drug (IND) submission to begin clinical testing.
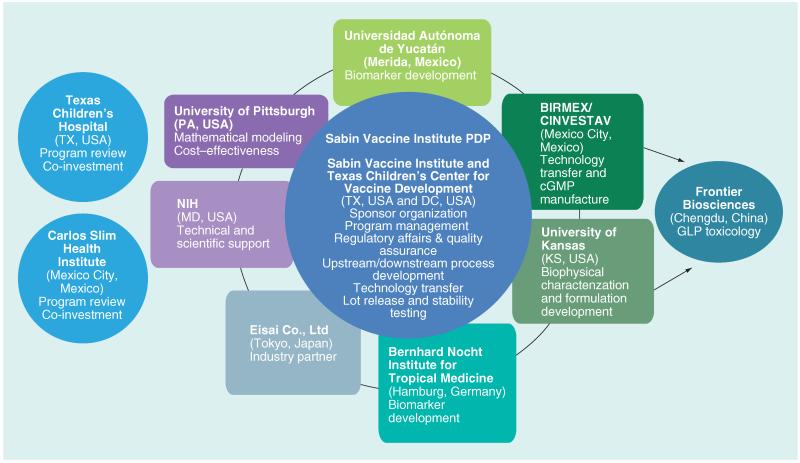
The major deliverable at the end of the project period is the concurrence of the US (FDA) and Mexican (COFEPRIS) national regulatory authorities with plans for initial Phase I clinical testing of the Chagas disease vaccine in Mexico, followed by further clinical testing throughout other areas of Latin America. Thus, the authors are advancing the development of two candidate antigens for the first vaccine against Chagas disease. The Carlos Slim Institute for Health (Instituto Carlos Slim de la Salud; Mexico City, Mexico), together with the Southwest Electronic Energy Medical Research Institute and Texas Children’s Hospital (TX, USA), has made an initial commitment to fund early development of the two T. cruzi vaccine candidate antigens. The vaccine development initiative is led by the nonprofit Sabin Vaccine Institute (DC, USA) Product Development Partnership (PDP), an internationally recognized PDP and one of the only PDPs with a specific mission to develop and test new vaccines to combat the neglected tropical diseases [59]. PDPs are nonprofit organizations that use industry practices to advance new neglected disease products, such as small-molecule drugs, vaccines, diagnostics, microbiocides and insecticides [59]. Worldwide there are approximately 16 PDPs including five committed to vaccine development. The Sabin Vaccine Institute PDP has built the infrastructure and capacity for research, development and scale-up technology transfer for potential vaccine candidates, especially to developing country manufacturers in Brazil (i.e., FIOCRUZ BioManguinhos [Rio de Janeiro, Brazil] and Instituto Butantan [Sao Paulo, Brazil]) and Mexico (see below), as well as a strong management and administrative core experienced in vaccine development, quality assurance, regulatory affairs and clinical trials. Sabin Vaccine Institute’s 11 years of research and development experience has generated a comprehensive, low-cost model that serves as a blueprint for vaccine development and ongoing efforts to fight public health threats that adversely impact more than 1 billion people worldwide. Such activities include new vaccines for hookworm infection and schistosomiasis, which have either entered or will soon enter clinical testing [59]. The partnership for accelerating the first therapeutic Chagas vaccine for human trials is illustrated in Figure 2 and summarized in Box 3. Briefly:
Sabin Vaccine Institute PDP, in collaboration with its affiliated Texas Children’s Hospital Center for Vaccine Development, will express the recombinant antigens (10–20 l fermentation scale for yeast, bacteria and other expression systems, together with downstream purification) and perform process development and technology transfer activities for manufacture in collaboration with Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV), the center for research and advanced studies in Mexico City (Mexico) [101]. Sabin will also provide regulatory affairs and quality assurance support. A hallmark of this activity is that the Sabin Vaccine Institute PDP selects processes – that is, microfiltration, ultrafiltration, centrifugation, column resins and assays – which are compatible with the pilot manufacturing facility of the GMP manufacturer at the 60–100 l scales. Upon execution of a manufacturing agreement for the production of Phase I clinical trial material, a productive research clone will be provided to the cGMP manufacturer for the generation of master/production cell banks;
UADY will evaluate the antigenicity, immunogenicity and preclinical efficacy of the Chagas vaccine candidates;
Birmex (Laboratorios de Biológicos y Reactivos de México – Mexico’s leading and public sector vaccine manufacturer) will perform cGMP manufacture. Birmex is a Mexican state-owned institution under the supervision of the Federal Secretary of Health, and is dedicated to research, development and production of venoms, different anti-venom purified polyclonal immunoglobulins and vaccines [102]. Birmex currently produces a number of vaccines for Mexico;
The partnership has obtained the access for the testing and evaluation of a novel adjuvant through the commitment from the Japanese company Eisai Co., Ltd, which has pioneered the development of the synthetic TLR4 agonist, E6020, as a vaccine adjuvant [45];
Additional partners include the Bernhard Nocht Institute for Tropical Medicine (BNI; Hamburg, Germany), which has extensive experience in the immunology of Chagas disease [60-62], University of Kansas (KS, USA) for biophysical and formulation assessments [63], and the PHICOR group of the University of Pittsburgh School of Medicine and Graduate School of Public Health (PA, USA) [3].
Figure 2. Partnership for the development of a human therapeutic Chagas disease vaccine.
cGMP: Current good manufacturing practice; GLP: Good laboratory practice; PDP: Product development partnership.
Based on our previous success with technology transfer, the authors expect that the production processes will yield Tc24 and TSA-1 drug substance and drug products of sufficient yield, purity and stability for use in Phase I testing. Following GLP toxicology testing, a regulatory filing for each of the candidate antigens will be prepared and submitted both to the US FDA and the Mexican national regulatory authority (COFEPRIS) (Box 4).
Box 4. Strengths of the partnership.
Sabin Vaccine Institute is a product development program with an 11-year successful track record of partnering with developing country manufacturers and transitioning discoveries to the clinic.
In preclinical testing studies conducted at Autonomous University of Yacutan (Yacutan, Mexico), proof-of-concept of protective efficacy of the two antigens when combined.
As a deliverable, the advancement in development of an urgently needed new medical intervention, a therapeutic vaccine for Chagas disease, one of the most important neglected tropical diseases in the Americas and a major maternal–child health threat.
High level of scientific innovation building on almost a decade of preliminary data, published in peer-reviewed journals.
Capacity building with key Mexican institutions including CINVESTAV and Birmex, the public sector vaccine manufacturers in Mexico.
Economic models confirming the cost–effectiveness of a therapeutic vaccine.
For quality control and assurance, an extensive array of assays has been developed and implemented for protein characterization. These assays are employed in the earliest stages of the candidate antigen and are refined for the specific antigen concurrently with the early process development. The Sabin Vaccine Institute PDP and Texas Children’s Center for Vaccine Development performs assay qualifications to establish that assays used for the recombinant vaccine antigen characterization are accurate, precise, sensitive, specific, reproducible and robust. Based on these qualification procedures, a set of release specifications will be established to support the regulatory submission to the FDA and COFEPRIS for the recombinant antigen vaccine. All assays use standard published procedures or modified procedures established by the Sabin Vaccine Institute PDP as a part of its hookworm vaccine development program and will be codeveloped with Birmex/CINVESTAV to assure smooth technology transfer and product release. These assays will establish criteria for the evaluation of the expression during ‘scale-up’ and ‘final lock-down’ as well as for the stability program of the recombinant proteins. Most of these assays are antigen-specific and will be developed through application and/or modification of methods currently existing in our laboratories. In addition, a variety of assays have been developed, maintained and qualified in order to analyze pH, protein concentration determination by UV absorbance and endotoxin content. All assays will complement biophysical methods for the generation of protein conformational maps or diagrams. This approach will provide confirmation of a successful, consistent and reproducible process development strategy.
Preclinical & clinical development of the vaccine
In addition to the product development challenges, the clinical development (and even preclinical development) of a therapeutic Chagas vaccine also presents a number of hurdles.
Autoimmunity
One potential concern is the possibility of inducing autoimmunity as a result of therapeutic vaccination. The role of autoimmunity in the pathogenesis of Chagas disease is considered controversial [5]. While it is generally accepted that persistence of parasites is a necessary requirement for the subsequent development of chronic complications of Chagas disease, including cardiomyopathy, it has not been well established whether tissue damage is ultimately caused by the parasites themselves versus immunopathology and autoimmune mechanisms [5,64,65]. However, no definitive pathogenic role has been ascribed from autoimmunity, which would require showing evidence of the disease from antibodies or T cells that result in chronic heart disease or myocarditis [5]. Indeed, the establishment of chronic T. cruzi infection can occur in the setting of depressed T-cell responses [5]. In a recent article, Machado et al. argue that there is “now a considerable body of evidence and broad consensus that parasite persistence is requisite for pathogenesis and that antiparasitic immunity can be protective against T. cruzi pathogenesis without eliciting autoimmune pathology.” However, it will be essential to consider and monitor autoimmune sequelae as a part of the clinical development plan of the Chagas Vaccine Initiative. It is likely that potential concerns about autoimmunity will result in a cautious and deliberate step-wise clinical development plan. One option will be to first test the vaccine in a non-human primate model prior to clinical testing [67,68].
Antigenic variation
Another challenge will be to examine each antigen for its genetic diversity and geographic variation in its amino acid sequence. Strain-specific immunity has been shown for some subunit preventive vaccines [34]. Currently T. cruzi is classified into six so-called ‘discrete taxonomic units’ based on a recently proposed nomenclature (TcI, TcII, TcIII, TcIV, TcV and TcVI) [69]. The impact of this taxonomy and genetic diversity on the two proposed antigens for the therapeutic vaccine is under evaluation.
Biomarkers
Novel biomarkers for disease progression will also need to be evaluated and standardized to allow for extensive vaccine evaluation, both in laboratory animals and in humans. These include measurement of plasma levels of natriuretic peptide [70], ApoA1 [71] or cardiac troponin T [72], as well as immunological markers such as T-cell profiles [73] and cytokine levels [74]. Recently, serum proteomic signatures were obtained from human Chagasic patients in order to identify novel protein biomarkers of cardiac muscle injury in Chagas disease patients, including myosin light chain 2, myosin heavy chain 11 and increased levels of vinculin and plasminogen [75]. Such observations also offer expanded possibilities for developing biomarkers for human study. Dog as well as non-human primate models will also be considered for further evaluation of the vaccine and biomarkers. To evaluate the success of vaccination, both for the mouse model as well as for the clinical Phase I trial, it will be important to measure the HLA-restricted activation of both CD4+ and CD8+ T cells in an autologous system. The authors propose to utilize a lentiviral transduction system currently used for gene transfer and adapt it to the expression of T. cruzi antigens in primary human APCs. The vaccine antigens will be cloned into the lentiviral system and the transduction into primary cells needs to be established.
Generally, parasitemia is an acceptable read out for acute infection in mice. Nevertheless, after the acute phase a PCR-based method will be used to measure the remaining tissue burden to assess whether a prophylactic vaccine will result in either sterile immunity or at least a reduced tissue burden. Similarly, parasite tissue burden will be assessed in the chronic mouse model. Furthermore, it will be desirable to monitor the function of the vaccine-induced T. cruzi-specific CD8+ T cells (in both acutely and chronically infected mice) by either measuring their cytokine production (enzyme-linked immunosorbent spot and/or intracellular flow cytometry) or by analyzing their cytotoxic potential using an in vivo killing assay (transfer of labeled target in vaccinated recipients and measurement of remaining cells by flow cytometry). At least two different strains will be used for challenge (Tulahuen and Y strain). Histology and/or release of marker enzymes (liver enzyme, troponin C) will be assessed as possible signs for inflammation. Biomarkers of a successful vaccination will be established and will be used for human volunteers enrolled in clinical trials.
In humans, the induction and functional capacity of T. cruzi-specific T cells will be assessed by measuring release of proinflammatory cytokines. For this purpose, peripheral blood leukocytes from vaccinated patients will be stimulated with T. cruzi lysate or the antigens in question at an early time point (T-effector cells) and at a late time point (T-memory cells) and the subsequent production of cytokines will be measured by enzyme-linked immunosorbent spot, intracellular flow cytometry or by multiplex ELISA. For this purpose, primary autologous cells will be transduced using a lentiviral vector encoding the protective antigens included in the vaccine. These cells will be used as target for the CD8+ cells of vaccinated patients in cytotoxicity or cytokine release assays. The assays in mice/patients will be established at an early stage of the project.
Expert commentary
A total of 8 years of published preclinical studies in mice and dogs provide a strong evidence base for a therapeutic effect of a bivalent DNA vaccine encoding the antigens Tc24 and TSA-1, with substantive reductions in parasitemia and tissue parasites, specific antiparasitic T-cell immunity, reduced cardiac inflammation and increased host survival. These observations provide a basis for accelerating the development of a human therapeutic Chagas vaccine. Because DNA vaccines have not yet translated into effective human vaccines, the therapeutic Chagas vaccine is being developed as a bivalent recombinant protein vaccine on alum, together with the synthetic TLR4 agonist E6020. Leading vaccine development efforts is a PDP, together with a consortium of Mexican and other institutions for formulation, process development, cGMP manufacture, lot release, preclinical and clinical testing and modeling. Ultimately, the vaccine would be manufactured and first tested in Mexico in order to initially address Latin America’s heavy burden of Chagas disease from vector-borne transmission, and MTCT downstream. Key scientific and technical challenges to overcome will include demonstrating that a therapeutic recombinant vaccine can induce protective Th1-type immunity while simultaneously avoiding the induction of autoimmunity, as well as the development and implementation of a satisfactory set of biomarkers to effectively monitor clinical efficacy. Ultimately, given the recent literature pointing overwhelmingly to the importance of parasite persistence in promoting progression of Chagas disease and cardiomyopathy, a therapeutic vaccine offers great promise for complementing or possibly replacing current drug therapy.
Five-year view
Pending success in process development and technology transfer to Birmex in Mexico, the two antigens comprising a prototype therapeutic vaccine for human Chagas disease could be manufactured under cGMP within the next 5 years. Formulation of the vaccine on alum, together with E6020, would need to stimulate Th1-type immunity in preclinical testing. Following product lot release, GLP toxicology testing and simultaneous regulatory filings in the USA (FDA) and in Mexico (COFEPRIS), Phase I clinical trials (for safety and immunogenicity) could commence in healthy volunteers followed by studies in patients with indeterminate or determinate Chagas disease. Success in the clinical development of a therapeutic Chagas disease vaccine will require advancement in the status of biomarkers to monitor its impact on halting the progression of disease, especially with regard to the vaccine’s ability to prevent or delay Chagasic cardiomyopathy.
Box 3. Activities of the Chagas vaccine initiative.
- Scale-up process development (10-20 l scale) and formulation of the two lead candidate protein antigens
- Development of a process for fermentation, purification and formulation of the recombinant antigens at high yield (>200 mg/l), purity (>98%), and binding to alum (>90%)
- Confirmatory preclinical efficacy testing for material generated during process development and re-engineering, including testing of E6020, an experimental adjuvant, and development of biomarkers
- Confirmation of protective immunity; independent confirmatory testing in at least two sites; and qualification of biomarkers for clinical assessment during Phase I and beyond
- Technology transfer to Mexico
- Transfer of batch production records for a scaled-up process at the 60–100 l scale for the cGMP manufacture of Tc24 and TSA-1 and the Chagas disease therapeutic vaccine
- cGMP manufacture
- cGMP manufacture of >1 g each of recombinant Tc24 and TSA-1 at purity >98% and formulated on alum + E6020 with >90% protein binding, passing lot release and 1-year stability testing
- Regulatory filing with US FDA and COFEPRIS
- Parallel filings to perform GLP toxicology and to obtain approval for future Phase I testing
- Clinical capacity building and site preparation for clinical testing
- Personnel trained, sites evaluated and selected in preparation for future clinical testing year 5 and beyond
- Mathematical modeling and cost–effectiveness
- Cost–effectiveness conferred
cGMP: Current good manufacturing practice; GLP: Good laboratory practice.
Key issues.
Chagas disease is a major neglected tropical disease affecting 10 million people living in poverty in Latin America.
All patients seroconvert following initial infection with Trypanosoma cruzi and 20–30% of these patients subsequently develop Chagasic cardiomyopathy or other heart sequelae.
The two major drugs currently used for Chagas disease, benznidazole and nifurtimox, have limited efficacy in adults with indeterminate status (patients who have seroconverted but do not yet show signs or symptoms) and determinate status (patients who have both seroconverted and have clinical disease). The drugs also require prolonged treatment courses, and are poorly tolerated and expensive.
An injectable therapeutic Chagas disease vaccine is under early development to prevent or delay Chagasic cardiomyopathy in patients with indeterminate or determinate status.
The proposed first-generation bivalent vaccine is comprised of two recombinant antigens, Tc24 and TSA-1, which are formulated on alum, together with a Toll-like receptor 4 agonist known as E6020.
Proof-of-concept for the efficacy of these antigens was obtained in preclinical testing in mice and dogs at the Autonomous University of Yucatan (location).
An important scientific hurdle is to reproduce the CD8+ immune responses generated using DNA vaccines with mice, but by substituting recombinant proteins together with a Toll-like receptor 4 agonist.
The vaccine is being developed by the Sabin Vaccine Institute Product Development Partnership and Texas Children’s Hospital Center for Vaccine Development in collaboration with CINVESTAV and Birmex, Mexico’s leading vaccine manufacturer, with funding from the Carlos Slim Health Institute.
Acknowledgments
Financial & competing interests disclosure
Michael Heffernan is a co-inventor on three patent applications pertaining to the pH-sensitive microparticles discussed in this manuscript. Several of the authors are involved in various aspects of the development and possible future manufacture of a potential vaccine currently in development against Chagas disease. The Carlos Slim Institute of Health and the Southwest Electronic Energy Medical Research Institute are providing financial support for the Chagas disease vaccine initiative. Eisai Co., Ltd is providing access to the E6020 adjuvant. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Eric Dumonteil, Laboratorio de Parasitología Centro De Investigaciones Regional, “Dr. Hideo Noguchi” Autonomous University of Yucatan (UADY), Merida, Mexico.
Maria Elena Bottazzi, Sabin Vaccine Institute and Texas Children’s Hospital Center for Vaccine Development, Departments of Pediatrics (Section of Pediatric Tropical Medicine) and Molecular Virology & Microbiology, National School of Tropical Medicine, Baylor College of Medicine, Houston, TX, USA.
Bin Zhan, Sabin Vaccine Institute and Texas Children’s Hospital Center for Vaccine Development, Department of Pediatrics (Section of Pediatric Tropical Medicine), National School of Tropical Medicine, Baylor College of Medicine, Houston, TX, USA.
Michael J Heffernan, Sabin Vaccine Institute and Texas Children’s Hospital Center for Vaccine Development, Department of Pediatrics (Section of Pediatric Tropical Medicine), National School of Tropical Medicine, Baylor College of Medicine, Houston, TX, USA.
Kathryn Jones, Sabin Vaccine Institute and Texas Children’s Hospital Center for Vaccine Development, Departments of Pediatrics (Section of Pediatric Tropical Medicine) and Molecular Virology & Microbiology, National School of Tropical Medicine, Baylor College of Medicine, Houston, TX, USA.
Jesus G Valenzuela, Vector Molecular Biology Section, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD, USA.
Shaden Kamhawi, Vector Molecular Biology Section, Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD, USA.
Jaime Ortega, Departamento de Biotecnología y Bioingeniería, Centro de Investigacion y de Estudios Avanzados - Instituto Politécnico Nacional (CINVESTAV-IPN), Mexico City, Mexico.
Samuel Ponce de Leon Rosales, Laboratorios de Biológicos y Reactivos de México (BIRMEX), Mexico City, Mexico.
Bruce Y Lee, Public Health Computational and Operations Research (PHICOR), University of Pittsburgh, Pittsburgh PA, USA.
Kristina M Bacon, Public Health Computational and Operations Research (PHICOR), University of Pittsburgh, Pittsburgh PA, USA.
Bernhard Fleischer, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany.
BT Slingsby, Eisai Co., Ltd, Tokyo, Japan.
Miguel Betancourt Cravioto, Instituto Carlos Slim de la Salud (ICSS), Mexico City, Mexico.
Roberto Tapia-Conyer, Instituto Carlos Slim de la Salud (ICSS), Mexico City, Mexico.
Peter J Hotez, Sabin Vaccine Institute and Texas Children’s Hospital Center for Vaccine Development, Departments of Pediatrics (Section of Pediatric Tropical Medicine) and Molecular Virology & Microbiology, National School of Tropical Medicine, Baylor College of Medicine, Houston, TX, USA.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl. Trop. Dis. 2008;2(9):e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . First WHO report on neglected tropical diseases: working to overcome the impact of neglected tropical diseases. WHO, Geneva, Switzerland: 2010. [Google Scholar]
- 3•.Lee BY, Bacon KM, Connor DL, Willig AM, Bailey RR. The potential economic value of a Trypanosoma cruzi (Chagas disease) vaccine in Latin America. PLoS Negl. Trop. Dis. 2010;4(12):e916. doi: 10.1371/journal.pntd.0000916. First published cost–effectiveness analysis of a preventive Chagas disease vaccine.
- 4.Hotez PJ, Dumonteil E, Heffernan MJ, Bottazzi ME. Innovation for “the bottom 100 million”: eliminating neglected tropical diseases in the Americas through mass drug administration and new vaccines for hookworm and Chagas disease. Adv. Exp. Biol. Med. 2012 doi: 10.1007/978-1-4614-4726-9_1. In Press. [DOI] [PubMed] [Google Scholar]
- 5•.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388–1402. doi: 10.1016/S0140-6736(10)60061-X. Excellent review of the clinical and epidemiological aspects of Chagas disease.
- 6.Hotez PJ, Bottazzi ME, Dumonteil E, et al. Texas and Mexico: sharing a legacy of poverty and neglected tropical diseases. PLoS Negl. Trop. Dis. 2012;6(3):e1497. doi: 10.1371/journal.pntd.0001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Reyes A, Pickering-López JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years – a review. Mem. Inst. Oswaldo Cruz. 2006;101(4):345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Ligonio A, López-Monteon A, Guzmán-Gómez D, Rosales-Encina JL, Limón-Flores Y, Dumonteil E. Identification of a hyperendemic area for Trypanosoma cruzi infection in central Veracruz, Mexico. Am. J. Trop. Med. Hyg. 2010;83(1):164–170. doi: 10.4269/ajtmh.2010.09-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. 2009;49(5):e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 10.Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin. Microbiol. Rev. 2011;24(4):655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl. Trop. Dis. 2008;2:e279. doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotez PJ, Gurwith M. Europe’s neglected infections of poverty. Int. J. Infect. Dis. 2011;15(9):e611–e619. doi: 10.1016/j.ijid.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115(1-2):22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115(1-2):14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Theiler RN, Rasmussen SA, Treadwell TA, Jamieson DJ. Emerging and zoonotic infections in women. Infect. Dis. Clin. North Am. 2008;22(4):755–72. vii. doi: 10.1016/j.idc.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siriano Lda R, Luquetti AO, Avelar JB, Marra NL, de Castro AM. Chagas disease: increased parasitemia during pregnancy detected by hemoculture. Am. J. Trop. Med. Hyg. 2011;84(4):569–574. doi: 10.4269/ajtmh.2011.10-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-López FR, Chedraui P. Chagas disease in pregnancy: a non-endemic problem in a globalized world. Arch. Gynecol. Obstet. 2010;282(6):595–599. doi: 10.1007/s00404-010-1553-7. [DOI] [PubMed] [Google Scholar]
- 18.Buekens P, Almendares O, Carlier Y, et al. Mother-to-child transmission of Chagas’ disease in North America: why don’t we do more? Matern. Child Health J. 2008;12(3):283–286. doi: 10.1007/s10995-007-0246-8. [DOI] [PubMed] [Google Scholar]
- 19.Roca C, Pinazo MJ, Lopez-Chejade P, et al. Chagas disease among the Latin American adult population attending in a primary care center in Barcelona. PLoS Negl. Trop. Dis. 2011;26:e1135. doi: 10.1371/journal.pntd.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarleton RL. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 2001;31(5-6):550–554. doi: 10.1016/s0020-7519(01)00158-8. [DOI] [PubMed] [Google Scholar]
- 21.Plessman Camargo E. Perspectives of vaccination in Chagas disease revisited. Mem. Inst. Oswaldo Cruz. 2009;104(Suppl. 1):275–280. doi: 10.1590/s0074-02762009000900036. [DOI] [PubMed] [Google Scholar]
- 22.Lescure FX, Le Loup G, Freilij H, et al. Chagas disease: changes in knowledge and management. Lancet Infect. Dis. 2010;10(8):556–570. doi: 10.1016/S1473-3099(10)70098-0. [DOI] [PubMed] [Google Scholar]
- 23.Bern C. Antitrypanosomal therapy for chronic Chagas’ disease. N. Engl. J. Med. 2011;364(26):2527–2534. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Molina JA, Pérez-Ayala A, Moreno S, Fernández-González MC, Zamora J, López-Velez R. Use of benznidazole to treat chronic Chagas’ disease: a systematic review with a meta-analysis. J. Antimicrob. Chemother. 2009;64(6):1139–1147. doi: 10.1093/jac/dkp357. [DOI] [PubMed] [Google Scholar]
- 25.Marin-Neto, Rassi A, Jr, Morillo CA, et al. on behalf of BENEFIT Investigators. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas cardiomyopathy: the Benznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) Am. Heart J. 2008;156:37–43. doi: 10.1016/j.ahj.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Sarli Issa V, Alcides Bocchi E. Antitrypanosomal agents: treatment or threat? Lancet. 2010;376:768–769. doi: 10.1016/S0140-6736(10)61372-4. [DOI] [PubMed] [Google Scholar]
- 27.Pinazo MJ, Muñoz J, Posada E, et al. Tolerance of benznidazole in treatment of Chagas’ disease in adults. Antimicrob. Agents Chemother. 2010;54(11):4896–4899. doi: 10.1128/AAC.00537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viotti R, Vigliano C, Lococo B, et al. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev. Anti. Infect. Ther. 2009;7(2):157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 29.Castillo-Riquelme M, Guhl F, Turriago B, et al. The costs of preventing and treating chagas disease in Colombia. PLoS Negl. Trop. Dis. 2008;2(11):e336. doi: 10.1371/journal.pntd.0000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallejo M, Montenegro P, Reyes PA. How much does the medical treatment of chronic Chagas cardiopathy cost? Direct costs in a cardiology hospital. Arch. Cardiol. Mex. 2002;72(2):129–137. [PubMed] [Google Scholar]
- 31.Apt W. Current and developing therapeutic agents in the treatment of Chagas disease. Drug Des. Devel. Ther. 2010;4:243–253. doi: 10.2147/dddt.s8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckner FS, Navabi N. Advances in Chagas disease drug development: 2009-2010. Curr. Opin. Infect. Dis. 2010;23(6):609–616. doi: 10.1097/QCO.0b013e3283402956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotez PJ, Dumonteil E, Woc-Colburn L, et al. Chagas disease: ‘The New HIV/AIDS of the Americas’. PLoS Negl. Trop. Dis. 2012;6(5):e1498. doi: 10.1371/journal.pntd.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cazorla SI, Frank FM, Malchiodi EL. Vaccination approaches against Trypanosoma cruzi infection. Expert Rev. Vaccines. 2009;8(7):921–935. doi: 10.1586/erv.09.45. [DOI] [PubMed] [Google Scholar]
- 35•.Vázquez-Chagoyán JC, Gupta S, Garg NJ. Vaccine development against Trypanosoma cruzi and Chagas disease. Adv. Parasitol. 2011;75:121–146. doi: 10.1016/B978-0-12-385863-4.00006-X. Major new review of the current status of Chagas disease vaccines.
- 36•.Quijano-Hernandez I, Dumonteil E. Advances and challenges towards a vaccine against Chagas disease. Hum. Vaccin. 2011;7(11):1184–1191. doi: 10.4161/hv.7.11.17016. Major new review of the current status of Chagas disease vaccines.
- 37.Lee BY, Bacon KM, Wateska AR, et al. Modeling the economic value of a Chagas’ disease therapeutic vaccine. Hum. Vaccines Immunther. 2012;8(9):1293–1301. doi: 10.4161/hv.20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Dumonteil E, Escobedo-Ortegon J, Reyes-Rodriguez N, Arjona-Torres A, Ramirez-Sierra MJ. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice. Infect. Immun. 2004;72(1):46–53. doi: 10.1128/IAI.72.1.46-53.2004. First paper describing the use of DNA vaccines as immunotherapy of Trypanosoma cruzi infection in mice.
- 39.Zapata-Estrella H, Hummel-Newell C, Sanchez-Burgos G, et al. Control of Trypanosoma cruzi infection and changes in T-cell populations induced by a therapeutic DNA vaccine in mice. Immunol. Lett. 2006;103(2):186–191. doi: 10.1016/j.imlet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 40••.Sanchez-Burgos G, Mezquita-Vega RG, Escobedo-Ortegon J, et al. Comparative evaluation of therapeutic DNA vaccines against Trypanosoma cruzi in mice. FEMS Immunol. Med. Microbiol. 2007;50(3):333–341. doi: 10.1111/j.1574-695X.2007.00251.x. Important paper comparing protection from different antigens when used as therapeutic vaccines.
- 41.Dumonteil E. DNA vaccines against protozoan-parasites: advances and challenges. J. Biomed. Biotechnol. 2007;2007:90520. doi: 10.1155/2007/90520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Quijano-Hernandez IA, Bolio-González ME, Rodríguez-Buenfil JC, Ramirez-Sierra MJ, Dumonteil E. Therapeutic DNA vaccine against Trypanosoma cruzi infection in dogs. Ann. NY Acad. Sci. 2008;1149:343–346. doi: 10.1196/annals.1428.098. First evidence showing protective effects of a therapeutic vaccine in a large animal model.
- 43.Dumonteil E. Vaccine development against Trypanosoma cruzi and Leishmania species in the post-genomic era. Infect. Genet. Evol. 2009;9(6):1075–1082. doi: 10.1016/j.meegid.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 44••.Limon-Flores AY, Cervera-Cetina R, Tzec-Arjona JL, et al. Effect of a combination DNA vaccine for the prevention and therapy of Trypanosoma cruzi infection in mice: role of CD4+ and CD8+ T cells. Vaccine. 2010;28(46):7414–7419. doi: 10.1016/j.vaccine.2010.08.104. Paper confirming the benefits of combining antigens in a therapeutic vaccine.
- 45.Ishizaka ST, Hawkins LD. E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev. Vaccines. 2007;6(5):773–784. doi: 10.1586/14760584.6.5.773. [DOI] [PubMed] [Google Scholar]
- 46.Baudner BC, Ronconi V, Casini D, et al. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020) Pharm. Res. 2009;26(6):1477–1485. doi: 10.1007/s11095-009-9859-5. [DOI] [PubMed] [Google Scholar]
- 47•.Wizel B, Garg N, Tarleton RL. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 1998;66(11):5073–5081. doi: 10.1128/iai.66.11.5073-5081.1998. Early proof-of-concept for protection using TSA-1.
- 48.Garg N, Tarleton RL. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 2002;70(10):5547–5555. doi: 10.1128/IAI.70.10.5547-5555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta S, Garg NJ. Prophylactic efficacy of TcVac2 against Trypanosoma cruzi in mice. PLoS Negl. Trop. Dis. 2010;4(8):e797. doi: 10.1371/journal.pntd.0000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aparicio-Burgos JE, Ochoa-García L, Zepeda-Escobar JA, et al. Testing the efficacy of a multi-component DNA-prime/DNA-boost vaccine against Trypanosoma cruzi infection in dogs. PLoS Negl. Trop. Dis. 2011;5(5):e1050. doi: 10.1371/journal.pntd.0001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin. Infect. Dis. 2011;53(3):296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant – ‘the long and winding road’. Drug Discov. Today. 2009;14(11-12):541–551. doi: 10.1016/j.drudis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines. 2010;9(9):1095–1107. doi: 10.1586/erv.10.89. Good review on nanoparticle formulations for vaccination.
- 54••.Heffernan MJ, Kasturi SP, Yang SC, Pulendran B, Murthy N. The stimulation of CD8+ T cells by dendritic cells pulsed with polyketal microparticles containing ion-paired protein antigen and poly(inosinic acid)-poly(cytidylic acid) Biomaterials. 2009;30(5):910–918. doi: 10.1016/j.biomaterials.2008.10.034. Paper showing proof of concept of improved CD8+ T-cell responses by formulating antigens into microparticles.
- 55.Heffernan MJ, Zaharoff DA, Fallon JK, Schlom J, Greiner JW. In vivo efficacy of a chitosan/IL-12 adjuvant system for protein-based vaccines. Biomaterials. 2011;32(3):926–932. doi: 10.1016/j.biomaterials.2010.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol. 2011;23(3):377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Cavenaugh JS, Awi D, Mendy M, Hill AV, Whittle H, McConkey SJ. Partially randomized, non-blinded trial of DNA and MVA therapeutic vaccines based on hepatitis B virus surface protein for chronic HBV infection. PLoS ONE. 2011;6(2):e14626. doi: 10.1371/journal.pone.0014626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill AV, Reyes-Sandoval A, O’Hara G, et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum. Vaccin. 2010;6(1):78–83. doi: 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- 59.Hotez P. A handful of ‘antipoverty’ vaccines exist for neglected diseases, but the world’s poorest billion people need more. Health Aff. (Millwood) 2011;30(6):1080–1087. doi: 10.1377/hlthaff.2011.0317. [DOI] [PubMed] [Google Scholar]
- 60.Lieke T, Graefe SE, Klauenberg U, Fleischer B, Jacobs T. NK cells contribute to the control of Trypanosoma cruzi infection by killing free parasites by perforin-independent mechanisms. Infect. Immun. 2004;72(12):6817–6825. doi: 10.1128/IAI.72.12.6817-6825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lieke T, Steeg C, Graefe SE, Fleischer B, Jacobs T. Interaction of natural killer cells with Trypanosoma cruzi-infected fibroblasts. Clin. Exp. Immunol. 2006;145(2):357–364. doi: 10.1111/j.1365-2249.2006.03118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobs T, Erdmann H, Fleischer B. Molecular interaction of Siglecs (sialic acid-binding Ig-like lectins) with sialylated ligands on Trypanosoma cruzi. Eur. J. Cell Biol. 2010;89(1):113–116. doi: 10.1016/j.ejcb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Maddux NR, Joshi SB, Volkin DB, Ralston JP, Middaugh CR. Multidimensional methods for the formulation of biopharmaceuticals and vaccines. J. Pharm. Sci. 2011 doi: 10.1002/jps.22618. doi:10.1002/jps.22618. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115(9):1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 65.Gutierrez FR, Guedes PM, Gazzinelli RT, Silva JS. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol. 2009;31(11):673–685. doi: 10.1111/j.1365-3024.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 66••.Machado FS, Tyler KM, Brant F, Esper L, Teixeira MM, Tanowitz HB. Pathogenesis of Chagas disease: time to move on. Front. Biosci. (Elite Ed.) 2012;4:1743–1758. doi: 10.2741/495. Viewpoint-type article about the important role of parasite persistence in the pathogenesis of Chagas disease and to not use potential concerns about autoimmunity as an excuse to paralyze the field of vaccine development.
- 67.Zabalgoitia M, Ventura J, Anderson L, Williams JT, Carey KD, Vandeberg JL. Electrocardiographic findings in naturally acquired chagasic heart disease in nonhuman primates. J. Electrocardiol. 2003;36(2):155–160. doi: 10.1054/jelc.2003.50019. [DOI] [PubMed] [Google Scholar]
- 68.Zabalgoitia M, Ventura J, Anderson L, Carey KD, Williams JT, Vandeberg JL. Morphologic and functional characterization of Chagasic heart disease in non-human primates. Am. J. Trop. Med. Hyg. 2003;68(2):248–252. [PubMed] [Google Scholar]
- 69.Ramírez JD, Guhl F, Rendón LM, Rosas F, Marin-Neto JA, Morillo CA. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic Chagasic patients. PLoS Negl. Trop. Dis. 2010;4(11):e899. doi: 10.1371/journal.pntd.0000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Alvarez A, Sitges M, Pinazo MJ, et al. Chagas cardiomyopathy: the potential of diastolic dysfunction and brain natriuretic peptide in the early identification of cardiac damage. PLoS Negl. Trop. Dis. 2010;4(9):e826. doi: 10.1371/journal.pntd.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ndao M, Spithill TW, Caffrey R, et al. Identification of novel diagnostic serum biomarkers for Chagas’ disease in asymptomatic subjects by mass spectrometric profiling. J. Clin. Microbiol. 2010;48(4):1139–1149. doi: 10.1128/JCM.02207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saravia SG, Haberland A, Bartel S, et al. Cardiac troponin T measured with a highly sensitive assay for diagnosis and monitoring of heart injury in chronic Chagas disease. Arch. Pathol. Lab. Med. 2011;135(2):243–248. doi: 10.5858/135.2.243. [DOI] [PubMed] [Google Scholar]
- 73.Laucella SA, Mazliah DP, Bertocchi G, et al. Changes in Trypanosoma cruzi-specific immune responses after treatment: surrogate markers of treatment efficacy. Clin. Infect. Dis. 2009;49(11):1675–1684. doi: 10.1086/648072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lorena VM, Lorena IM, Braz SC, et al. Cytokine levels in serious cardiopathy of Chagas disease after in vitro stimulation with recombinant antigens from Trypanosoma cruzi. Scand. J. Immunol. 2010;72(6):529–539. doi: 10.1111/j.1365-3083.2010.02462.x. [DOI] [PubMed] [Google Scholar]
- 75.Wen JJ, Zago MP, Nunez S, Gupta S, Nunez Burgos F, Garg NJ. Serum proteomic signature of human chagasic patients for the identification of novel protein biomarkers of disease. Mol. Cell. Proteomics. 2012 doi: 10.1074/mcp.M112.017640. doi:10.1074/mcp.M112.017640. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional www.cinvestav.mx.
- 102.Laboratories de Biológicos Reactivos de México www.birmex.gob.mx.
- 103.Wikipedia File: Trypanosomiasis world map. http://en.wikipedia.org/wiki/File:Chagas_disease_world_map_-_DALY_-_WHO2004.svg. [Google Scholar]