Abstract
Background:
The aim of preemptive analgesia is to reduce central sensitization that arises from noxious inputs across the entire perioperative period. N-methyl d-aspartate receptor antagonists have the potential for attenuating central sensitization and preventing central neuroplasticity.
Materials and Methods:
Patients undergoing laparoscopic cholecystectomy were randomized into four groups of 20 patients each, who were administered the study drug intravenously 30 min before incision. Groups A, B, and C received ketamine in a dose of 1.00, 0.75 and 0.50 mg/kg, respectively, whereas group D received isotonic saline. Anesthetic and surgical techniques were standardized. Postoperatively, the degree of pain at rest, movement, and deep breathing using visual analogue scale, time of request for first analgesic, total opioid consumption, and postoperative nausea and vomiting were recorded in postanesthesia care unit for 24 h.
Results:
Pain scores were highest in Group D at 0 h. Groups A, B, and C had significantly decreased postoperative pain scores at 0, 0.5, 3, 4, 5, 6, and 12 h. Postoperative analgesic consumption was significantly less in groups A, B, and C as compared with group D. There was no significant difference in the pain scores among groups A, B, and C. Group A had a significantly higher heart rate and blood pressure than groups B and C at 0 and 0.5 h along with 10% incidence of hallucinations.
Conclusion:
Preemptive ketamine has a definitive role in reducing postoperative pain and analgesic requirement in patients undergoing laparoscopic cholecystectomy. The lower dose of 0.5 mg/kg being devoid of any adverse effects and hemodynamic changes is an optimal dose for preemptive analgesia in patients undergoing laparoscopic cholecystectomy.
Keywords: Ketamine, pain relief laparoscopic cholecystectomy, preemptive analgesia
Introduction
Pain control is pertinent for optimal care in surgical patients. Various treatment modalities and their combinations have been used; however, adequate pain control is still not achieved in a majority of patients.[1] Preemptive analgesia has been proposed to result in better pain management, reduced analgesic consumption, and improved patient satisfaction.[1]
Laparoscopic cholecystectomy is associated with less pain and disability, nonetheless many patients experience considerable pain in the postoperative period and improvement in analgesia is desirable. Pain after laparoscopic cholecystectomy involves several components and may be due to peritoneal stretching due to insufflation and diaphragmatic irritation.[2,3]
Preemptive analgesia is defined as an antinociceptive treatment that prevents the establishment of altered central processing of afferent input, which amplifies postoperative pain.[4] Although many drugs have demonstrated the evidence of preemptive analgesic benefit, N-methyl d-aspartate (NMDA) receptor antagonists have received greatest attention because NMDA receptors have a role in central sensitization and neural modulation.[5]
Evidence about the effectiveness of the NMDA antagonist ketamine to reduce postoperative hyperalgesia and acute and long-lasting pain is inconclusive.[6,7] There is some evidence that a single dose of perioperatively administered ketamine can reduce postoperative analgesic requirement,[8,9,10,11,12] whereas other studies have demonstrated no beneficial effect of preemptive ketamine.[4,5,6] These contradictory results could be because the studies were performed in different types of surgeries, which can influence the degree of pain perceived and thus the dose requirement of preemptive analgesic.[5]
It is thus plausible that a low dose of preemptive ketamine, which could be insufficient for a major surgery may be adequate for minimally invasive surgery such as laparoscopic cholecystectomy, which causes less tissue trauma.[11] Conceivably, a smaller dose may have the benefit of minimal hemodynamic effects without additional psychotomimetic adverse effects.[11]
With these considerations, the present study was designed to evaluate preemptive analgesic efficacy of intravenous ketamine in patients undergoing laparoscopic cholecystectomy by administering it 30 min before the incision. The research question also included comparing the preemptive analgesic efficacy of varying doses of ketamine to derive the optimal dose.
Materials and Methods
After obtaining the approval from the Hospital Ethics Committee and written informed consent from the patient, the study was conducted in a randomized double-blinded manner on a total of 80 adult patients of either gender belonging to ASA grades I and II and scheduled for elective laparoscopic cholecystectomy using a standardized general anesthesia technique.
Patients who were allergic to opioids or ketamine, with a history of drug abuse, being unable to comprehend visual analogue scale (VAS), patients with psychiatric illness or communication difficulties, morbidly obese patients, or ASA grade IV or V were excluded from the study.
Patients were divided into four groups of 20 patients each. Group A patients received ketamine in dose of 1.00 mg/kg, Group B patients were given ketamine 0.75 mg/kg, Group C was given 0.50 mg/kg ketamine, whereas Group D received isotonic saline [Figure 1].
Figure 1.
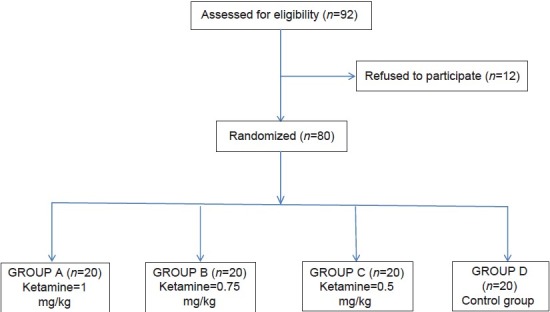
Study flow diagram
The study drug was drawn and diluted to a fixed volume of 10 mL by an anesthesiologist who was not involved in the administration of anesthesia and monitoring of the patient.
A thorough preanesthetic check-up, including detailed history, general and systemic examination, and review of routine investigations, was conducted a day before surgery. All the patients were shown the VAS scale preoperatively and instructed of its use as a tool for measuring postoperative pain. After ensuring nil per oral status of at least 6 h preoperatively, all patients were premedicated with Tab diazepam 10 mg and Tab ranitidine 150 mg the night before surgery.
After shifting the patients to operation theater, preinduction vital parameters, such as heart rate (HR), blood pressure, respiratory rate, temperature, and SpO2, were recorded. An intravenous access was achieved and all patients received Inj midazolam 1 mg and Inj glycopyrolate 0.2 mg intravenously (i.v.). Patients were then randomly allocated using computer-generated random numbers and concealed opaque envelopes to one of the four groups. After preoxygenation with 100% O2 for 3 min, anesthesia was induced with thiopentone sodium (2.5%) 4-6 mg/kg i.v. till the abolition of eyelash reflex. Endotracheal intubation with an adequate-sized, cuffed endotracheal tube was facilitated by neuromuscular blocker suxamethonium 1.5 mg/kg i.v. The study drug diluted to a volume of 10 mL with a coded label was administered i.v. by an anesthesiologist who was blinded to the nature of the study and nature of the drug being administered.
Anesthesia was maintained with oxygen and nitrous in a ratio of 1:2 along with isoflurane (0.8-2%) on controlled mechanical ventilation. Injection fentanyl citrate 1.5 g/kg i.v. was given immediately after induction for intraoperative analgesia in all patients. Vital parameters, including HR, blood pressure, electrocardiogram, temperature, end-tidal CO2 and O2 saturation, were monitored throughout the procedure. Neuromuscular blockade was achieved with atracurium besylate. Intraoperatively injection ondansetron 0.1 mg/kg i.v. was given to all patients about 20 min before closure.
At the completion of the surgery, the neuromuscular blockade was reversed with inj. neostigmine 0.04 mg/kg i.v. plus glycopyrrolate 0.01 mg/kg i.v. Trachea was extubated once the patient was awake after establishment of adequate spontaneous respiration. Patients were then shifted to recovery room where the continuing observations were made and recorded by an anesthesiologist who was unaware of the group to which the patient belonged.
All enrolled patients were observed for pain intensity and relief in postoperative recovery room using visual analogue scale (VAS) and verbal rating scale (VRS) every half-hour for first 2 h, every 1 h for the next 4 h, and then at 12 hand 24 h postoperatively. The time 0 h was taken as the time of shifting the patient to postoperative recovery room. Each time the pain was evaluated at rest, at slight movement, and at deep breathing. Patients were informed before surgery that they can request an analgesic if they feel pain, which was administered using i.v. fentanyl 1 g/kg. The first dose was given when the VAS at rest was ≥5 or VRS ≥2.[13] Furthermore, supplemental analgesia was administered using i.v. boluses of fentanyl 1 g/kg as and when the patient requested. Analgesics other than i.v. fentanyl were not permitted during the first 24 h to allow comparisons of opioid consumption between groups. After 24 h, analgesics at the discretion of anesthesiologist/surgeon were allowed.
Total number of patients requiring supplemental analgesia, time of first request of supplemental analgesia, and the total opioid dose required in the first 24 h in postoperative period was recorded. Baseline HR, systolic blood pressure (SBP), diastolic blood pressure (DBP) respiratory rate (RR), and saturation (SpO2) were recorded before shifting patient to operating table. Postoperative recording of these vital parameters was performed every half-hour for the first 2 h, every 1 h for the next 4 h and then at 12 h and 24 h in the postanesthesia care unit. Adverse effects associated with ketamine, such as nausea, vomiting, and hallucinations were also recorded. Hallucination was defined as a false sensory experience in which the patients reported they saw, heard, smelled, tasted, or felt something that was non-existent.
Statistical analysis
Sample size was determined taking into consideration that a sample size of 20 patients per group would give a power of 80% at an -level of 0.05 (two tailed). Data were analyzed with the SPSS statistical program (SPSS Inc, Chicago, IL, USA) using Student's t-test, and analysis of variance (ANOVA) test. Data of the different groups were compared by Student's t test for unpaired comparisons for normally distributed data, Kruskal-Wallis test for nonnormally distributed data or Chi-square test for qualitative data. Differences among group means were compared using ANOVA. Incidence of side effects and number of patients receiving rescue analgesia were analyzed using Fisher's exact test. Significance levels throughout this study were considered at P > 0.05.
Results
The demographic profile was found to be comparable with no statistically significant difference between the groups.
The mean VAS score at rest was significantly higher in Group D at 0 and 0.5 h postoperatively with maximum value of 4.75 ± 1.41 cm and 4.25 ± 1.77 cm, respectively [Figure 2].
Figure 2.
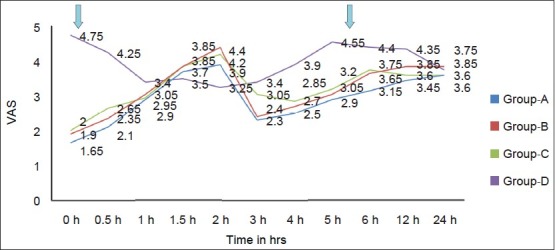
Trends in VAS at rest in different groups. VAS, visual analog scale
The VAS score in groups A, B, and C at 0, 0.5, 1, 1.5, and 2 h was comparable with no significant intergroup variation in between them.
At 1, 1.5, and 2 h the mean VAS score at rest was comparable in all the groups with no significant intergroup variation.
Mean VAS scores at 3, 4, 5, 6, and 12 h were significantly higher in Group D when compared with mean VAS scores in groups A, B, and C. At 24 h the mean VAS score at rest was comparable in all the groups with no significant intergroup variation [Figure 2].
VAS scores on deep breathing were higher than VAS scores at rest and on slight movement. The highest VAS scores on deep breathing were observed in Group D at 0 h with a mean of 7.40 ± 0.99 followed by VAS score of 6.40 ± 1.43 at 30 min again in Group D [Figure 3].
Figure 3.

Trends in VAS at deep breathing in different groups. VAS, visual analog scale
VAS and VRS were significantly more in Group D as compared with groups A, B, and C at most of the time intervals [Figures 4 and 5].
Figure 4.
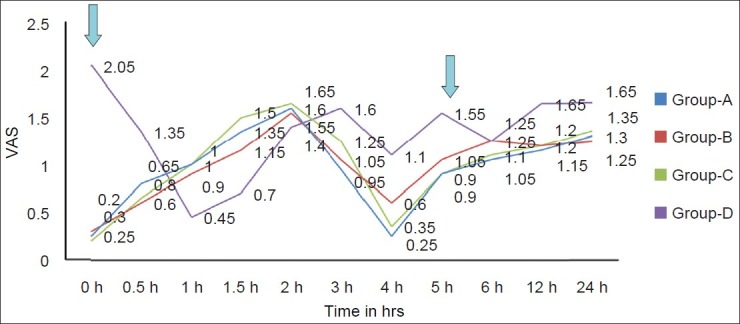
Trends in VRS at rest in different groups at various time intervals. VRS, verbal rating scale
Figure 5.
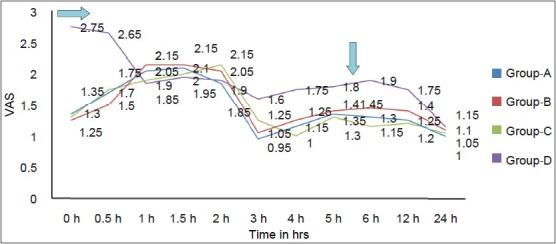
Trends in VRS at deep breathing in different groups at various time intervals. VRS, verbal rating scale
Total opioid consumption was significantly more in Group D as compared with groups A, B, and C [Figure 6].
Figure 6.

Mean number of analgesic doses given to subjects in different groups.
The mean time to receive rescue analgesic was significantly more in the groups A, B, and C as compared with Group D [Figure 7]. As the patients in Group D received the rescue analgesic earlier than the other three groups, the pain scores were statistically similar in all the groups at most times between 1 and 2 h.
Figure 7.

Mean time to the requirement of the rescue analgesic
The mean time to request of rescue analgesia and the mean number of doses of opioid required were similar in groups A, B, and C with no statistically significant intergroup difference.
The HR, SBP, DBP and RR were significantly more in Group D than groups B and C at most times in first 1.5 h. HR was significantly greater in Group A than groups B and C at 0 h [Figure 8]. SBP was significantly greater at 0 and 0.5 h in Group A as compared with groups B and C.
Figure 8.
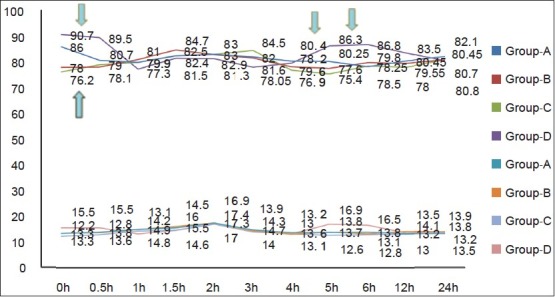
Comparison of respiratory and heart rate between different groups at different time intervals postoperatively
The incidence of nausea and vomiting was comparable in all the groups.
Group A had an incidence of hallucinations in 10% of its subjects, whereas none of the patients in any other group reported hallucinations [Table 1].
Table 1.
Distribution of subjects according to side effects in different groups

Discussion
Intraoperative and postoperative noxious inputs cause prolonged firing of C-fiber nociceptors resulting in the release of glutamate. Glutamate is a major excitatory transmitter in the central nervous system, which activates postsynaptic NMDA receptors. NMDA receptor activation contributes to pain processing and pain phenomena such as wind up, spinal neural plasticity. Enhanced NMDA receptor activation plays a role in inflammatory and neuropathic pain states and results in the activation and exacerbation of secondary hyperalgesia. This also initiates translational changes of the second-order neurones, which might be a crucial link in the pathogenesis of chronic pain.[14] Analgesic intervention before the noxious stimulus, that is, preemptive analgesia may attenuate or block sensitization and hence reduce acute pain.[15]
Although many drugs have demonstrated the evidence of preemptive analgesic benefit,[8] treatments that are likely to prevent the development of central excitability may have the greatest benefit. Ketamine, an NMDA receptor antagonist, is an interesting option for this purpose.
Our study demonstrated that low-dose i.v. ketamine administered before surgical incision has preemptive effect on postoperative pain and reduced analgesic requirements during the first 24 h after laparoscopic cholecystectomy. We assessed VAS and VRS at rest, on slight movement, and on deep breathing as the VAS assessment at slight movement and at deep breathing is more reproducible than the VAS at rest.[5] The VRS scale was used as it is easier for the patient to use.[5]
VAS and VRS at rest, on deep breathing and on slight movement were higher in saline group at most time intervals except at 1.5, 2, and 2.5 h. This implies that groups that received ketamine had significantly less pain than the group that received saline. Interestingly, VAS and VRS in the three groups receiving different doses of ketamine were similar, implying that a lower dose of 0.5 mg/kg was equi-efficacious to a higher dose of 1.00 mg/kg for preemptive analgesia. The reason for similar VAS and VRS at 1.5, 2, and 2.5 h is that the control group received rescue analgesia earlier than the ketamine groups so that at the time intervals mentioned, the control group had already received rescue analgesics accounting for the statistically similar VAS and VRS.
We used i.v. fentanyl for rescue analgesia as it is a highly potent and short-acting drug thereby allowing us to make repeated unbiased assessments of the efficacy of study drug.
The hemodynamic parameters were studied as an indirect reflection of the pain control in the various groups. Patients in Group D had higher VAS and VRS scores and early requirement of rescue analgesics. This implies that patients in Group D had more pain earlier and could account for the higher HR and blood pressure in this group. Ketamine is known to cause tachycardia and increased blood pressure.[5] This could explain the significantly higher HR in Group A. The low mean HR in the groups B and C at 0, 0.5, 2, 3, and 4 h correspond well with the preemptive analgesic effects of ketamine as compared with the control.
The role of preemptive analgesia with ketamine has been previously reported in laparoscopic gynecological surgery.[11] Preemptive ketamine also had an opioid sparing effect in opioid abusers undergoing lithotripsy.[16]
Our results, however, are at a variance with the results of Darabi et al. who could not demonstrate any reduction in analgesic consumption in the groups receiving ketamine.[17] This could be ascribed to both a smaller dose of ketamine used in their study along with simultaneous use of rectal diclofenac and i.v. paracetamol. Inguinal herniorraphy being a less painful procedure, these analgesics may be sufficient to take care of postoperative pain masking any preemptive analgesic effects of ketamine.
Similarly, Dullenkopf et al. did not find any preemptive analgesic effects of ketamine. However, they had a heterogenous population of general surgical and orthopedic patients, and dose of ketamine used in the study may have been inadequate for the painful surgical procedures performed.[5] It is proposed that adequate sensory blockade is necessary for preemptive effect of ketamine to be exhibited. In a study on patients undergoing gastrectomy, ketamine alone was ineffective for preemptive analgesia and preemptive effect was only demonstrated once epidural morphine was also added.[18] The authors explained that small dose administered was ineffective to block sensory outputs, which occurred once epidural morphine was also added.
In our study, two patients (10%) experienced hallucinations in Group A. This corresponds to the incidence reported in the literature with similar preemptive doses.[19] Patients receiving smaller doses of ketamine did not experience any hallucinations.
The incidence of nausea and vomiting was similar in all the groups. Recently a small dose of ketamine has been shown to decrease postoperative nausea and vomiting, but a similar decrease was not seen in our study, as the laparoscopic nature of surgery might have masked the antiemetic property at small doses.[20]
One limitation of our study is that we did not follow up the patients to evaluate whether chronic pain was reduced with the small doses of ketamine used in the study. Ketamine is known to alter the neuroplasticity and reduce the development of chronic pain syndromes.[14] Future research should also focus on the long-term effects of ketamine in reducing the incidence of chronic pain syndromes.
We conclude that preemptive ketamine has a definitive role in reducing postoperative pain and analgesic requirement in patients undergoing laparoscopic cholecystectomy. A low dose of 0.5 mg/kg was devoid of any adverse effects and hemodynamic changes. Hence, it is an optimal dose for preemptive analgesia in patients undergoing laparoscopic cholecystectomy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hariharan S, Moseley H, Kumar A, Raju S. The effect of preemptive analgesia in postoperative pain relief: A prospective double-blind randomized study. Pain Med. 2009;10:49–53. doi: 10.1111/j.1526-4637.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 2.Ingelmo PM, Bucciero M, Somaini M, Sahillioglu E, Garbagnati A, Charton A, et al. Intraperitoneal nebulization of ropivacaine for pain control after laparoscopic cholecystectomy: A double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2013;110:800–6. doi: 10.1093/bja/aes495. [DOI] [PubMed] [Google Scholar]
- 3.Mouton WG, Bessell JR, Otten KT, Maddern GJ. Pain after laparoscopy. Surg Endosc. 1999;13:445–8. doi: 10.1007/s004649901011. [DOI] [PubMed] [Google Scholar]
- 4.Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: A meta-analysis. Anesth Analg. 2005;100:757–73. doi: 10.1213/01.ANE.0000144428.98767.0E. [DOI] [PubMed] [Google Scholar]
- 5.Dullenkop FA, Müller R, Dillmann F, Wiedemeier P, Hegi TR, Gautschi S. An intraoperative pre-incision single dose of intravenous ketamine does not have an effect on postoperative analgesic requirements under clinical conditions. Anaesth Intensive Care. 2009;37:753–7. doi: 10.1177/0310057X0903700519. [DOI] [PubMed] [Google Scholar]
- 6.Carstensen M, Møller AM. Adding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: A qualitative review of randomized trials. Br J Anaesth. 2010;104:401–6. doi: 10.1093/bja/aeq041. [DOI] [PubMed] [Google Scholar]
- 7.Elia N, Tramer MR. Ketamine and postoperative pain: A quantitative systematic review of randomised trials. Pain. 2005;113:61–7. doi: 10.1016/j.pain.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: A meta-analysis. Anesth Analg. 2005;100:757–73. doi: 10.1213/01.ANE.0000144428.98767.0E. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Tsueda K, Lansing PS, Tolan MM, Fuhrman TM, Ignacio CI, et al. Small-dose ketamine enhances morphine-induced analgesia after outpatient surgery. Anesth Analg. 1999;89:98–103. doi: 10.1097/00000539-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Menigaux C, Guignard B, Fletcher D, Sessler DI, Dupont X, Chauvin M. Intraoperative smalldose ketamine enhances analgesia after outpatient knee arthroscopy. Anesth Analg. 2001;93:606–12. doi: 10.1097/00000539-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Kwok RF, Lim J, Chan MT, Gin T, Chiu WK. Preoperative ketamine improves postoperative analgesia after gynaecologic laparoscopic surgery. Anesth Analg. 2004;98:1044–9. doi: 10.1213/01.ANE.0000105911.66089.59. [DOI] [PubMed] [Google Scholar]
- 12.Truta E, Vartic M, Cristea AN. Clinical study regarding preemptive analgesic effect of ketamine and remifentanyl in laparoscopic cholecystectomy. Farmacia. 2011;59:239–45. [Google Scholar]
- 13.Krishna R, Nataraj MS. Efficacy of a single dose of a transdermal diclofenac patch as pre-emptive postoperative analgesia: A comparison with intramuscular diclofenac. South Afr J Anesth Analg. 2012;18:194–7. [Google Scholar]
- 14.Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105(suppl 1):i69–85. doi: 10.1093/bja/aeq323. [DOI] [PubMed] [Google Scholar]
- 15.Warncke T, Stubhaug A, Jørum E. Ketamine, an NMDA receptor antagonist, suppresses spatial and temporal properties of burn-induced secondary hyperalgesia in man: A double-blind, cross-over comparison with morphine and placebo. Pain. 1997;72:99–106. doi: 10.1016/s0304-3959(97)00006-7. [DOI] [PubMed] [Google Scholar]
- 16.Gharaei B, Jafari A, Aghamohammadi H, Kamranmanesh M, Poorzamani M, Elyassi H, et al. Opioid-Sparing Effect of Preemptive Bolus Low-Dose Ketamine for Moderate Sedation in Opioid Abusers Undergoing Extracorporeal Shock Wave Lithotripsy: A Randomized Clinical Trial. Anesth Analg. 2013;116:75–80. doi: 10.1213/ANE.0b013e31826f0622. [DOI] [PubMed] [Google Scholar]
- 17.Darabi ME, Mireskandari SM, Sadeghi M, Salamati P, Rahimi E. Ketamine has no pre-emptive analgesic effect In children undergoing inguinal hernia repair. Acta Med Iran. 2008;46:451–6. [Google Scholar]
- 18.Aida S, Yamakura T, Baba H, Taga K, Fukuda S, Shimoji K. Preemptive analgesia by intravenous low-dose ketamine and epidural morphine in gastrectomy: A randomized double-blind study. Anesthesiology. 2000;92:1624–30. doi: 10.1097/00000542-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Launo C, Bassi C, Spagnolo L, Badano S, Ricci C, Lizzi A, et al. Preemptive ketamine during general anesthesia for postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Minerva Anestesiol. 2004;70:727–38. [PubMed] [Google Scholar]
- 20.Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. 2011;107(Suppl 1):i27–40. doi: 10.1093/bja/aer358. [DOI] [PubMed] [Google Scholar]


